Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review
Abstract
1. Introduction
1.1. Definition and Clinical Features of POTS
1.2. Current Clinical Spectrum
2. COVID-19-Related POTS
2.1. Definition
2.2. Presumed Mechanism for COVID-19-Related POTS
2.2.1. Renin–Angiotensin–Aldosterone System (RAAS) Dysregulation
2.2.2. Hyperadrenergic Reaction
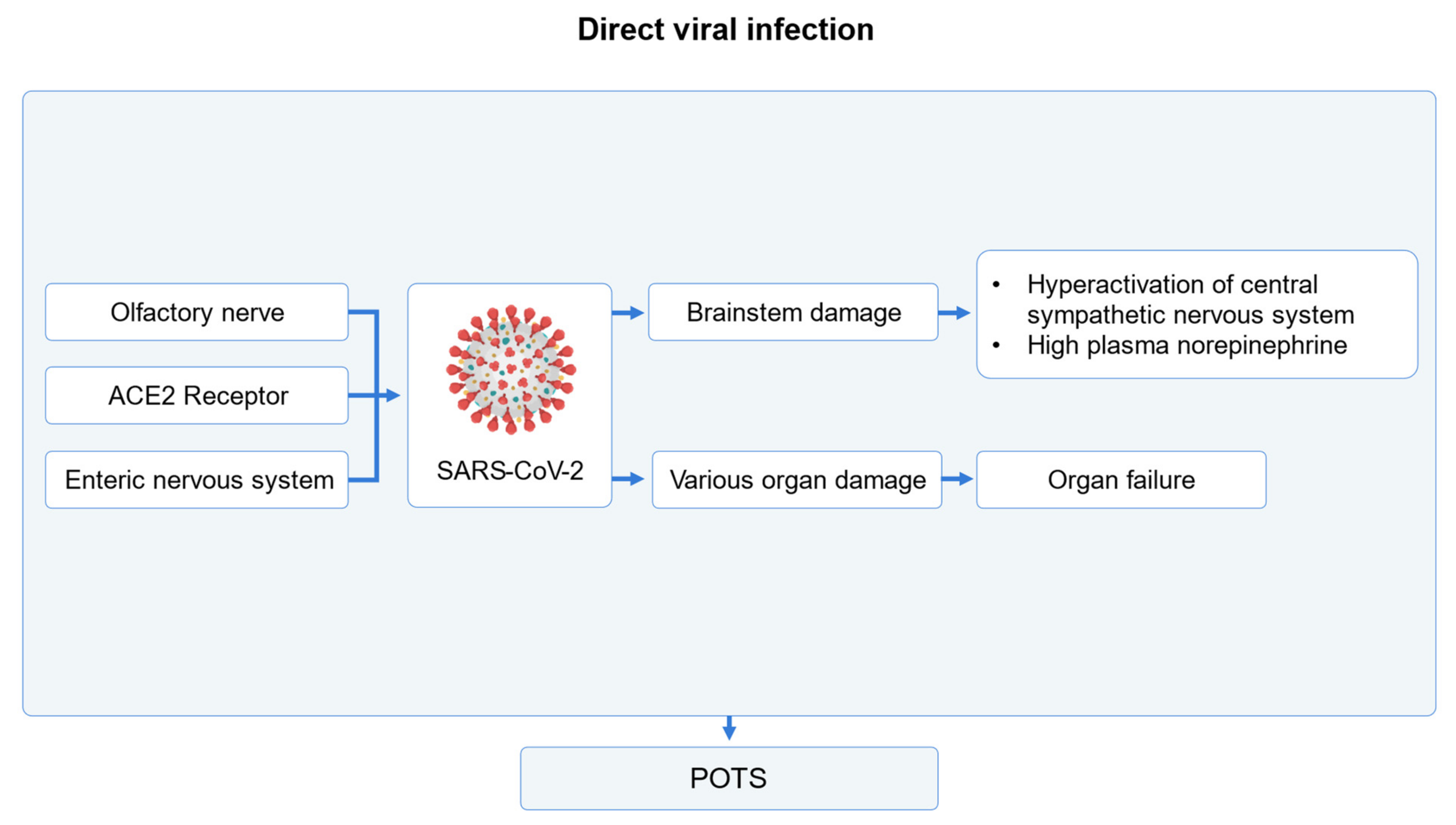
2.2.3. Direct Viral Infection
2.2.4. Mechanism of Post-COVID-19 Tachycardia Syndrome
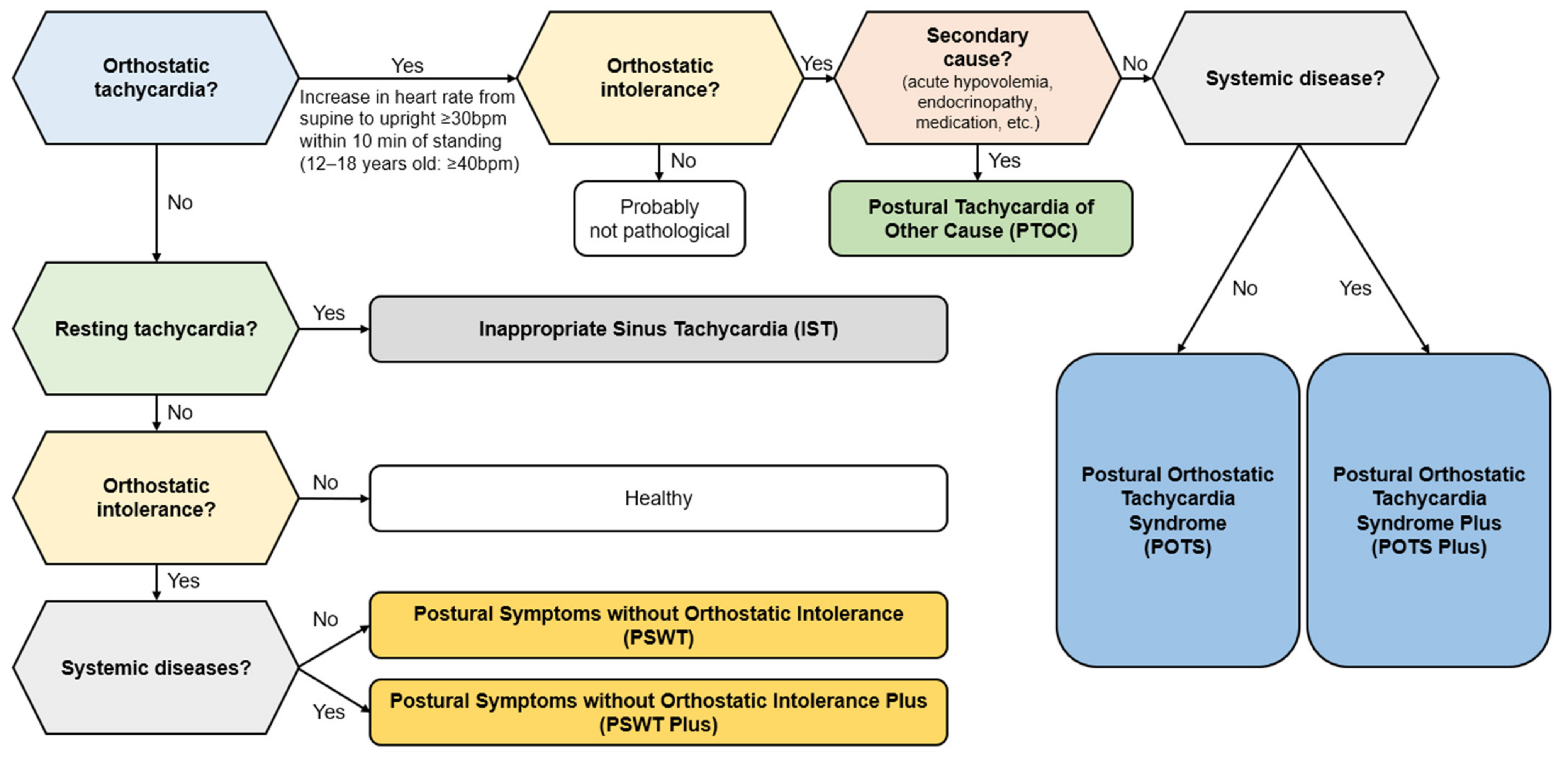
3. Diagnosis of COVID-19-Related POTS
Differential Diagnosis
4. Management
4.1. Patient Education
4.2. Exercise
4.3. Treatment for COVID-19-Related POTS Due to Hypovolemia
4.4. Treatment for COVID-19-Related POTS Due to Intravenous or Splanchnic Pooling
4.5. Treatments for COVID-19-Related POTS Due to Increased Norepinephrine in the Blood
4.6. Treatment for COVID-19-Related POTS Due to an Autoimmune Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, J.H.; Jeon, J.; Kim, J.; Song, T.J. Risk of COVID-19 Infection and of Severe Complications Among People with Epilepsy: A Nationwide Cohort Study. Neurology 2022, 98, e1886–e1892. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jeon, J.; Song, T.J.; Kim, J. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: A nationwide retrospective cohort study. BMC Infect. Dis. 2022, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Chang, Y.; Jeon, J.; Shin, J.I.; Song, T.J.; Kim, J. Association of Alzheimer’s Disease with COVID-19 Susceptibility and Severe Complications: A Nationwide Cohort Study. J. Alzheimers Dis. 2022, 87, 701–710. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, J.; Song, T.J.; Kim, J. Association of triglyceride-glucose index with prognosis of COVID-19: A population-based study. J. Infect. Public Health 2022, 15, 837–844. [Google Scholar] [CrossRef]
- Ju, H.; Kim, Y.H.; Seok, J.M.; Kim, B.J. Unusual Demyelinating Disease of the Central Nervous System Involving Bilateral Corticospinal Tracts Following COVID-19 Infection: A Case Report. J. Clin. Neurol. 2023, 19, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yu, W.; Sun, M.; Zhang, W.; Zhou, D.; Sun, J.; Wang, M. Outcome of COVID-19 Infection in Patients with Multiple Sclerosis Who Received Disease-Modifying Therapies: A Systematic Review and Meta-Analysis. J. Clin. Neurol. 2023, 19, 381–391. [Google Scholar] [CrossRef]
- Cadilhac, D.A.; Kim, J.; Cloud, G.; Anderson, C.S.; Tod, E.K.; Breen, S.J.; Faux, S.; Kleinig, T.; Castley, H.; Lindley, R.I.; et al. Effect of the Coronavirus Disease 2019 Pandemic on the Quality of Stroke Care in Stroke Units and Alternative Wards: A National Comparative Analysis. J. Stroke 2022, 24, 79–87. [Google Scholar] [CrossRef]
- Larsen, N.W.; Stiles, L.E.; Miglis, M.G. Preparing for the long-haul: Autonomic complications of COVID-19. Auton. Neurosci. 2021, 235, 102841. [Google Scholar] [CrossRef]
- Kwan, A.C.; Ebinger, J.E.; Wei, J.; Le, C.N.; Oft, J.R.; Zabner, R.; Teodorescu, D.; Botting, P.G.; Navarrette, J.; Ouyang, D.; et al. Apparent Risks of Postural Orthostatic Tachycardia Syndrome Diagnoses after COVID-19 Vaccination and SARS-CoV-2 Infection. Nat. Cardiovasc. Res. 2022, 1, 1187–1194. [Google Scholar] [CrossRef]
- Narasimhan, B.; Calambur, A.; Moras, E.; Wu, L.; Aronow, W. Postural Orthostatic Tachycardia Syndrome in COVID-19: A Contemporary Review of Mechanisms, Clinical Course and Management. Vasc. Health Risk Manag. 2023, 19, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting-Part 1. Auton. Neurosci. 2021, 235, 102828. [Google Scholar] [CrossRef]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021, 31, 365–368. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Bryarly, M.; Phillips, L.T.; Fu, Q.; Vernino, S.; Levine, B.D. Postural orthostatic tachycardia syndrome: JACC focus seminar. J. Am. Coll. Cardiol. 2019, 73, 1207–1228. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J. Neurol. 2022, 269, 725–732. [Google Scholar] [CrossRef]
- Peggs, K.J.; Nguyen, H.; Enayat, D.; Keller, N.R.; Al-Hendy, A.; Raj, S.R. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int. J. Gynecol. Obstet. 2012, 118, 242–246. [Google Scholar] [CrossRef]
- Tu, Y.; Abell, T.L.; Raj, S.R.; Mar, P.L. Mechanisms and management of gastrointestinal symptoms in postural orthostatic tachycardia syndrome. Neurogastroenterol. Motil. 2020, 32, e14031. [Google Scholar] [CrossRef]
- Stewart, J.M.; Montgomery, L.D. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1319–H1327. [Google Scholar] [CrossRef]
- Wells, R.; Spurrier, A.J.; Linz, D.; Gallagher, C.; Mahajan, R.; Sanders, P.; Page, A.; Lau, D.H. Postural tachycardia syndrome: Current perspectives. Vasc. Health Risk Manag. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Opie, M.; Arnold, A.C. Cognitive and psychological issues in postural tachycardia syndrome. Auton. Neurosci. 2018, 215, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.R.; Chang-Kit, L.; Raj, S.R.; Black, B.K.; Milam, D.F.; Reynolds, W.S.; Biaggioni, I.; Robertson, D.; Dmochowski, R.R. Overactive bladder and autonomic dysfunction: Lower urinary tract symptoms in females with postural tachycardia syndrome. Neurourol. Urodyn. 2017, 36, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Deb, A.; Culbertson, C.; Morgenshtern, K.; DePold Hohler, A. Dermatological Manifestations of Postural Tachycardia Syndrome Are Common and Diverse. J. Clin. Neurol. 2016, 12, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Watari, M.; Nakane, S.; Mukaino, A.; Nakajima, M.; Mori, Y.; Maeda, Y.; Masuda, T.; Takamatsu, K.; Kouzaki, Y.; Higuchi, O.; et al. Autoimmune postural orthostatic tachycardia syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 486–492. [Google Scholar] [CrossRef]
- Raj, S.R.; Guzman, J.C.; Harvey, P.; Richer, L.; Schondorf, R.; Seifer, C.; Thibodeau-Jarry, N.; Sheldon, R.S. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can. J. Cardiol. 2020, 36, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Zubchenko, S.; Kril, I.; Nadizhko, O.; Matsyura, O.; Chopyak, V. Herpesvirus infections and post-COVID-19 manifestations: A pilot observational study. Rheumatol. Int. 2022, 42, 1523–1530. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci. 2021, 22, 6703. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune post-COVID vaccine syndromes: Does the spectrum of autoimmune/inflammatory syndrome expand? Clin. Rheumatol. 2022, 41, 1603–1609. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Guilmot, A.; Maldonado Slootjes, S.; Sellimi, A.; Bronchain, M.; Hanseeuw, B.; Belkhir, L.; Yombi, J.C.; De Greef, J.; Pothen, L.; Yildiz, H.; et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021, 268, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T., 3rd; Kvale, H.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Is Associated with Elevated G-Protein Coupled Receptor Autoantibodies. J. Am. Heart Assoc. 2019, 8, e013602. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Andargie, T.E.; Tsuji, N.; Seifuddin, F.; Jang, M.K.; Yuen, P.S.; Kong, H.; Tunc, I.; Singh, K.; Charya, A.; Wilkins, K.; et al. Cell-free DNA maps COVID-19 tissue injury and risk of death and can cause tissue injury. JCI Insight 2021, 6, e147610. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Gyebi, G.A.; Batiha, G.E. COVID-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro 2021, 13, 17590914211057635. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Bucchi, A.; Milanesi, R.; Paina, M.; Barbuti, A.; Gnecchi-Ruscone, T.; Bianco, E.; Vitali-Serdoz, L.; Cappato, R.; DiFrancesco, D. A gain-of-function mutation in the cardiac pacemaker HCN4 channel increasing cAMP sensitivity is associated with familial Inappropriate Sinus Tachycardia. Eur. Heart J. 2017, 38, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, B.; Sullivan, R.M. Inappropriate sinus tachycardia. EP Europace 2019, 21, 194–207. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, J.; Almskog, L.; Liliequist, A.; Grip, J.; Fux, T.; Rysz, S.; Ågren, A.; Oldner, A.; Ståhlberg, M. Thromboembolism, Hypercoagulopathy, and Antiphospholipid Antibodies in Critically Ill Coronavirus Disease 2019 Patients: A Before and after Study of Enhanced Anticoagulation. Crit. Care Explor. 2020, 2, e0308. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2023, 401, e21–e33. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Schondorf, R.; Low, P.A. Idiopathic postural orthostatic tachycardia syndrome: An attenuated form of acute pandysautonomia? Neurology 1993, 43, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Mallick, D.; Goyal, L.; Chourasia, P.; Zapata, M.R.; Yashi, K.; Surani, S. COVID-19 Induced Postural Orthostatic Tachycardia Syndrome (POTS): A Review. Cureus 2023, 15, e36955. [Google Scholar] [CrossRef] [PubMed]
- Bosco, J.; Titano, R. Severe Post-COVID-19 dysautonomia: A case report. BMC Infect. Dis. 2022, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, S.; Zou, R.; Li, F.; Zhang, J.; Wang, Y.; Xu, Y.; Wang, C. Diagnostic value of diurnal variability of orthostatic heart rate increment in children and adolescents with POTS. Front. Pediatr. 2021, 9, 644461. [Google Scholar] [CrossRef] [PubMed]
- Magkas, N.; Tsioufis, C.; Thomopoulos, C.; Dilaveris, P.; Georgiopoulos, G.; Sanidas, E.; Papademetriou, V.; Tousoulis, D. Orthostatic hypotension: From pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens. 2019, 21, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Rivasi, G.; Rafanelli, M.; Mossello, E.; Brignole, M.; Ungar, A. Drug-Related Orthostatic Hypotension: Beyond Anti-Hypertensive Medications. Drugs Aging 2020, 37, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Vanneman, M.W.; Madhok, J.; Weimer, J.M.; Dalia, A.A. Perioperative Implications of the 2020 American Heart Association Scientific Statement on Drug-Induced Arrhythmias—A Focused Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef]
- Fu, Q.; Levine, B.D. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. 2018, 215, 20–27. [Google Scholar] [CrossRef]
- Arnold, A.C.; Ng, J.; Raj, S.R. Postural tachycardia syndrome—Diagnosis, physiology, and prognosis. Auton. Neurosci. 2018, 215, 3–11. [Google Scholar] [CrossRef]
- Stewart, J.M.; Boris, J.R.; Chelimsky, G.; Fischer, P.R.; Fortunato, J.E.; Grubb, B.P.; Heyer, G.L.; Jarjour, I.T.; Medow, M.S.; Numan, M.T.; et al. Pediatric Disorders of Orthostatic Intolerance. Pediatrics 2018, 141, e20171673. [Google Scholar] [CrossRef]
- Goodman, B.P. Evaluation of postural tachycardia syndrome (POTS). Auton. Neurosci. 2018, 215, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zadourian, A.; Doherty, T.A.; Swiatkiewicz, I.; Taub, P.R. Postural Orthostatic Tachycardia Syndrome: Prevalence, Pathophysiology, and Management. Drugs 2018, 78, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Liao, Y.; Tian, H.; Huang, M.; Dong, X.; Shi, L.; Sun, J.; Jin, H.; Du, J.; et al. 2018 Chinese Pediatric Cardiology Society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci. Bull. 2018, 63, 1558–1564. [Google Scholar] [CrossRef]
- Rudofker, E.W.; Parker, H.; Cornwell, W.K., 3rd. An Exercise Prescription as a Novel Management Strategy for Treatment of Long COVID. JACC Case Rep. 2022, 4, 1344–1347. [Google Scholar] [CrossRef]
- Coffin, S.T.; Black, B.K.; Biaggioni, I.; Paranjape, S.Y.; Orozco, C.; Black, P.W.; Dupont, W.D.; Robertson, D.; Raj, S.R. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012, 9, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Sun, J.; Qin, J.; Tang, C.; Jin, H.; Du, J. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ. J. 2011, 75, 927–931. [Google Scholar] [CrossRef]
- Rowell, L.B.; Detry, J.M.; Blackmon, J.R.; Wyss, C. Importance of the splanchnic vascular bed in human blood pressure regulation. J. Appl. Physiol. 1972, 32, 213–220. [Google Scholar] [CrossRef]
- Bourne, K.M.; Sheldon, R.S.; Hall, J.; Lloyd, M.; Kogut, K.; Sheikh, N.; Jorge, J.; Ng, J.; Exner, D.V.; Tyberg, J.V.; et al. Compression Garment Reduces Orthostatic Tachycardia and Symptoms in Patients with Postural Orthostatic Tachycardia Syndrome. J. Am. Coll. Cardiol. 2021, 77, 285–296. [Google Scholar] [CrossRef]
- Ruzieh, M.; Dasa, O.; Pacenta, A.; Karabin, B.; Grubb, B. Droxidopa in the Treatment of Postural Orthostatic Tachycardia Syndrome. Am. J. Ther. 2017, 24, e157–e161. [Google Scholar] [CrossRef]
- Kaufmann, H. L-dihydroxyphenylserine (Droxidopa): A new therapy for neurogenic orthostatic hypotension: The US experience. Clin. Auton. Res. 2008, 18 (Suppl. 1), 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Saeed, B.; Karabin, B.; Kanjwal, Y.; Grubb, B.P. Use of octreotide in the treatment of refractory orthostatic intolerance. Am. J. Ther. 2012, 19, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ouyang, J.; Perkins, K.; Somauroo, J.; Joseph, F. Treatment of Refractory Postural Tachycardia Syndrome with Subcutaneous Octreotide Delivered Using an Insulin Pump. Case Rep. Med. 2015, 2015, 545029. [Google Scholar] [CrossRef] [PubMed]
- Tahir, F.; Arif, T.B.; Majid, Z.; Ahmed, J.; Khalid, M. Ivabradine in postural orthostatic tachycardia syndrome: A review of the literature. Cureus 2020, 12, e7868. [Google Scholar] [CrossRef]
- Mar, P.L.; Raj, S.R. Postural orthostatic tachycardia syndrome: Mechanisms and new therapies. Annu. Rev. Med. 2020, 71, 235–248. [Google Scholar] [CrossRef]
- Stewart, J.M.; Warsy, I.A.; Visintainer, P.; Terilli, C.; Medow, M.S. Supine parasympathetic withdrawal and upright sympathetic activation underly abnormalities of the baroreflex in postural tachycardia syndrome: Effects of pyridostigmine and digoxin. Hypertension 2021, 77, 1234–1244. [Google Scholar] [CrossRef]
| Common | Hypovolemia | Intravenous/Splanchnic Pooling | Blood Norepinephrine Level Increase | Autoimmunity | |
|---|---|---|---|---|---|
| Non-pharmacological treatment | Avoid lying down for a long time. Stand up gradually, especially after meals and urination. Avoid exposure to high temperature and humidity. Avoid eating a large amount at once. Physical counter-pressure maneuvering and contraction of gastrocnemius muscle and tensor muscle. Maintain good sleep hygiene. Exercises: aerobic exercise and thigh resistance training. | 10 g or more of sodium per day/water 3 L or more. Elevating head-end of the bed 10 cm for sleeping. Sleeping in seated position. | Compression stockings, clothes. Eating habits: consuming small amounts of food multiple times per day or conducting food elimination. | ||
| Pharmacological treatment | Fludrocortisone Desmopressin Midodrine Withdrawal of drugs that interfere with liquid balance | Midodrine Droxidopa Octreotide | Propranolol Bisoprolol Metoprolol Atenolol Ivabradine Clonidine Methyldopa | Pyridostigmine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Park, S.; Kim, N.-H.; Lee, Y.; Chang, Y.; Song, T.-J. Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review. Medicina 2024, 60, 1325. https://doi.org/10.3390/medicina60081325
Park J-H, Park S, Kim N-H, Lee Y, Chang Y, Song T-J. Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review. Medicina. 2024; 60(8):1325. https://doi.org/10.3390/medicina60081325
Chicago/Turabian StylePark, Jung-Hyun, Somin Park, Na-Hye Kim, Yoonjin Lee, Yoonkyung Chang, and Tae-Jin Song. 2024. "Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review" Medicina 60, no. 8: 1325. https://doi.org/10.3390/medicina60081325
APA StylePark, J.-H., Park, S., Kim, N.-H., Lee, Y., Chang, Y., & Song, T.-J. (2024). Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review. Medicina, 60(8), 1325. https://doi.org/10.3390/medicina60081325






