A Comparison of Pre- and Post-Treatment Cranial MRI Characteristics in Patients with Pediatric Epilepsy Receiving Levetiracetam
Abstract
:1. Introduction
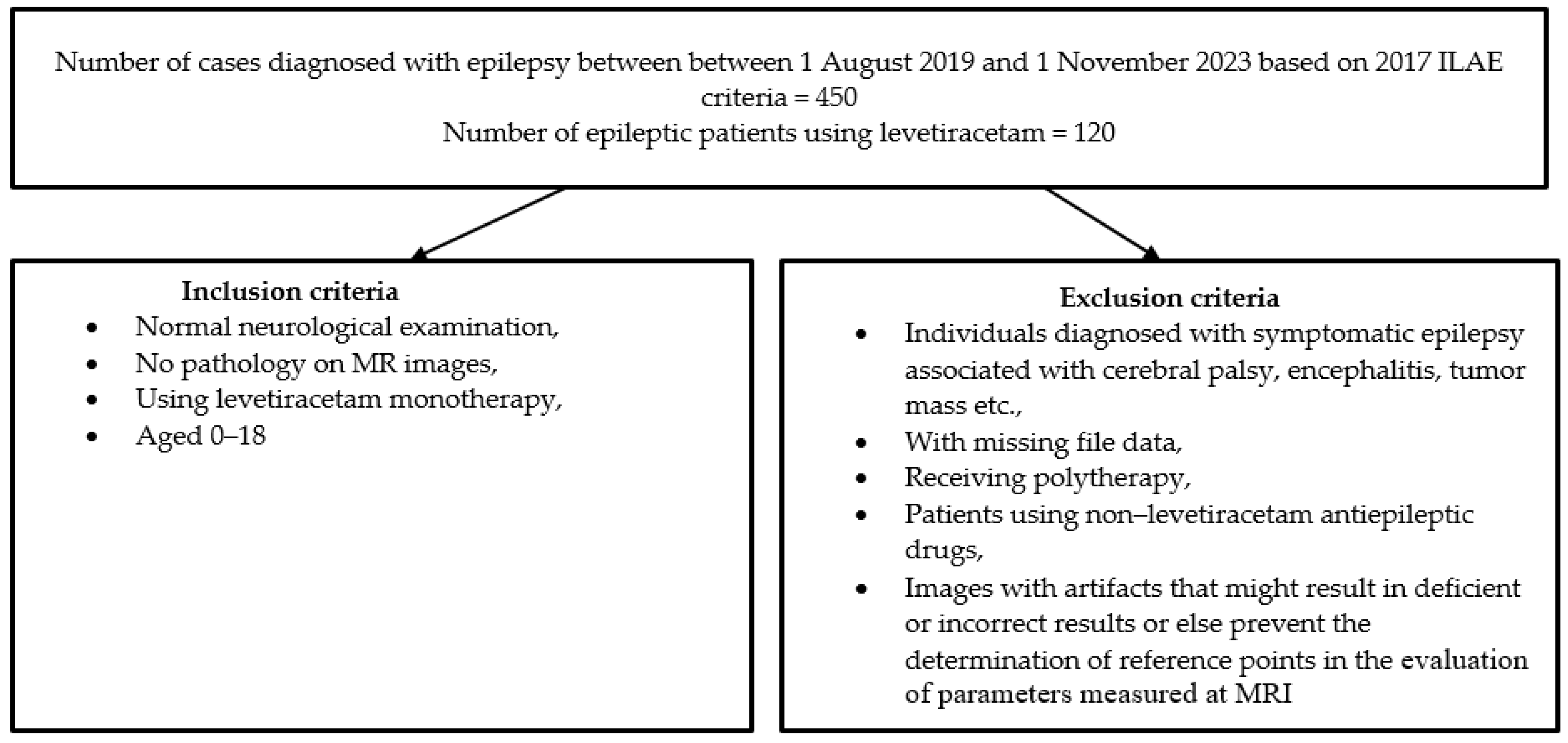
2. Materials and Methods
2.1. MR Imaging Procedures and Image Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Gaillard, W.D.; Chiron, C.; Cross, J.H.; Harvey, A.S.; Kuzniecky, R.; Hertz-Pannier, L.; Vezina, L.G. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia 2009, 50, 2147–2153. [Google Scholar] [CrossRef]
- Guissard, G.; Damry, N.; Dan, B.; David, P.; Sekhara, T.; Ziereisen, F.; Christophe, C. Imaging in paediatric epilepsy. Arch. Pediatr. 2005, 12, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Costantino, M.M.; Stratford, T. Basal ganglia: Anatomy, pathology, and imaging characteristics. Curr. Probl. Diagn. Radiol. 2004, 33, 28–41. [Google Scholar] [CrossRef]
- DeLong, M.R. The basal ganglia. In Principles of Neural Science, 4th ed.; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; McGraw-Hill: New York, NY, USA, 2000; pp. 853–867. [Google Scholar]
- Gray, T.S. Functional and anatomical relationships among the amygdala, basal forebrain, ventral striatum, and cortex: An integrative discussion. Ann. N. Y. Acad. Sci. 1999, 877, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Bajjalieh, S.M.; Peterson, K.; Linial, M.; Scheller, R.H. Brain contains two forms of synaptic vesicle protein 2. Proc. Natl. Acad. Sci. USA 1993, 90, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Vogrin, S.; Bleasel, A.F.; Cook, M.J.; Burns, L.; McAnally, L.; Pereira, J.; Bye, A.M. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia 2000, 41, 1540–1545. [Google Scholar] [CrossRef]
- Caplan, R.; Levitt, J.; Siddarth, P.; Taylor, J.; Daley, M.; Wu, K.N.; Gurbani, S.; Shields, W.D.; Sankar, R. Thought disorder and frontotemporal volumes in pediatric epilepsy. Epilepsy Behav. 2008, 13, 593–599. [Google Scholar] [CrossRef]
- Daley, M.; Levitt, J.; Siddarth, P.; Mormino, E.; Hojatkashani, C.; Gurbani, S.; Shields, W.D.; Sankar, R.; Toga, A.; Caplan, R. Frontal and temporal volumes in children with epilepsy. Epilepsy Behav. 2007, 10, 470–476. [Google Scholar] [CrossRef]
- Lawson, J.A.; Cook, M.J.; Vogrin, S.; Litewka, L.; Strong, D.; Bleasel, A.F.; Bye, A. Clinical, EEG, and quantitative MRI differences in pediatric frontal and temporal lobe epilepsy. Neurology 2002, 58, 723–729. [Google Scholar] [CrossRef]
- Ternigan, T.L.; Trauner, D.A.; Hesselink, J.R.; Tallal, P.A. Maturation of human cerebrum observed in vivo during adolescence. Brain 1991, 114 Pt 5, 2037–2049. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Thompson, P.M.; Leonard, C.M.; Welcome, S.E.; Kan, E.; Toga, A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef]
- Herrero, M.T.; Barcia, C.; Navarro, J.M. Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst. 2002, 18, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; Dimitrakopoulos, I.; Eleftheriades, M.; Agorastos, T.; Makrydimas, G. Thalamic volume measurement in normal fetuses using three-dimensional sonography. J. Clin. Ultrasound 2012, 40, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Y.A.; De Bellis, M.D. The relationship of age, gender, and IQ with the brainstem and thalamus in healthy children and adolescents: A magnetic resonance imaging volumetric study. J. Child Neurol. 2012, 27, 325–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sussman, D.; Leung, R.C.; Chakravarty, M.M.; Lerch, J.P.; Taylor, M.J. The developing human brain: Age-related changes in cortical, subcortical, and cerebellar anatomy. Brain Behav. 2016, 6, e00457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sowell, E.R.; Trauner, D.A.; Gamst, A.; Jernigan, T.L. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev. Med. Child Neurol. 2002, 44, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Rosenbloom, M.; Serventi, K.L.; Pfefferbaum, A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol. Aging 2004, 25, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Hosseini, S.H.; Golalipour, M.J. Morphometric measurements of the thalamus and interthalamic adhesion by MRI in the South-East of the Caspian Sea border. Neurosciences 2008, 13, 272–275. [Google Scholar] [PubMed]
- Tutunji, R.; El Homsi, M.; Saaybi, S.; Al Arab, N.; Tamim, H.; Makki, M.; Hourani, M.; Hourani, R. Thalamic volume and dimensions on MRI in the pediatric population: Normative values and correlations: (A cross sectional study). Eur. J. Radiol. 2018, 109, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yoshiura, T.; Hiwatashi, A.; Noguchi, T.; Togao, O.; Takayama, Y.; Nagao, E.; Kamano, H.; Hatakenaka, M.; Honda, H. Volumetric asymmetry and differential aging effect of the human caudate nucleus in normal individuals: A prospective MR imaging study. J. Neuroimaging 2001, 21, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.S.; Riddle, M.A.; Cohen, D.J.; Katz, L.D.; Smith, J.; Leckman, J.F. Human basal ganglia volume asymmetries on magnetic resonance images. Magn. Reson. Imaging 1993, 11, 493–498. [Google Scholar] [CrossRef]
- Ifthikharuddin, S.F.; Shrier, D.A.; Numaguchi, Y.; Tang, X.; Ning, R.; Shibata, D.K.; Kurlan, R. MR volumetric analysis of the human basal ganglia: Normative data. Acad. Radiol. 2000, 7, 627–634. [Google Scholar] [CrossRef]
- Abedelahi, A.; Hasanzadeh, H.; Hadizadeh, H.; Joghataie, M.T. Morphometric and volumetric study of caudate and putamen nuclei in normal individuals by MRI: Effect of normal aging, gender and hemispheric differences. Pol. J. Radiol. 2013, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Vernaleken, I.; Weibrich, C.; Siessmeier, T.; Buchholz, H.-G.; Rösch, F.; Heinz, A.; Cumming, P.; Stoeter, P.; Bartenstein, P.; Gründer, G. Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage 2007, 34, 870–878. [Google Scholar] [CrossRef]
- Giedd, J.N.; Snell, J.W.; Lange, N.; Rajapakse, J.C.; Casey, B.J.; Kozuch, P.L.; Vaituzis, A.C.; Vauss, Y.C.; Hamburger, S.D.; Kaysen, D.; et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb. Cortex 1996, 6, 551–560. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Head, D.; McQuain, J.; Acker, J.D.; Raz, N. Differential aging of the human striatum: A prospective MR imaging study. Am. J. Neuroradiol. 1998, 19, 1501–1507. [Google Scholar]
- Raz, N.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Gunning-Dixon, F.; Acker, J.D. Differential aging of the human striatum: Longitudinal evidence. Am. J. Neuroradiol. 2003, 24, 1849–1856. [Google Scholar]
- Amirsalari, S.; Saburi, A.; Hadi, R.; Torkaman, M.; Beiraghdar, F.; Afsharpayman, S.; Ghazavi, Y. Magnetic resonance imaging findings in epileptic children and its relation to clinical and demographic findings. Acta Medica Iran. 2012, 50, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.L.; Abrams, M.T.; Singer, H.S.; Ross, J.L.; Denckla, M.B. Brain development, gender and IQ in children. A volumetric imaging study. Brain 1996, 119, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Horton, N.J.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex 2001, 11, 490–497. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Wong, D.F.; Oswald, L.M.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W.; et al. Sex differences in striatal dopamine release in healthy adults. Biol. Psychiatry 2006, 59, 966–974. [Google Scholar] [CrossRef]
- Pfaff, D.; Keiner, M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 1973, 151, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Van Hartesveldt, C.; Joyce, J.N. Effects of estrogen on the basal ganglia. Neurosci. Biobehav. Rev. 1986, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wyciszkiewicz, A.; Pawlak, M.A. Basal Ganglia Volumes: MR-Derived Reference Ranges and Lateralization Indices for Children and Young Adults. Neuroradiol. J. 2014, 27, 595–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salmah, J.W.M.; Noorfizura, A.; Shafie, A.M.; Helmy, A.; Salmi, A.R.; Naing, L. Hippocampal MR Volumetric Studies in Paediatric Patients with Epilepsy and Normal Controls. Neuroradiol. J. 2011, 24, 503–510. [Google Scholar] [CrossRef]
- Santos, V.R.; Melo, I.S.; Pacheco, A.L.D.; Castro, O.W. Life and death in the hippocampus: What’s bad? Epilepsy Behav. 2021, 121 Pt B, 106595. [Google Scholar] [CrossRef] [PubMed]
- Hakyemez, B.; Yucel, K.; Yildirim, N.; Erdogan, C.; Bora, I.; Parlak, M. Morphologic and volumetric analysis of amygdala, hippocampus, fornix and mamillary body with MRI in patients with temporal lobe epilepsy. Neuroradiol. J. 2006, 19, 289–296. [Google Scholar] [CrossRef]
- Cascino, G.D. Progressive damage in epilepsy. Epilepsy Curr. 2003, 3, 214–215. [Google Scholar] [CrossRef]
- Galovic, M.; van Dooren, V.Q.; Postma, T.S.; Vos, S.B.; Caciagli, L.; Borzì, G.; Rosillo, J.C.; Vuong, K.A.; de Tisi, J.; Nachev, P.; et al. Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol. 2019, 76, 1230–1239. [Google Scholar] [CrossRef]
- Tondelli, M.; Vaudano, A.E.; Sisodiya, S.M.; Meletti, S.J. Valproate use is associated with posterior cortical thinning and ventricular enlargement in epilepsy patients. Front. Neurol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Liu, R.S.N.; Lemieux, L.; Bell, G.S.; Sisodiya, S.M.; Bartlett, P.A.; Shorvon, S.D.; Sander, J.W.A.S.; Duncan, J.S. Cerebral Damage in Epilepsy: A Population-Based Longitudinal Quantitative MRI Study. Epilepsia 2005, 46, 1482–1494. [Google Scholar] [CrossRef]
- Woźniak, M.M.; Zbroja, M.; Matuszek, M.; Pustelniak, O.; Cyranka, W.; Drelich, K.; Kopyto, E.; Materniak, A.; Słomka, T.; Cebula, M.; et al. Epilepsy in Pediatric Patients-Evaluation of Brain Structures’ Volume Using VolBrain Software. J. Clin. Med. 2022, 11, 4657. [Google Scholar] [CrossRef]
- Tondelli, M.; Vaudano, A.E.; Ruggieri, A.; Meletti, S. Cortical and subcortical brain alterations in Juvenile Absence Epilepsy. Neuroimage Clin. 2016, 12, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Bekkelund, S.I.; Pierre-Jerome, C.; Mellgren, S.I. Quantitative Cerebral MRI in Epileptic Patients. Acta Neurol. Scand. 1996, 94, 378–382. [Google Scholar] [CrossRef]
- Alruwaili, A.R.; Pannek, K.; Coulthard, A.; Henderson, R.; Kurniawan, N.D.; Mccombe, P. A combined tract-based spatial statistics and voxel-based morphometry study of the first MRI scan after diagnosis of amyotrophic lateral sclerosis with subgroup analysis. J. Neuroradiol. 2018, 45, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Baltruschat, S.A.; Ventura-Campos, N.; Cruz-Gómez, A.J.; Belenguer, A.; Forn, C. Gray matter atrophy is associated with functional connectivity reorganization during the Paced Auditory Serial Addition Test (PASAT) execution in Multiple Sclerosis (MS). J. Neuroradiol. 2015, 42, 141–149. [Google Scholar]
- Diaz, J.; Schain, R.J. Phenobarbital: Effects of long-term administration on behaviour and brain of artificially reared rats. Science 1978, 199, 901. [Google Scholar]
- Yanai, J.; Rosselli-Austin, L.; Tabakoff, B. Neuronal deficits in mice following prenatal exposure to phenobarbital. Exp. Neurol. 1979, 64, 23744. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Bosch, F.; Miksa, M.; Bittigau, P.; Vöckler, J.; Dikranian, K.; Tenkova, T.I.; Stefovska, V.; Turski, L.; Olney, J.W. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1991, 283, 70–74. [Google Scholar] [CrossRef]
- Bittigau, P.; Sifringer, M.; Genz, K.; Reith, E.; Pospischil, D.; Govindarajalu, S.; Dzietko, M.; Pesditschek, S.; Mai, I.; Dikranian, K.; et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15089–15094. [Google Scholar] [CrossRef] [PubMed]
- Crepeau, A.Z.; Treiman, D.M. Levetiracetam: A comprehensive review. Expert Rev. Neurother. 2010, 10, 159–171. [Google Scholar] [CrossRef]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Loscher, W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004, 5, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Arjmand, S.; Bærentzen, S.L.; Gjedde, A.; Landau, A.M. Synaptic Vesicle Glycoprotein 2A: Features and Functions. Front. Neurosci. 2022, 16, 864514. [Google Scholar] [CrossRef]
- Margineanu, D.G.; Matagne, A.; Kaminski, R.M.; Klitgaard, H. Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res. Bull. 2008, 77, 282–285. [Google Scholar] [CrossRef]
- Talos, D.M.; Chang, M.; Kosaras, B.; Fitzgerald, E.; Murphy, A.; Folkerth, R.D.; Jensen, F.E. Antiepileptic effects of levetiracetam in a rodent neonatal seizure model. Pediatr. Res. 2013, 73, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Nguyen, D.K.; Zaatreh, M.; Singh, S.P.; Westerveld, M.; Thompson, J.L.; Mattson, R.; Blumenfeld, H.; Novotny, E.; Spencer, S.S. Levetiracetam efficacy in refractory partial-onset seizures, especially after failed epilepsy surgery. Epilepsia 2003, 44, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Janszky, J.; Pannek, H.W.; Janszky, I.; Schulz, R.; Behne, F.; Hoppe, M.; Ebner, A. Failed surgery for temporal lobe epilepsy: Predictors of long-term seizure-free course. Epilepsy Res. 2005, 64, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jehi, L.E.; Irwin, A.I.; Kayyali, H.; Vadera, S.; Bingaman, W.; Najm, I. Levetiracetam may favorably affect seizure outcome after temporal lobectomy. Epilepsia 2012, 53, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chen, C.C.; Liou, H.H. Levetiracetam inhibits glutamate transmission through presynaptic P/Q-type calcium channels on the granule cells of the dentate gyrus. Br. J. Pharmacol. 2009, 158, 1753–1762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.E.; Choi, H.C.; Song, H.K.; Jo, S.M.; Kim, D.S.; Choi, S.Y.; Kim, Y.I.; Kang, T.C. Levetiracetam inhibits interleukin-1 beta inflammatory responses in the hippocampus and piriform cortex of epileptic rats. Neurosci. Lett. 2010, 471, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Haghikia, A.; Dambach, H.; Thöne, J.; Wiemann, M.; Gold, R.; Chan, A.; Dermietzel, R.; Faustmann, P.M.; Hinkerohe, D.; et al. Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFβ1 regulation. Br. J. Pharmacol. 2011, 162, 491–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wandschneider, B.; Stretton, J.; Sidhu, M.; Centeno, M.; Kozak, L.R.; Symms, M.; Thompson, P.J.; Duncan, J.S.; Koepp, M.J. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology 2014, 83, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, X.; Zhang, X.; Xia, W.; Wu, X.; Zou, X.; Li, H.; Huang, X.; Stefan, H.; Chen, Q.; et al. Single-dose intravenous administration of antiepileptic drugs induces rapid and reversible remodeling in the brain: Evidence from a voxel-based morphometry evaluation of valproate and levetiracetam in rhesus monkeys. Neuroscience 2015, 303, 595–603. [Google Scholar] [CrossRef] [PubMed]
| Age | 13 ± 2.18 (8–15) |
| Gender | |
| Female | 11 (78.6%) |
| Male | 3 (21.4%) |
| Type of epilepsy | |
| Focal | 9 (64.3%) |
| Generalized | 5 (35.7%) |
| EEG findings | |
| Normal | 3 (21.4%) |
| Abnormal | 6 (42.9%) |
| Epileptiform | 5 (35.7%) |
| Family history of epilepsy | 5 (35.7%) |
| Patient | Pre-Treatment Volume (cm3) | Post-Treatment Volume (cm3) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | R, CN | L, CN | R, PTM | L, PTM | R, GP | L, GP | R, Th | L, Th | R, HCM | L, HCM | R, CN | L, CN | R, PTM | L, PTM | R, GP | L, GP | R, Th | L, Th | R, HCM | L, HCM |
| 1 | 3.915 | 4.012 | 6.223 | 6.158 | 1.289 | 1.175 | 4.307 | 4.234 | 2.568 | 2.407 | 4.017 | 4.089 | 6.513 | 6.491 | 1.349 | 1.264 | 4.442 | 4.216 | 2.590 | 2.447 |
| 2 | 4.703 | 4.660 | 6.601 | 6.811 | 1.873 | 1.803 | 5.003 | 5.141 | 2.826 | 2.904 | 4.759 | 4.716 | 6.903 | 7.025 | 1.829 | 1.859 | 5.419 | 5.284 | 2.773 | 2.802 |
| 3 | 3.573 | 3.751 | 6.169 | 6.112 | 1.652 | 1.492 | 4.433 | 4.280 | 2.502 | 2.635 | 3.512 | 3.552 | 6.271 | 6.317 | 1.711 | 1.505 | 4.486 | 4.429 | 2.534 | 2.589 |
| 4 | 4.663 | 4.690 | 6.529 | 6.469 | 1.903 | 1.839 | 5.091 | 5.524 | 2.639 | 2.555 | 4.672 | 4.912 | 6.905 | 6.996 | 2.004 | 1.904 | 5.088 | 5.690 | 2.785 | 2.690 |
| 5 | 4.126 | 4.317 | 6.515 | 6.258 | 1.713 | 1.814 | 5.142 | 5.253 | 2.489 | 2.557 | 4.235 | 4.699 | 6.445 | 6.557 | 1.729 | 1.956 | 5.214 | 5.238 | 2.875 | 2.947 |
| 6 | 4.451 | 4.391 | 6.251 | 6.124 | 1.759 | 1.741 | 4.568 | 4.696 | 2.711 | 2.735 | 4.830 | 4.721 | 6.382 | 6.235 | 1.779 | 1.813 | 4.817 | 4.794 | 2.736 | 2.795 |
| 7 | 3.515 | 3.485 | 5.904 | 5.842 | 1.413 | 1.284 | 4.480 | 4.655 | 2.479 | 2.364 | 3.603 | 3.712 | 6.204 | 6.312 | 1.454 | 1.481 | 4.518 | 4.774 | 2.490 | 2.412 |
| 8 | 4.729 | 4.518 | 6.572 | 6.719 | 1.789 | 1.932 | 4.572 | 4.713 | 2.446 | 2.561 | 4.594 | 4.821 | 6.610 | 6.880 | 1.816 | 1.953 | 4.858 | 4.890 | 2.411 | 2.510 |
| 9 | 4.442 | 4.321 | 6.148 | 6.312 | 1.585 | 1.502 | 4.311 | 4.257 | 2.616 | 2.544 | 4.311 | 4.382 | 6.231 | 6.309 | 1.615 | 1.546 | 4.501 | 4.315 | 2.772 | 2.591 |
| 10 | 4.414 | 4.566 | 6.573 | 6.048 | 1.376 | 1.359 | 4.316 | 4.119 | 2.444 | 2.650 | 4.741 | 4.832 | 6.846 | 6.370 | 1.220 | 1.245 | 4.650 | 4.595 | 2.559 | 2.616 |
| 11 | 4.886 | 4.784 | 6.786 | 6.317 | 1.685 | 1.699 | 4.913 | 4.754 | 2.690 | 2.557 | 4.534 | 4.963 | 6.800 | 6.805 | 1.663 | 1.612 | 5.214 | 5.095 | 2.901 | 2.954 |
| 12 | 3.687 | 3.788 | 6.217 | 6.018 | 1.136 | 1.184 | 4.305 | 4.630 | 2.475 | 2.506 | 3.855 | 3.961 | 6.325 | 6.346 | 1.289 | 1.275 | 4.413 | 4.527 | 2.377 | 2.435 |
| 13 | 4.780 | 4.874 | 6.884 | 6.732 | 1.873 | 1.719 | 4.776 | 4.890 | 2.220 | 2.175 | 4.528 | 4.729 | 6.552 | 6.718 | 1.813 | 1.749 | 4.635 | 4.904 | 2.457 | 2.519 |
| 14 | 4.269 | 4.342 | 6.542 | 6.438 | 1.691 | 1.712 | 4.693 | 4.524 | 2.776 | 2.684 | 4.371 | 4.402 | 6.623 | 6.501 | 1.700 | 1.721 | 4.917 | 4.905 | 2.777 | 2.746 |
| Pre-Treatment Volume (cm3) Mean ± SD (min–max) | Post-Treatment Volume (cm3) Mean ± SD (min–max) | p | |
|---|---|---|---|
| R, CN | 4.29 ± 0.46 (3.515–4.886) | 4.32 ± 0.43 (3.512–4.830) | 0.594 |
| L, CN | 4.32 ± 0.41 (3.485–4.874) | 4.46 ± 0.46 (3.552–4.963) | 0.013 |
| R, PTM | 6.422 ± 0.27 (5.904–6.884) | 6.54 ± 0.24 (6.204–6.905) | 0.022 |
| L, PTM | 6.31 ± 0.29 (5.842–6.811) | 6.56 ± 0.27 (6.235–7.025) | 0.02 |
| R, GP | 1.62 ± 0.23 (1.136–1.903) | 1.64 ± 0.22 (1.220–2.004) | 0.331 |
| L, GP | 1.58 ± 0.25 (1.175–1.932) | 1.63 ± 0.255 (1.245–1.956) | 0.048 |
| R, Th | 4.63 ± 0.30 (4.305–5.142) | 4.97 ± 0.33 (4.413–5.419) | 0.005 |
| L, Th | 4.69 ± 0.40 (4.11–5.52) | 4.83 ± 0.40 (4.216–5.690) | 0.009 |
| R, HCM | 2.56 ± 0.15 (2.220–2.826) | 2.64 ± 0.17 (2.377–2.901) | 0.048 |
| L, HCM | 2.55 ± 0.17 (2.175–2.904) | 2.64 ± 0.18 (2.412–2.954) | 0.140 |
| Age (years) | Thalamus Volume (cm3) Median | IQR |
|---|---|---|
| 1 | 4.77 | 1.38 |
| 2 | 4.45 | 1.21 |
| 3 | 5.19 | 1.69 |
| 4 | 5.32 | 0.60 |
| 5 | 5.10 | 2.01 |
| 6 | 4.78 | 1.99 |
| 7 | 6.01 | 0.5 |
| 8 | 5.90 | 1.78 |
| 9 | 5.51 | 1.39 |
| 10 | 5.22 | 1.45 |
| 11 | 5.81 | 1.43 |
| 12 | 4.78 | 1.68 |
| 13 | 5.66 | 0.64 |
| 14 | 5.60 | 0.75 |
| 15 | 5.69 | 1.69 |
| 16 | 5.09 | 0.97 |
| 17 | 5.71 | 0.87 |
| 18 | 5.13 | 1.58 |
| Age Group (years) | 7–9 | 10–12 | 13–15 | 16–18 |
|---|---|---|---|---|
| Right caudate nucleus | 4.25 ± 0.58 | 4.25 ± 0.53 | 4.26 ± 0.58 | 4.24 ± 0.61 |
| Left caudate nucleus | 4.09 ± 0.49 | 4.03 ± 0.46 | 4.03 ± 0.51 | 4.04 ± 0.49 |
| Right putamen | 6.48 ± 0.84 | 6.52 ± 0.77 | 6.53 ± 0.73 | 6.27 ± 0.78 |
| Left putamen | 6.76 ± 0.84 | 6.77 ± 0.79 | 6.75 ± 0.80 | 6.47 ± 0.85 |
| Right globus pallidus | 1.81 ± 0.24 | 1.81 ± 0.22 | 1.79 ± 0.22 | 1.79 ± 0.25 |
| Left globus pallidus | 1.98 ± 0.24 | 1.99 ± 0.27 | 1.97 ± 0.30 | 1.90 ± 0.31 |
| Right hippocampus | 2.76 ± 0.29 | 2.75 ± 0.40 | 2.69 ± 0.27 | 3.04 ± 0.46 |
| Left hippocampus | 2.56 ± 0.27 | 2.68 ± 0.45 | 2.50 ± 0.32 | 2.85 ± 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, H.; Aytac, A.; Bulbul, E.; Yanik, B.; Korkut, O.; Gulcen, B. A Comparison of Pre- and Post-Treatment Cranial MRI Characteristics in Patients with Pediatric Epilepsy Receiving Levetiracetam. Medicina 2024, 60, 1355. https://doi.org/10.3390/medicina60081355
Aydin H, Aytac A, Bulbul E, Yanik B, Korkut O, Gulcen B. A Comparison of Pre- and Post-Treatment Cranial MRI Characteristics in Patients with Pediatric Epilepsy Receiving Levetiracetam. Medicina. 2024; 60(8):1355. https://doi.org/10.3390/medicina60081355
Chicago/Turabian StyleAydin, Hilal, Adil Aytac, Erdogan Bulbul, Bahar Yanik, Oguzhan Korkut, and Burak Gulcen. 2024. "A Comparison of Pre- and Post-Treatment Cranial MRI Characteristics in Patients with Pediatric Epilepsy Receiving Levetiracetam" Medicina 60, no. 8: 1355. https://doi.org/10.3390/medicina60081355





