Impact of Surgical and Anesthetic Procedures after Colorectal Cancer Surgery: A Propensity Score-Matched Cohort Study (The PROCOL Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Non-Eligibility Criteria
2.4. Data Collection
2.4.1. Demographic Data
2.4.2. Preoperative Data
2.4.3. Intraoperative Data
2.4.4. Postoperative Data
2.5. Outcomes
2.6. Definition
2.7. Statistical Analyses
2.7.1. Groups and Sub-Groups
2.7.2. Propensity Score Matching
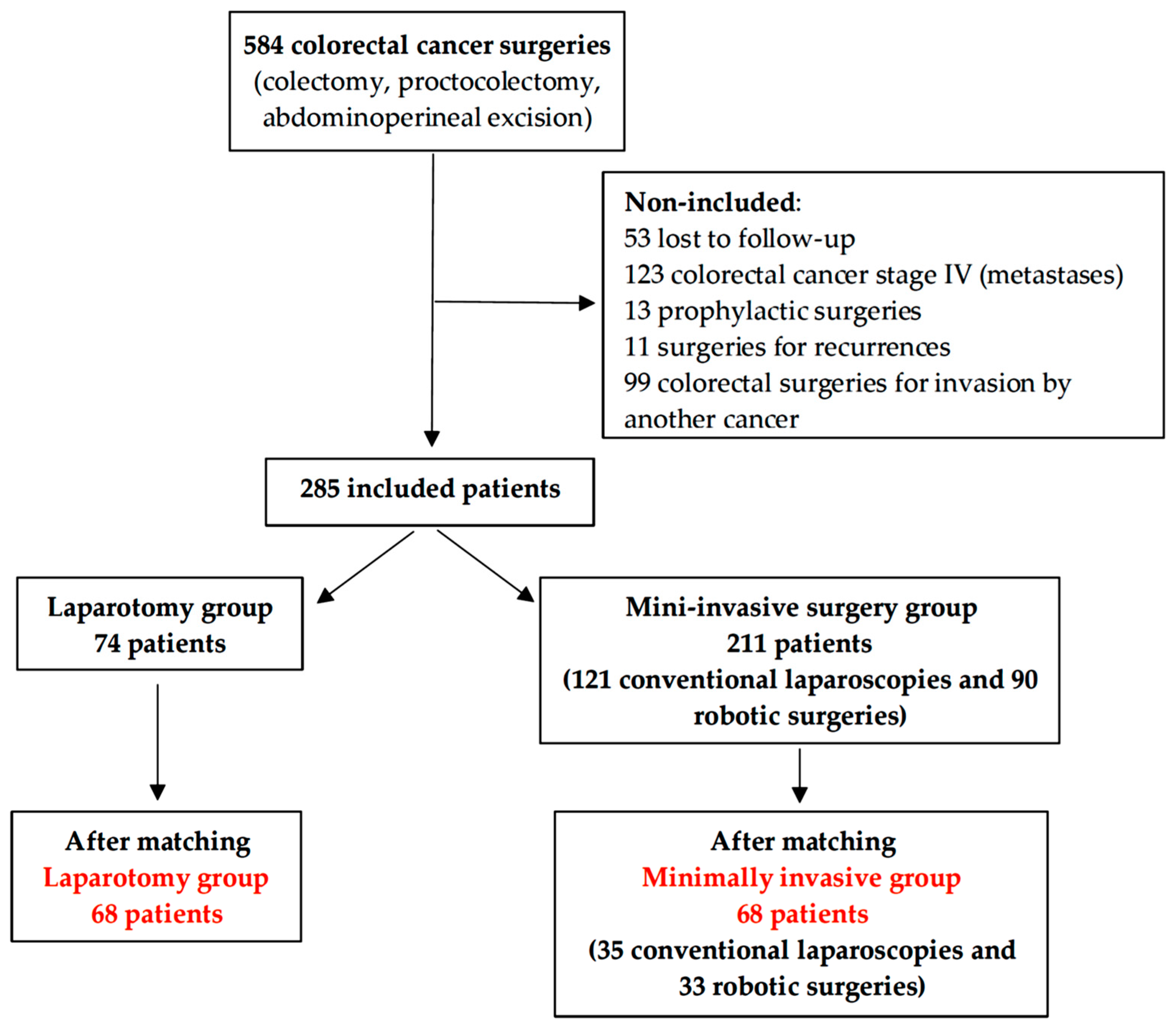
3. Results
3.1. Patients’ Characteristics
3.2. Intraoperative and Postoperative Variables
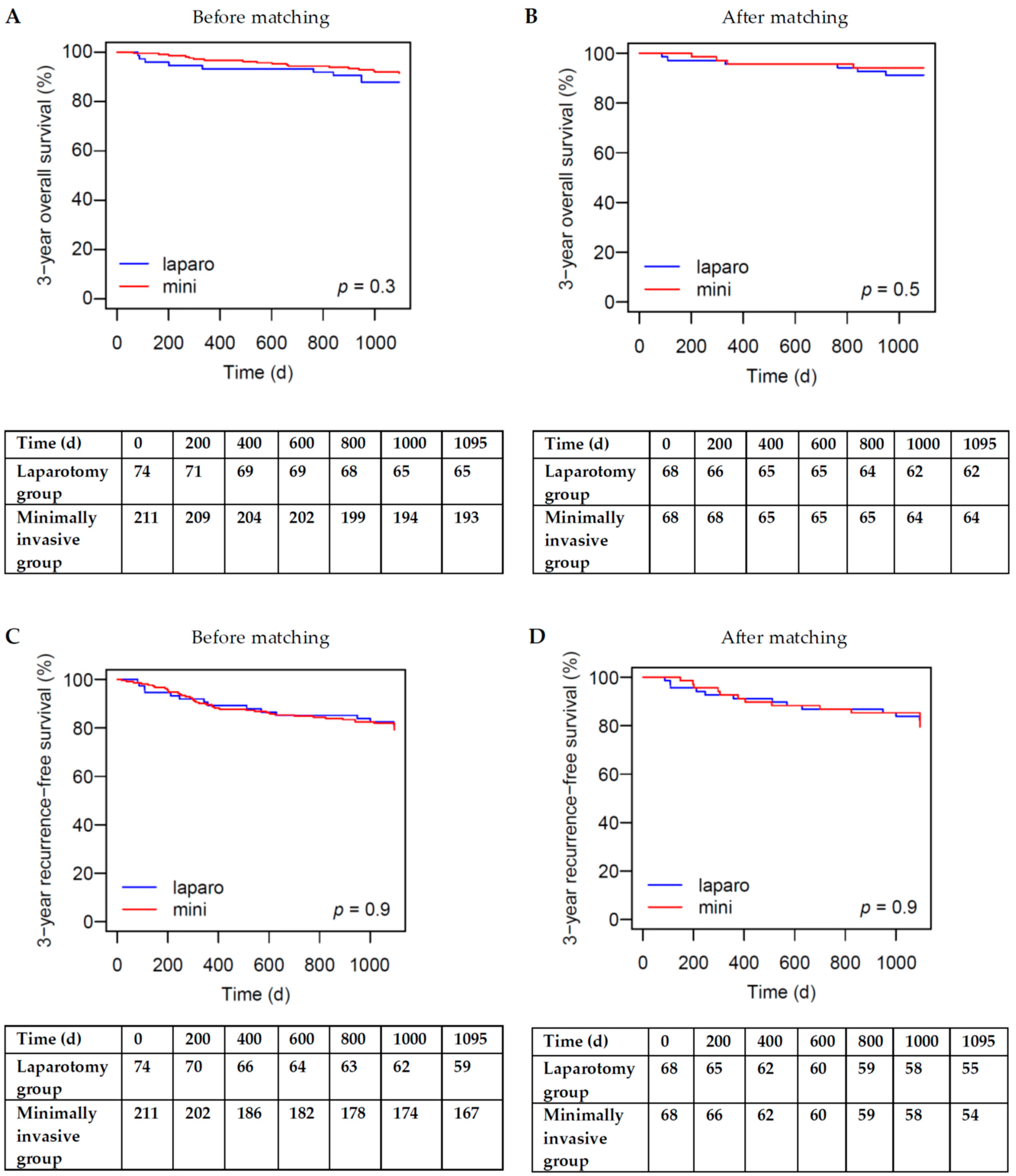
3.3. Overall Survival
3.4. Recurrence-Free Survival
3.5. Predictors for Overall Survival
3.6. Predictors for Recurrence-Free Survival
3.7. Colon Cancer Surgery Versus Rectum Cancer Surgery
3.7.1. Colon Cancer Surgery
3.7.2. Rectum Cancer Surgery
3.8. Conventional Laparoscopy versus Robotic Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer#:~:text=Colon%20cancer%20is%20the%20second,estimated%20to%20have%20occurred%20worldwide (accessed on 11 July 2023).
- Rentsch, M.; Schiergens, T.; Khandoga, A.; Werner, J. Surgery for Colorectal Cancer—Trends, Developments, and Future Perspectives. Visc. Med. 2016, 32, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. JPEN J. Parenter. Enter. Nutr. 2013, 37, 21S–29S. [Google Scholar] [CrossRef]
- Yang, H.; Xia, L.; Chen, J.; Zhang, S.; Martin, V.; Li, Q.; Lin, S.; Chen, J.; Calmette, J.; Lu, M.; et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 2019, 25, 1428–1441. [Google Scholar] [CrossRef]
- Petrik, J.; Verbanac, D.; Fabijanec, M.; Hulina-Tomaskovic, A.; Ceri, A.; Somborac-Bacura, A.; Petlevski, R.; Grdic Rajkovic, M.; Rumora, L.; Kruslin, B.; et al. Circulating Tumor Cells in Colorectal Cancer: Detection Systems and Clinical Utility. Int. J. Mol. Sci. 2022, 23, 13582. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef]
- Jacobs, M.; Verdeja, J.C.; Goldstein, H.S. Minimally invasive colon resection (laparoscopic colectomy). Surg. Laparosc. Endosc. 1991, 1, 144–150. [Google Scholar]
- Schiedeck, T.H.; Schwandner, O.; Baca, I.; Baehrlehner, E.; Konradt, J.; Kockerling, F.; Kuthe, A.; Buerk, C.; Herold, A.; Bruch, H.P. Laparoscopic surgery for the cure of colorectal cancer: Results of a German five-center study. Dis. Colon. Rectum 2000, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamabe, A.; Takemasa, I.; Kotake, M.; Nakano, D.; Hasegawa, S.; Shiomi, A.; Numata, M.; Sakamoto, K.; Kimura, K.; Hanai, T.; et al. Feasibility of robotic-assisted surgery in advanced rectal cancer: A multicentre prospective phase II study (VITRUVIANO trial). BJS Open 2024, 8, zrae048. [Google Scholar] [CrossRef] [PubMed]
- Hahnloser, D.; Rrupa, D.; Grass, F. Feasibility of on-demand robotics in colorectal surgery: First cases. Surg. Endosc. 2023, 37, 8594–8600. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Yamashita, K.; Hasegawa, H.; Oshikiri, T.; Hosono, M.; Higashino, N.; Yamamoto, M.; Matsuda, Y.; Kanaji, S.; Nakamura, T.; et al. Recent updates in the surgical treatment of colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 129–136. [Google Scholar] [CrossRef]
- Nazzal, K.; Hasan, L.; Alqaseer, A.; Abdulla, H.A.; Majed, A.S.; Abushwemeh, M.A.; Salman, E.S.; Arafa, M.; Jawad, A. Laparoscopic Versus Open Surgery for Colorectal Cancers: Clinical and Pathological Outcomes from a Single Institution in Bahrain. Gulf J. Oncol. 2024, 1, 64–68. [Google Scholar]
- Bezu, L.; Wu Chuang, A.; Sauvat, A.; Humeau, J.; Xie, W.; Cerrato, G.; Liu, P.; Zhao, L.; Zhang, S.; Le Naour, J.; et al. Local anesthetics elicit immune-dependent anticancer effects. J. Immunother. Cancer 2022, 10, e004151. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Liu, L.; Shi, Z.; Zhang, L.; Yang, Y. Ropivacaine Inhibits Cell Proliferation, Migration and Invasion, Whereas Induces Oxidative Stress and Cell Apoptosis by circSCAF11/miR-145-5p Axis in Glioma. Cancer Manag. Res. 2020, 12, 11145–11155. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, S.T.; Huang, S.M.; Wu, Z.F. Apoptosis, Proliferation, and Autophagy Are Involved in Local Anesthetic-Induced Cytotoxicity of Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15455. [Google Scholar] [CrossRef]
- Abdelaatti, A.; Buggy, D.J.; Wall, T.P. Local anaesthetics and chemotherapeutic agents: A systematic review of preclinical evidence of interactions and cancer biology. BJA Open 2024, 10, 100284. [Google Scholar] [CrossRef]
- Wu Chuang, A.; Kepp, O.; Kroemer, G.; Bezu, L. Direct Cytotoxic and Indirect, Immune-Mediated Effects of Local Anesthetics Against Cancer. Front. Oncol. 2021, 11, 821785. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, F.J.; Abegg, R.; van der Linden, J.C.; Cornelisse, H.G.; van Dorsten, F.R.; Lemmens, V.E.; Bosscha, K. Epidural analgesia associated with better survival in colon cancer. Int. J. Color. Dis. 2015, 30, 1103–1107. [Google Scholar] [CrossRef]
- Holler, J.P.; Ahlbrandt, J.; Burkhardt, E.; Gruss, M.; Rohrig, R.; Knapheide, J.; Hecker, A.; Padberg, W.; Weigand, M.A. Peridural analgesia may affect long-term survival in patients with colorectal cancer after surgery (PACO-RAS-Study): An analysis of a cancer registry. Ann. Surg. 2013, 258, 989–993. [Google Scholar] [CrossRef]
- Cummings, K.C., 3rd; Xu, F.; Cummings, L.C.; Cooper, G.S. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: A population-based study. Anesthesiology 2012, 116, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Zimmitti, G.; Soliz, J.; Aloia, T.A.; Gottumukkala, V.; Cata, J.P.; Tzeng, C.W.; Vauthey, J.N. Positive Impact of Epidural Analgesia on Oncologic Outcomes in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2016, 23, 1003–1011. [Google Scholar] [CrossRef]
- Gottschalk, A.; Ford, J.G.; Regelin, C.C.; You, J.; Mascha, E.J.; Sessler, D.I.; Durieux, M.E.; Nemergut, E.C. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology 2010, 113, 27–34. [Google Scholar] [CrossRef]
- Gupta, A.; Bjornsson, A.; Fredriksson, M.; Hallbook, O.; Eintrei, C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: A retrospective analysis of data from 655 patients in central Sweden. Br. J. Anaesth. 2011, 107, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Tai, Y.H.; Mandell, M.S.; Tsou, M.Y.; Yang, S.H.; Chen, T.H.; Chang, K.Y. Effect of epidural analgesia on cancer prognosis after colon cancer resection: A single-centre cohort study in Taiwan. BMJ Open 2020, 10, e036577. [Google Scholar] [CrossRef] [PubMed]
- Wurster, E.F.; Pianka, F.; Warschkow, R.; Antony, P.; Brenner, T.; Weigand, M.A.; Schmied, B.M.; Buchler, M.W.; Tarantino, I.; Ulrich, A. Peridural analgesia does not impact survival in patients after colon cancer resection: A retrospective propensity score-adjusted analysis. Int. J. Color. Dis. 2019, 34, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Day, A.; Smith, R.; Jourdan, I.; Fawcett, W.; Scott, M.; Rockall, T. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. Br. J. Anaesth. 2012, 109, 185–190. [Google Scholar] [CrossRef]
- Christopherson, R.; James, K.E.; Tableman, M.; Marshall, P.; Johnson, F.E. Long-term survival after colon cancer surgery: A variation associated with choice of anesthesia. Anesth. Analg. 2008, 107, 325–332. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Ammirati, C.A.; Passera, R.; Beltrami, E.; Peluso, C.; Francis, N.; Arezzo, A. Laparoscopic and robotic surgery for colorectal cancer in older patients: A systematic review and meta-analysis. Minim. Invasive Ther. Allied Technol. 2024, 1–17. [Google Scholar] [CrossRef]
- Duhoky, R.; Rutgers, M.L.W.; Burghgraef, T.A.; Stefan, S.; Masum, S.; Piozzi, G.N.; Sagias, F.; Khan, J.S. Long-Term Outcomes of Robotic Versus Laparoscopic Total Mesorectal Excisions: A Propensity-Score Matched Cohort study of 5-year survival outcomes. Ann. Surg. Open 2024, 5, e404. [Google Scholar] [CrossRef]
- Watanabe, T.; Sasaki, K.; Nozawa, H.; Murono, K.; Emoto, S.; Matsuzaki, H.; Yokoyama, Y.; Abe, S.; Nagai, Y.; Shinagawa, T.; et al. Robotic Versus Laparoscopic Abdominoperineal Resection for Locally Advanced Rectal Cancer Following Preoperative Chemoradiotherapy. Vivo 2024, 38, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, R.; Hou, T.; Xu, H.; Zhang, C.; Ye, C. Robotic versus laparoscopic surgery for colorectal cancer in older patients: A systematic review and meta-analysis. Minim. Invasive Ther. Allied Technol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Manfredi, B.; Bianchi, M.; Panerai, A.E. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav. Immun. 1994, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G.; Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain 1993, 54, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Yamaguchi, Y. Autologous tumor killing activity as a prognostic factor in primary resected nonsmall cell carcinoma of the lung. Cancer 1997, 79, 474–481. [Google Scholar] [CrossRef]
- Badwe, R.A.; Parmar, V.; Nair, N.; Joshi, S.; Hawaldar, R.; Pawar, S.; Kadayaprath, G.; Borthakur, B.B.; Rao Thammineedi, S.; Pandya, S.; et al. Effect of Peritumoral Infiltration of Local Anesthetic Before Surgery on Survival in Early Breast Cancer. J. Clin. Oncol. 2023, 41, 3318–3328. [Google Scholar] [CrossRef]
- Carli, F.; Webster, J.; Pearson, M.; Pearson, J.; Bartlett, S.; Bannister, P.; Halliday, D. Protein metabolism after abdominal surgery: Effect of 24-h extradural block with local anaesthetic. Br. J. Anaesth. 1991, 67, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, H.K.; Kwon, J.Y.; Baek, S.H.; Ri, H.S.; Choi, H.J.; Cho, H.R.; Lee, Y.S.; Kim, J.Y.; Kim, J.; et al. Role of Sevoflurane on Natural Killer Group 2, Member D-Mediated Immune Response in Non-Small-Cell Lung Cancer: An In Vitro Study. Med. Sci. Monit. 2020, 26, e926395. [Google Scholar] [CrossRef] [PubMed]
- Tabnak, P.; Masrouri, S.; Geraylow, K.R.; Zarei, M.; Esmailpoor, Z.H. Targeting miRNAs with anesthetics in cancer: Current understanding and future perspectives. Biomed. Pharmacother. 2021, 144, 112309. [Google Scholar] [CrossRef] [PubMed]
- Enlund, M.; Berglund, A.; Enlund, A.; Lundberg, J.; Warnberg, F.; Wang, D.X.; Ekman, A.; Ahlstrand, R.; Flisberg, P.; Hedlund, L.; et al. Impact of general anaesthesia on breast cancer survival: A 5-year follow up of a pragmatic, randomised, controlled trial, the CAN-study, comparing propofol and sevoflurane. eClinicalMedicine 2023, 60, 102037. [Google Scholar] [CrossRef]
- Levi, L.; Hikri, E.; Popovtzer, A.; Dayan, A.; Levi, A.; Bachar, G.; Mizrachi, A.; Shoffel-Havakuk, H. Effect of Opioid Receptor Activation and Blockage on the Progression and Response to Treatment of Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, T.; Sano, M.; Kajiwara, I.; Oshima, Y.; Itaya, T.; Kim, J.; Ichimaru, Y.; Kitajima, O.; Masamune, A.; Ijichi, H.; et al. Effects of tramadol via a micro-opioid receptor on pancreatic ductal adenocarcinoma in vitro and in vivo. Reg. Anesth. Pain Med. 2024, 49, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Yuval, J.B.; Lee, J.; Wu, F.; Thompson, H.M.; Verheij, F.S.; Gupta, H.V.; Irie, T.; Scarpa, J.R.; McCormick, P.J.; Smith, J.J.; et al. Intraoperative opioids are associated with decreased recurrence rates in colon adenocarcinoma: A retrospective observational cohort study. Br. J. Anaesth. 2022, 129, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Tai, Y.H.; Chang, K.Y.; Lin, S.P. Dose-dependent association between morphine requirement and mortality risk after resection for hepatocellular carcinoma. Eur. J. Anaesthesiol. 2021, 38, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Khabbazi, S.; Hassanshahi, M.; Hassanshahi, A.; Peymanfar, Y.; Su, Y.W.; Xian, C.J. Opioids and matrix metalloproteinases: The influence of morphine on MMP-9 production and cancer progression. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 123–133. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, S.; Fu, G.; Ji, F.; Wang, C.; Cao, M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci. 2020, 251, 117604. [Google Scholar] [CrossRef]
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Laparo (74) | Mini (211) | SMD | p-Value | Laparo (68) | Mini (68) | SMD | p-Value | |
| Demographic data | ||||||||
| Female, n (%) | 26 (35.1) | 103 (48.8) | 0.280 | 0.06 | 24 (35.3) | 23 (33.8) | 0.031 | 1 |
| Age (mean (SD)) | 64.20 (12.66) | 62.69 (13.77) | 0.114 | 0.39 | 66.5 [54.75; 70.25] | 61.5 [53; 71.25] | 0.173 | 0.32 |
| Age > 70 years old, n (%) | 20 (27) | 59 (28) | 0.021 | 1 | 18 (26.5) | 18 (26.5) | <0.001 | 1 |
| BMI, kg/m2 (mean (SD)) | 26.34 (5.07) | 25.37 (4.32) | 0.207 | 0.14 | 26.18 [23.41; 29.06] | 25.74 [22.66; 27.69] | 0.066 | 0.59 |
| BMI > 25 kg/m2, n (%) | 43 (58.1) | 91 (43.1) | 0.303 | <0.001 | 38 (55.9) | 38 (55.9) | <0.001 | 1 |
| ASA 3-4, n (%) | 14 (18.9) | 26 (12.3) | 0.182 | 0.23 | 13 (19.1) | 10 (14.7) | 0.118 | 0.65 |
| Tobacco, n (%) | 27 (36.5) | 55 (26.1) | 0.226 | 0.12 | 25 (36.8) | 19 (27.9) | 0.189 | 0.36 |
| Alcohol, n (%) | 12 (16.2) | 20 (9.5) | 0.202 | 0.17 | 9 (13.2) | 8 (11.8) | 0.044 | 1 |
| Undernutrition, n (%) | 20 (27) | 41 (19.4) | 0.181 | 0.23 | 19 (27.9) | 18 (26.5) | 0.033 | 1 |
| History | ||||||||
| Stroke, n (%) | 1 (1.4) | 8 (3.8) | 0.155 | 0.45 | 1 (1.5) | 1 (1.5) | <0.001 | 1 |
| Hypertension, n (%) | 27 (36.5) | 63 (29.9) | 0.141 | 0.36 | 23 (33.8) | 22 (32.4) | 0.031 | 1 |
| Diabetes, n (%) | 12 (16.2) | 26 (12.3) | 0.112 | 0.52 | 11 (16.2) | 16 (23.5) | 0.185 | 0.39 |
| COPD, n (%) | 4 (5.4) | 4 (1.9) | 0.188 | 0.21 | 4 (5.9) | 0 (0) | 0.354 | 0.12 |
| Cardiopathy, n (%) | 3 (4.1) | 9 (4.3) | 0.011 | 1 | 3 (4.4) | 4 (5.9) | 0.067 | 1 |
| Cardiac failure, n (%) | 2 (2.7) | 1 (0.5) | 0.179 | 0.17 | 2 (2.9) | 0 (0) | 0.246 | 0.5 |
| NYHA | 0.198 | 0.289 | ||||||
| I, n (%) | 54 (73) | 149 (70.6) | 0.81 | 48 (70.6) | 44 (64.7) | 0.58 | ||
| II, n (%) | 15 (20.3) | 55 (26.1) | 0.40 | 15 (22.1) | 22 (32.4) | 0.25 | ||
| III, n (%) | 5 (6.8) | 7 (3.3) | 0.35 | 5 (7.4) | 2 (2.9) | 0.44 | ||
| Kidney insufficiency, n (%) | 3 (4.1) | 8 (3.8) | 0.014 | 1 | 2 (2.9) | 3 (4.4) | 0.078 | 1 |
| Family history of CRC, n (%) | 8 (10.8) | 30 (14.2) | 0.103 | 0.59 | 7 (10.3) | 9 (13.2) | 0.091 | 0.79 |
| Medication | ||||||||
| Statin, n (%) | 8 (10.8) | 40 (19) | 0.230 | 0.15 | 8 (11.8) | 15 (22.1) | 0.277 | 0.17 |
| Beta-blocker, n (%) | 7 (9.5) | 29 (13.7) | 0.134 | 0.46 | 7 (10.3) | 9 (13.2) | 0.091 | 0.79 |
| ACEi/ARB, n (%) | 15 (20.3) | 38 (18) | 0.057 | 0.80 | 11 (16.2) | 17 (25) | 0.220 | 0.29 |
| Aspirin, n (%) | 8 (10.8) | 25 (11.8) | 0.033 | 0.98 | 8 (11.8) | 8(11.8) | <0.001 | 1 |
| Opiate analgesics, n (%) | 2 (2.7) | 10 (4.7) | 0.108 | 0.74 | 1 (1.5) | 3 (4.4) | 0.175 | 0.62 |
| ERAS, n (%) | 2 (2.7) | 4 (1.9) | 0.054 | 0.65 | 2 (2.9) | 1 (1.5) | 0.100 | 1 |
| Neoadjuvant therapy | ||||||||
| Chemotherapy, n (%) | 33 (44.6) | 53 (25.1) | 0.418 | <0.003 | 30 (44.1) | 31 (45.6) | 0.030 | 1 |
| Radiotherapy, n (%) | 28 (37.8) | 56 (26.5) | 0.244 | 0.09 | 26 (38.2) | 31 (45.6) | 0.149 | 0.49 |
| Biology | ||||||||
| Creatinine, µmol/L (mean (SD)) | 73.36 (32.63) | 71.03 (21.5) | 0.085 | 0.57 | 70 [59; 79.25] | 67 [59; 80.25] | 0.010 | 0.94 |
| Albumin, g/L (mean (SD)) | 34.34 (6.45) | 33.08 (7.08) | 0.186 | 0.16 | 34 [29; 40] | 36.5 [29; 41] | 0.022 | 0.99 |
| Leukocytes, G/L (mean (SD)) | 8.29 (6.90) | 7.72 (3.14) | 0.106 | 0.49 | 6.8 [5.48; 9.18] | 7.2 [5.8; 9.5] | 0.119 | 0.64 |
| Hb, g/dL (mean (SD)) | 12.46 (3.60) | 12.45 (2.08) | 0.003 | 0.98 | 12.10 [11; 13.53] | 12.9 [11.3; 14] | 0.049 | 0.13 |
| CEA, mg/L (median [IQR]) | 5.6 [2; 79] | 6.10 [2; 79.7] | 0.218 | 0.50 | 6 [2; 79] | 4.3 [1.5; 79] | 0.061 | 0.46 |
| Tumor site | 0.254 | 0.490 | ||||||
| Left colon, n (%) | 32 (43.2) | 85 (40.3) | 0.76 | 30 (44.1) | 25 (36.8) | 0.48 | ||
| Right colon, n (%) | 12 (16.2) | 33 (15.6) | 1 | 11 (16.2) | 6 (8.8) | 0.30 | ||
| Transverse colon, n (%) | 4 (5.4) | 3 (1.4) | 0.21 | 3 (4.4) | 0 (0) | 0.24 | ||
| Rectum, n (%) | 26 (35.1) | 90 (42.7) | <0.001 | 24 (35.3) | 37 (54.4) | 0.04 | ||
| TNM | ||||||||
| T3-T4 (vs. T1-T2), n (%) | 33 (44.6) | 63 (29.9) | 0.308 | 0.03 | 30 (44.1) | 29 (42.6) | 0.030 | 1 |
| N+ (vs. N0), n (%) | 41 (55.4) | 148 (70.1) | 0.308 | 0.006 | 39 (57.4) | 37 (54.4) | 0.059 | 0.86 |
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Laparo (74) | Mini (211) | SMD | p-Value | Laparo (68) | Mini (68) | SMD | p-Value | |
| pTNM | ||||||||
| pT3-T4 (vs. pT1-T2), n (%) | 49 (66.2) | 135 (64) | 0.047 | 0.84 | 45 (66.2) | 37 (54.4) | 0.242 | 0.22 |
| pN+ (vs. pN0), n (%) | 19 (25.7) | 72 (34.1) | 0.185 | 0.23 | 17 (25) | 23 (30.8) | 0.195 | 0.35 |
| R-category | 0.273 | 0.354 | ||||||
| R0, n (%) | 70 (94.6) | 209 (99.1) | 0.07 | 64 (94.1) | 68 (100) | 0.12 | ||
| R1, n (%) | 2 (2.7) | 0 (0) | 0.07 | 2 (2.9) | 0 (0) | 0.49 | ||
| R2, n (%) | 2 (2.7) | 2 (0.9) | 0.28 | 2 (2.9) | 0 (0) | 0.49 | ||
| Microscopic resection margin | 0.211 | 0.398 | ||||||
| 0–1 mm, n (%) | 3 (4.1) | 3 (1.4) | 0.18 | 3 (4.4) | 0 (0) | 0.24 | ||
| 1–2 mm, n (%) | 2 (2.7) | 2 (0.9) | 0.28 | 2 (2.9) | 0 (0) | 0.49 | ||
| >2 mm, n (%) | 69 (93.2) | 206 (97.6) | 0.16 | 63 (92.6) | 68 (100) | 0.06 | ||
| Surgery | ||||||||
| Duration, min (mean (SD)) | 268.95 (112.83) | 261.79 (103.99) | 0.066 | 0.86 | 260.5 [205.5; 326.5] | 251 [179; 331] | 0.110 | 0.47 |
| Blood loss, ml (median [IQR]) | 60 [0; 300] | 0 [0; 55] | 0.504 | <0.001 | 75 [0; 350] | 0 [0; 85] | 0.554 | <0.001 |
| Transfusion, n (%) | 6 (8.1) | 7 (3.3) | 0.208 | 0.17 | 6 (8.8) | 3 (4.4) | 0.178 | 0.49 |
| Anesthesia | ||||||||
| TIVA (vs. volatile), n (%) | 2 (2.7) | 1 (0.5) | 0.179 | 0.17 | 2 (2.9) | 1 (1.5) | 0.100 | 1 |
| Remifentanil, µg (median [IQR]) | 1047 [560.2; 1573] | 1265 [914; 1854] | 0.162 | <0.01 | 1047 [627.8; 1589] | 1424 [960; 2107] | 0.239 | 0.02 |
| Lidocaine infusion, n (%) | 23 (31.1) | 140 (66.4) | 0.754 | <0.001 | 21 (30.9) | 40 (58.8) | 0.585 | 0.002 |
| Ketamine infusion, n (%) | 65 (87.8) | 182 (86.3) | 0.047 | 0.89 | 61 (89.7) | 58 (85.3) | 0.134 | 0.6 |
| PCEA, n (%) | 43 (58.1) | 17 (8.1) | 1.256 | <0.001 | 39 (57.4) | 7 (10.3) | 1.147 | <0.001 |
| TAP block, n (%) | 5 (6.8) | 3 (1.4) | 0.272 | 0.03 | 5 (7.4) | 2 (2.9) | 0.201 | 0.44 |
| Infiltration, n (%) | 2 (2.7) | 25 (11.8) | 0.358 | 0.02 | 1 (1.5) | 6 (8.8) | 0.337 | 0.12 |
| Acetaminophen, n (%) | 71 (95.9) | 210 (99.5) | 0.242 | 0.06 | 65 (95.6) | 67 (98.5) | 0.175 | 0.62 |
| Nefopam, n (%) | 63 (85.1) | 196 (92.9) | 0.250 | 0.08 | 58 (85.3) | 60 (88.2) | 0.087 | 0.8 |
| Tramadol, n (%) | 49 (66.2) | 122 (57.8) | 0.174 | 0.26 | 45 (66.2) | 41 (60.3) | 0.122 | 0.59 |
| Morphine IV, mg (median [IQR]) | 0 [0; 9.75] | 8 [6; 10] | 0.618 | <0.001 | 0 [0; 10] | 7.5 [5; 10] | 0.502 | 0.001 |
| Postoperative period | ||||||||
| Acetaminophen, n (%) | 70 (94.6) | 202 (95.7) | 0.053 | 0.75 | 64 (94.1) | 64 (94.1) | <0.001 | 1 |
| Nefopam, n (%) | 61 (82.4) | 149 (70.6) | 0.282 | 0.07 | 57 (83.8) | 55 (80.9) | 0.077 | 0.82 |
| Tramadol, n (%) | 49 (66.2) | 121 (57.3) | 0.183 | 0.23 | 46 (67.6) | 38 (55.9) | 0.244 | 0.22 |
| NSAID, n (%) | 11 (14.9) | 26 (12.3) | 0.074 | 0.72 | 11 (16.2) | 10 (14.7) | 0.041 | 1 |
| Total morphine intra- and postoperative OME, mg (median [IQR]) | 45 [0; 135.68] | 52.50 [27; 117.45] | 0.001 | 0.04 | 49.05 [0; 144.07] | 69.30 [24; 140.47] | 0.051 | 0.13 |
| VAS POD1 (median [IQR]) | 3 [1; 4] | 3 [2; 5] | 0.245 | 0.07 | 3 [1; 4] | 3 [2; 5] | 0.135 | 0.35 |
| VAS POD2 (median [IQR]) | 2 [1; 4] | 2 [1; 4] | 0.046 | 0.76 | 2 [1; 4] | 2.5 [1; 4] | 0.014 | 0.97 |
| Chemotherapy, n (%) | 38 (51.4) | 105 (49.8) | 0.032 | 0.89 | 35 (51.5) | 37 (54.4) | 0.059 | 0.86 |
| Chemotherapy | 0.370 | 0.289 | ||||||
| folfiri, n (%) | 1 (1.4) | 0 (0) | 0.26 | 1 (1.5) | 0 (0) | 1 | ||
| folfirinox, n (%) | 0 (0) | 1 (0.5) | 1 | 0 (0) | 0 (0) | 1 | ||
| folfox, n (%) | 25 (33.8) | 54 (25.6) | 0.23 | 23 (33.8) | 24 (35.3) | 1 | ||
| LV5FU2, n (%) | 1 (1.4) | 0 (0) | 0.58 | 1 (1.5) | 0 (0) | 1 | ||
| xeloda, n (%) | 6 (8.1) | 23 (10.9) | 0.65 | 5 (7.4) | 5 (7.4) | 1 | ||
| xelox, n (%) | 5 (6.8) | 27 (12.8) | 0.23 | 5 (7.4) | 8 (11.8) | 0.40 | ||
| Time between surgery and chemotherapy, d (median [IQR]) | 39 [34; 57.50] | 45 [39; 56] | 0.207 | 0.41 | 39 [34.5; 56.75] | 47 [40; 57] | 0.148 | 0.42 |
| Immunotherapy, n (%) | 0 (0) | 1 (0.5) | 0.098 | 1 | 0 (0) | 0 (0) | <0.001 | 1 |
| CEA at 3 years, mg/L (median [IQR]) | 1.9 [0.72; 2.36] | 1.8 [0.66; 2] | 0.160 | 0.49 | 1.77 [0.56; 2.32] | 1.8 [0.6; 2] | 0.227 | 0.59 |
| Length of stay, d (median [IQR]) | 12 [8.25; 19] | 8 [5.5; 11] | 0.567 | <0.001 | 12 [8.75; 19] | 8 [7; 12.25] | 0.342 | <0.001 |
| Complications | ||||||||
| > or =1 complication at POD7, n (%) | 23 (31.1) | 34 (16.1) | 0.358 | 0.009 | 22 (32.4) | 16 (23.5) | 0.198 | 0.34 |
| Clavien-Dindo at POD7 major (3b-5), n (%) | 6 (8.1) | 9 (4.3) | 0.160 | 0.33 | 5 (7.4) | 3 (4.4) | 0.125 | 0.72 |
| > or =1 complication at 3 months, n (%) | 23 (31.1) | 15 (7.1) | 0.640 | <0.001 | 20 (29.4) | 8 (11.8) | 0.447 | 0.02 |
| Clavien-Dindo at 3 months major (3b-5), n (%) | 15 (20.3) | 11 (5.2) | 0.464 | <0.001 | 12 (17.6) | 7 (10.3) | 0.213 | 0.32 |
| Recurrence at 3 years, n (%) | 15 (20.3) | 44 (20.9) | 0.014 | 1 | 13 (19.1) | 14 (20.6) | 0.037 | 0.83 |
| Local recurrence, n (%) | 3 (4.1) | 6 (2.8) | 0.066 | 0.70 | 3 (4.4) | 1 (1.5) | 0.175 | 0.62 |
| Node recurrence, n (%) | 0 (0) | 3 (1.4) | 0.170 | 0.57 | 0 (0) | 0 (0) | <0.001 | 1 |
| Metastases, n (%) | 12 (16.2) | 35 (16.6) | 0.010 | 1 | 10 (14.7) | 13 (19.1) | 0.118 | 0.65 |
| Time between surgery and recurrence, d (median [IQR]) | 350.5 [134.5; 614] | 308.5 [201; 578.2] | 0.166 | 0.80 | 435 [185.5; 709] | 340.5 [225; 483.5] | 0.308 | 0.77 |
| Death at 3 years, n (%) | 9 (12.2) | 18 (8.5) | 0.119 | 0.36 | 6 (8.8) | 4 (5.9) | 0.113 | 0.74 |
| Time between surgery and death, d (median [IQR]) | 331 [109; 840] | 572.5 [284.2; 880.2] | 0.262 | 0.47 | 547 [164.5; 820.8] | 318 [273; 460.2] | 0.292 | 0.91 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Demographic variables | ||||||
| Age | 1.1 | [1–1.2] | <0.01 | 1.08 | [1.02–1.15] | <0.01 |
| Female | 0.81 | [0.21–3.1] | 0.76 | |||
| ASA > 3 | 3.5 | [0.99–12] | 0.05 | 1.86 | [0.44–7.74] | 0.40 |
| BMI > 25 kg/m2 | 1.2 | [0.33–4.2] | 0.80 | |||
| Tumor | ||||||
| pT3-T4 (vs. pT1-pT2) | 0.97 | [0.27–3.4] | 0.96 | 1.52 | [0.37–6.26] | 0.56 |
| pN+ (vs. pN0) | 0.6 | [0.13–2.8] | 0.51 | |||
| R1-2 (vs. R0) | <0.001 | [0-inf] | 1 | |||
| Surgery | ||||||
| Mini (vs Laparo) | 0.66 | [0.19–2.3] | 0.52 | 0.43 | [0.11–1.72] | 0.23 |
| Surgical duration > 6 h | 2.9 | [0.81–10] | 0.1 | |||
| Blood loss > 350 mL | 1.2 | [0.26–5.8] | 0.79 | |||
| Transfusion | 1.5 | [0.19–12] | 0.70 | |||
| Intraoperative analgesia | ||||||
| Remifentanil > 2100 µg | 1.6 | [0.41–6.2] | 0.50 | |||
| Tramadol | 1.4 | [0.35–5.3] | 0.65 | |||
| Morphine > 10 mg (IV) | 0.86 | [0.18–4] | 0.84 | |||
| PCEA | 0.48 | [0.1–2.3] | 0.36 | 0.12 | [0.02–0.86] | 0.03 |
| Lidocaine infusion | 0.81 | [0.23–2.9] | 0.75 | 0.34 | [0.08–1.57] | 0.17 |
| Postoperative analgesia | ||||||
| NSAID | 1.4 | [0.3–6.6] | 0.66 | |||
| Tramadol | 0.6 | [0.17–2.1] | 0.42 | |||
| Total morphine at POD7 > 145 mg (OME) | 2 | [0.57–7.2] | 0.27 | |||
| VAS > 3 within 72 h postoperative | 1.6 | [0.42–6.3] | 0.47 | |||
| Adjuvant chemotherapy | 0.21 | [0.046–1] | 0.05 | 0.27 | [0.05–1.53] | 0.14 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Demographic variables | ||||||
| Age | 1 | [0.98–1] | 0.37 | |||
| Female | 1.3 | [0.61–2.8] | 0.48 | |||
| ASA > 3 | 1.2 | [0.45–3.1] | 0.72 | |||
| BMI > 25 kg/m2 | 1.4 | [0.63–3] | 0.41 | |||
| Tumor | ||||||
| pT3-T4 (vs. pT1-pT2) | 2.5 | [1–6.3] | 0.045 | 2.17 | [0.79–5.96] | 0.13 |
| pN+ (vs. pN0) | 1.8 | [0.83–3.9] | 0.14 | 1.38 | [0.55–3.48] | 0.50 |
| R1-R2 (vs. R0) | <0.001 | [0-inf] | 1 | |||
| Surgery | ||||||
| Mini (vs. Laparo) | 1.1 | [0.5–2.3] | 0.87 | 0.81 | [0.32–2.04] | 0.65 |
| Surgical duration > 6 h | 0.73 | [0.25–2.1] | 0.56 | |||
| Blood loss >350 mL | 1.8 | [0.76–4.3] | 0.18 | |||
| Transfusion | 0.5 | [0.07–3.7] | 0.50 | |||
| Intraoperative analgesia | ||||||
| Remifentanil > 2100 µg | 1.1 | [0.42–2.6] | 0.92 | |||
| Tramadol | 0.97 | [0.44–2.1] | 0.93 | |||
| Morphine > 10 mg (IV) | 0.4 | [0.12–1.3] | 0.14 | |||
| PCEA | 0.67 | [0.28–1.6] | 0.35 | 0.40 | [0.13–1.28] | 0.12 |
| Lidocaine infusion | 0.59 | [0.26–1.3] | 0.19 | 0.39 | [0.16–0.97] | 0.04 |
| Postoperative analgesia | ||||||
| NSAID | 0.43 | [0.1–1.8] | 0.25 | |||
| Tramadol | 1.4 | [0.63–3.3] | 0.38 | |||
| Total morphine at POD7 > 145 mg (OME) | 1 | [0.44–2.5] | 0.93 | |||
| VAS > 3 within 72 h postoperative | 2.7 | [1.1–6.6] | 0.03 | 2.29 | [0.89–5.90] | 0.08 |
| Adjuvant chemotherapy | 1.6 | [0.72–3.4] | 0.25 | 0.85 | [0.33–2.14] | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuoch, C.; Bezu, L. Impact of Surgical and Anesthetic Procedures after Colorectal Cancer Surgery: A Propensity Score-Matched Cohort Study (The PROCOL Study). Medicina 2024, 60, 1362. https://doi.org/10.3390/medicina60081362
Kuoch C, Bezu L. Impact of Surgical and Anesthetic Procedures after Colorectal Cancer Surgery: A Propensity Score-Matched Cohort Study (The PROCOL Study). Medicina. 2024; 60(8):1362. https://doi.org/10.3390/medicina60081362
Chicago/Turabian StyleKuoch, Céline, and Lucillia Bezu. 2024. "Impact of Surgical and Anesthetic Procedures after Colorectal Cancer Surgery: A Propensity Score-Matched Cohort Study (The PROCOL Study)" Medicina 60, no. 8: 1362. https://doi.org/10.3390/medicina60081362
APA StyleKuoch, C., & Bezu, L. (2024). Impact of Surgical and Anesthetic Procedures after Colorectal Cancer Surgery: A Propensity Score-Matched Cohort Study (The PROCOL Study). Medicina, 60(8), 1362. https://doi.org/10.3390/medicina60081362








