Cardiac Angiosarcoma in the Right Atrium Treated by Surgical Resection
Abstract
:1. Introduction
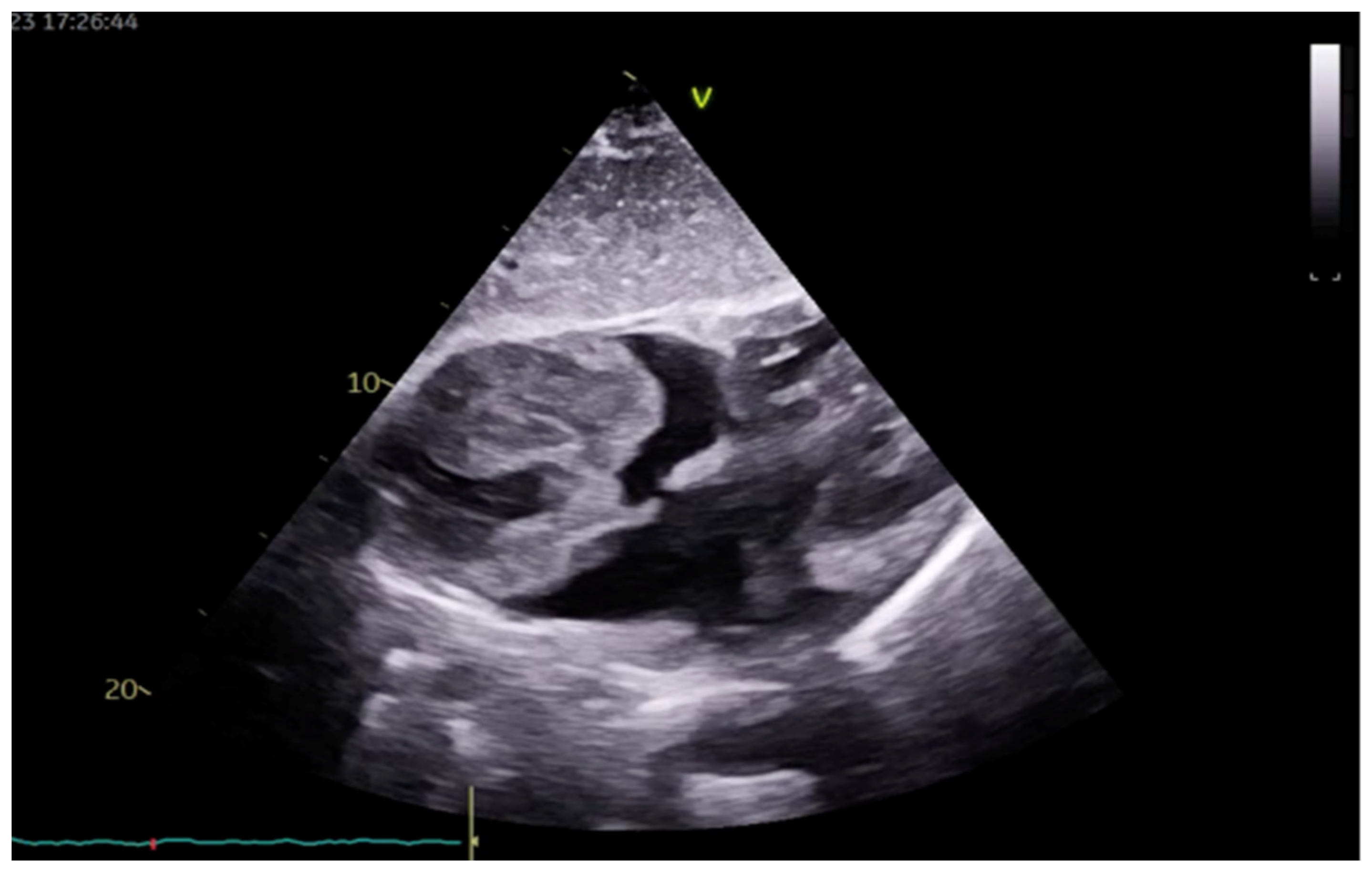
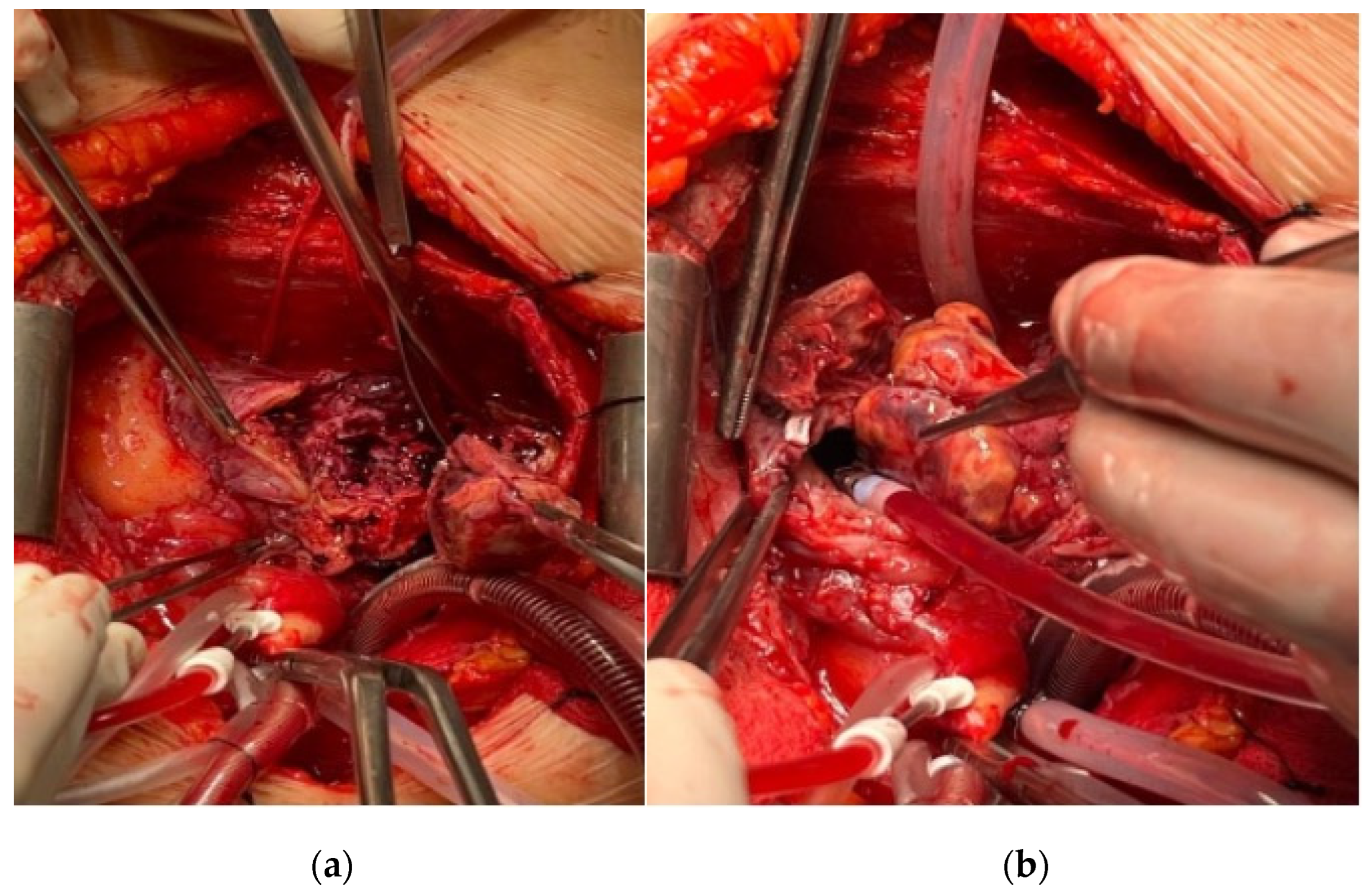
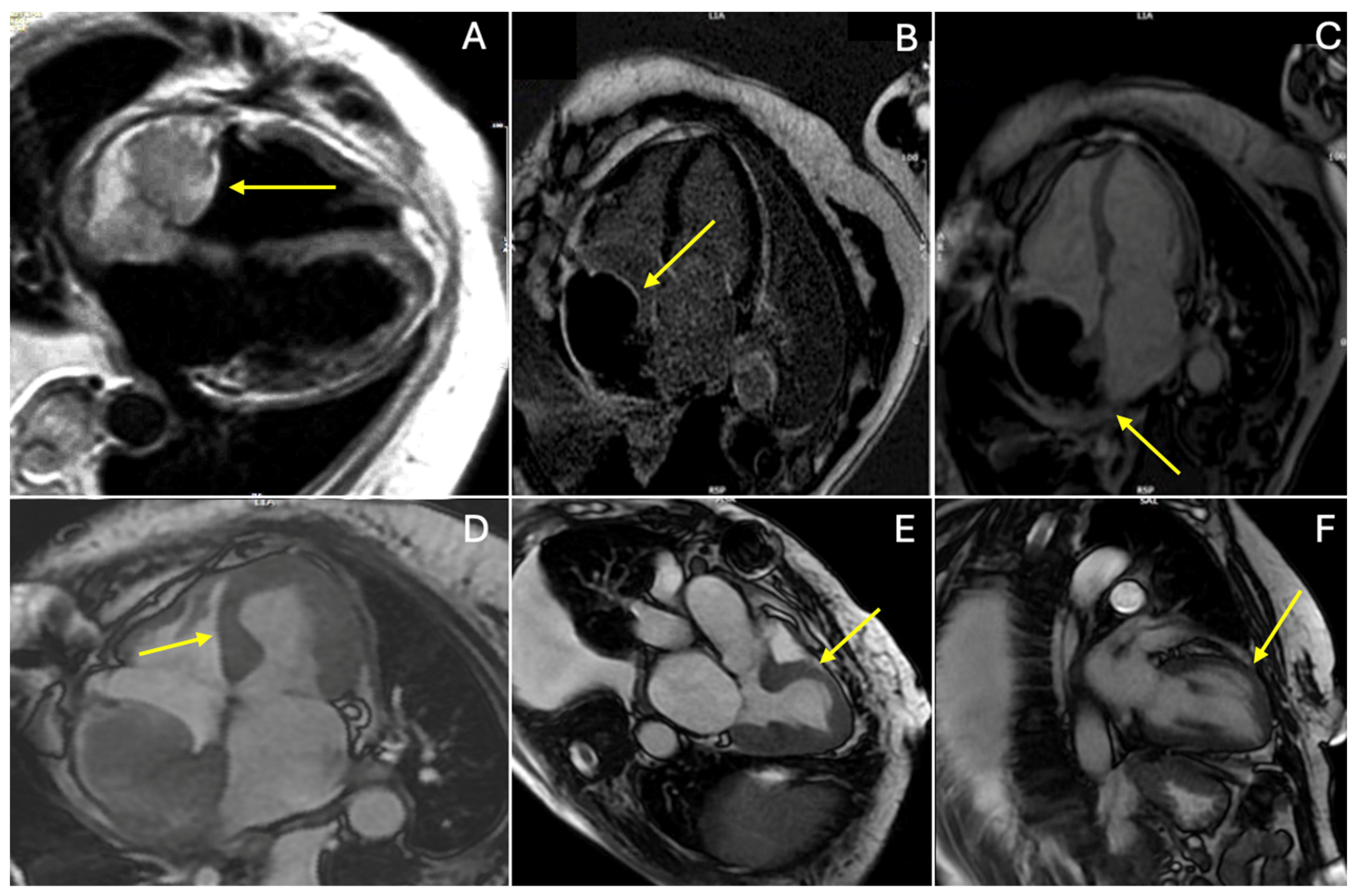
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatti, M.; D’Angelo, T.; Muscogiuri, G.; Dell’aversana, S.; Andreis, A.; Carisio, A.; Darvizeh, F.; Tore, D.; Pontone, G.; Faletti, R. Cardiovascular magnetic resonance of cardiac tumors and masses. WJC 2021, 13, 628–649. [Google Scholar] [CrossRef]
- Walker, E.A.; Salesky, J.S.; Fenton, M.E.; Murphey, M.D. Magnetic Resonance Imaging of Malignant Soft Tissue Neoplasms in the Adult. Radiol. Clin. N. Am. 2011, 49, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Bendel, E.C.; Maleszewski, J.J.; Araoz, P.A. Imaging Sarcomas of the Great Vessels and Heart. Semin. Ultrasound CT MRI 2011, 32, 377–404. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Lai, H.; Lima, J.; Tong, W.; Qian, Y.; Lai, S. Echocardiographic and pathologic characteristics of primary cardiac tumors: A study of 149 cases. Int. J. Cardiol. 2002, 84, 69–75. [Google Scholar] [CrossRef]
- Gong, Y.; Hong, T.; Chen, M.; Huo, Y. A Right Heart Angiosarcoma with Rapidly Progressing Hemorrhagic Pericardial Effusion. Intern. Med. 2011, 50, 455–458. [Google Scholar] [CrossRef]
- Göbölös, L.; Bhatnagar, G. Angiosarcoma of the Heart. JACC Case Rep. 2021, 3, 950–953. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Bois, M.C.; Bois, J.P.; Young, P.M.; Stulak, J.M.; Klarich, K.W. Neoplasia and the Heart. J. Am. Coll. Cardiol. 2018, 72, 202–227. [Google Scholar] [CrossRef]
- Vaporciyan, A.; Reardon, M.J. Right heart sarcomas. Methodist. DeBakey Cardiovasc. J. 2010, 6, 44. [Google Scholar] [CrossRef]
- Strohl, K.P. Angiosarcoma of the Heart. Arch. Intern. Med. 1976, 136, 928–929. [Google Scholar] [CrossRef]
- Kupsky, D.F.; Newman, D.B.; Kumar, G.; Maleszewski, J.J.; Edwards, W.D.; Klarich, K.W. Echocardiographic Features of Cardiac Angiosarcomas: The Mayo Clinic Experience (1976–2013). Echocardiography 2016, 33, 186–192. [Google Scholar] [CrossRef]
- Romero-Farina, G.; Candell-Riera, J.; Beltrán-Ror, Á.; González-Moreno, J.B.; Bigalli, D.; Stratta, A. Primary Cardiac Angiosarcoma: Diagnostic Utility of Computed Tomography and Cardiac Magnetic Resonance. Rev. Española Cardiol. (Engl. Ed.) 2004, 57, 1234–1237. [Google Scholar] [CrossRef]
- Loukas, M. Primary cardiac angiosarcoma—A review. Med. Sci. Monit. 2014, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pigott, C.; Welker, M.; Khosla, P.; Higgins, R.S. Improved outcome with multimodality therapy in primary cardiac angiosarcoma. Nat. Rev. Clin. Oncol. 2008, 5, 112–115. [Google Scholar] [CrossRef]
- Fields, E.C.; Squires, B.; Lomas, H. Treating the Critically Ill with Radiotherapy: Lessons Learned from a Young Woman with Cardiac Angiosarcoma. Front. Oncol. 2017, 7, 29. [Google Scholar] [CrossRef]
- Bhukar, R.K.; Gowda, D.; Rao, J.N.; Desai, N. Management of atrial thrombus formation following surgical closure of an atrial septal defect. J. Card. Surg. 2017, 32, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, S.H.; Reardon, M.J. Surgical treatment of primary cardiac sarcomas. Tex. Heart Inst. J. 2009, 36, 451–452. [Google Scholar] [PubMed] [PubMed Central]
- Filippiadis, D.K.; Kapetanakis, E.I.; Spiliopoulos, S.; Kostopanagiotou, K.; Tomos, P.; Kelekis, A. Bleeding Remission with Microwave Ablation in a Transfusion-Dependent Patient with Hemorrhaging Angiosarcoma of the Pleura. J. Vasc. Interv. Radiol. 2018, 29, 1298–1300. [Google Scholar] [CrossRef]
- Szczeklik, M.; Gupta, P.; Amersey, R.; Lall, K.S. Reconstruction of the Right Atrium and Superior Vena Cava with Extracellular Matrix: Extracellular matrix in cardiac reconstruction. J. Card. Surg. 2015, 30, 351–354. [Google Scholar] [CrossRef]
- Woo, J.S.; Fishbein, M.C.; Reemtsen, B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc. Pathol. 2016, 25, 12–17. [Google Scholar] [CrossRef]
- Weis, J.; Geiger, R.; Kilo, J.; Zimpfer, D. Cormatrix® for vessel reconstruction in paediatric cardiac surgery—A word of caution. Interact. CardioVascular Thorac. Surg. 2022, 34, 597–603. [Google Scholar] [CrossRef]
- Gent, D.G.; Rebecca, D. The 2022 European Society of Cardiology Cardio-oncology Guidelines in Focus. Eur Cardiol. 2023, 18, e16. [Google Scholar] [CrossRef]
- Buckley, O.; Madan, R.; Kwong, R.; Rybicki, F.J.; Hunsaker, A. Cardiac Masses, Part 1: Imaging Strategies and Technical Considerations. Am. J. Roentgenol. 2011, 197, W837–W841. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, J.; Xu, L.; Li, Y.; Liu, D.; Sun, Z.; Wen, Z. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant. Imaging Med. Surg. 2020, 10, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Hoey, E.T.D.; Shahid, M.; Ganeshan, A.; Baijal, S.; Simpson, H. MRI assessment of cardiac tumours: Part 2, spectrum of appearances of histologically malignant lesions and tumour mimics. Quant. Imaging Med. Surg. 2014, 4, 489. [Google Scholar] [PubMed]
- Chen, Y.; Li, Y.; Zhang, N.; Shang, J.; Li, X.; Liu, J.; Xu, L.; Liu, D.; Sun, Z.; Wen, Z. Clinical and Imaging Features of Primary Cardiac Angiosarcoma. Diagnostics 2020, 10, 776. [Google Scholar] [CrossRef]
- O’Donnell, D.H.; Abbara, S.; Chaithiraphan, V.; Yared, K.; Killeen, R.P.; Cury, R.C.; Dodd, J.D. Cardiac Tumors: Optimal Cardiac MR Sequences and Spectrum of Imaging Appearances. Am. J. Roentgenol. 2009, 193, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, G.A.; Bruno, P.; Colizzi, C.; Crea, F.; Massetti, M. Takotsubo Cardiomyopathy Following Cardiac Surgery: Takotsubo and cardiac surgery. J. Card. Surg. 2016, 31, 89–95. [Google Scholar] [CrossRef]
- Plácido, R.; Cunha Lopes, B.; Almeida, A.G.; Rochitte, C.E. The role of cardiovascular magnetic resonance in takotsubo syndrome. J. Cardiovasc. Magn. Reson. 2016, 18, 68. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragicevic-Antonic, M.; Rankovic-Nicic, L.; Stamenkovic, G.; Petrovic, M.; Loncar, G.; Markovic, N.; Dimitrijevic, A.; Bulatovic, S.; Cirkovic, M.; Borzanovic, B.; et al. Cardiac Angiosarcoma in the Right Atrium Treated by Surgical Resection. Medicina 2024, 60, 1321. https://doi.org/10.3390/medicina60081321
Dragicevic-Antonic M, Rankovic-Nicic L, Stamenkovic G, Petrovic M, Loncar G, Markovic N, Dimitrijevic A, Bulatovic S, Cirkovic M, Borzanovic B, et al. Cardiac Angiosarcoma in the Right Atrium Treated by Surgical Resection. Medicina. 2024; 60(8):1321. https://doi.org/10.3390/medicina60081321
Chicago/Turabian StyleDragicevic-Antonic, Milica, Ljiljana Rankovic-Nicic, Gordana Stamenkovic, Masa Petrovic, Goran Loncar, Nikola Markovic, Ana Dimitrijevic, Sulin Bulatovic, Milan Cirkovic, Branislava Borzanovic, and et al. 2024. "Cardiac Angiosarcoma in the Right Atrium Treated by Surgical Resection" Medicina 60, no. 8: 1321. https://doi.org/10.3390/medicina60081321




