Monitoring of Cerebral Blood Flow Autoregulation after Cardiac Arrest
Abstract
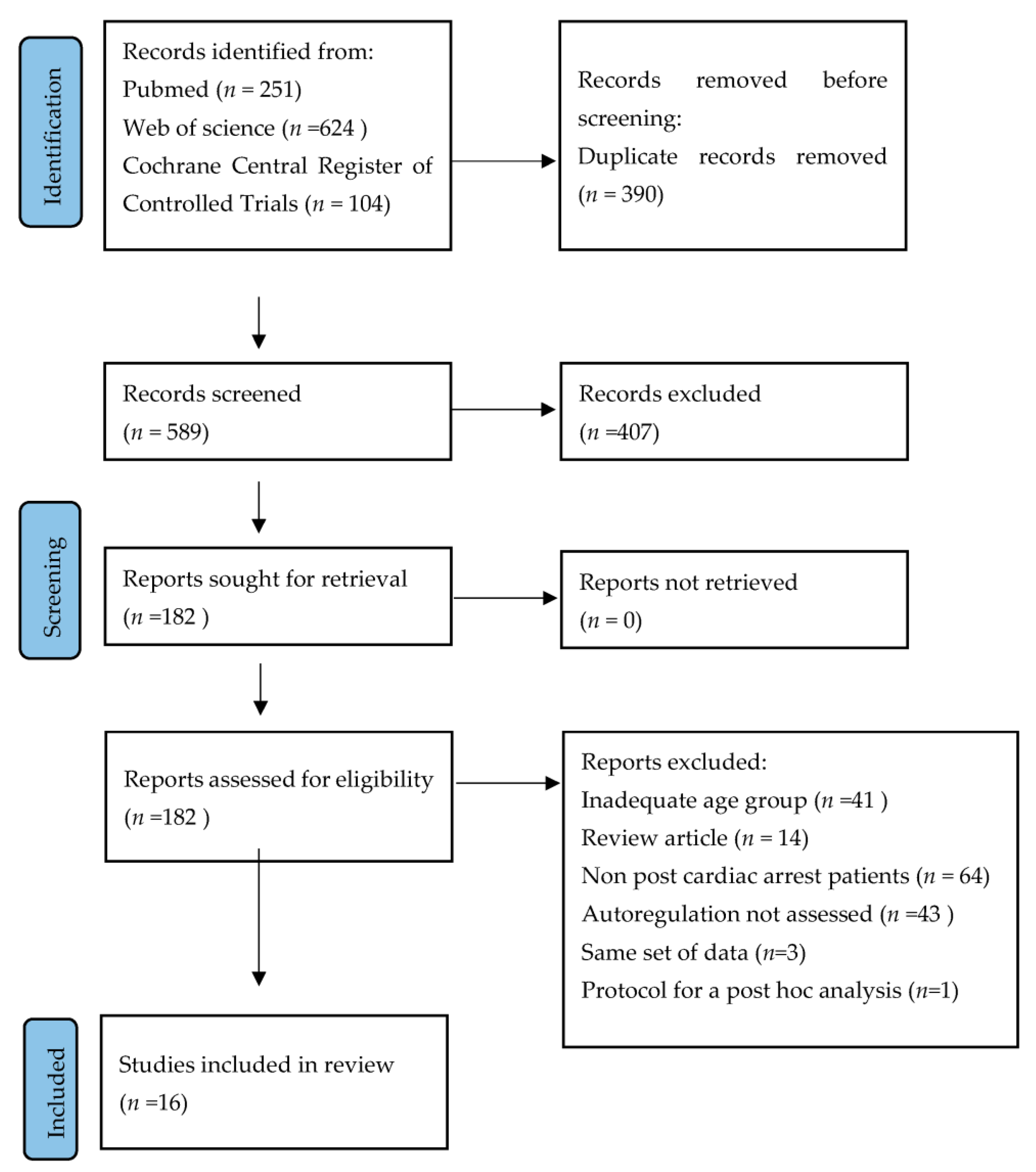
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Data Collection
2.3. Endpoints
3. Results
4. Discussion
- Heterogeneity in Monitoring Approaches
- Association with Clinical Outcomes
- Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gräsner, J.-T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after Out-of-Hospital Cardiac Arrest in Europe-Results of the EuReCa TWO Study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Lefering, R.; Koster, R.W.; Masterson, S.; Böttiger, B.W.; Herlitz, J.; Wnent, J.; Tjelmeland, I.B.M.; Ortiz, F.R.; Maurer, H.; et al. EuReCa ONE⿿27 Nations, ONE Europe, ONE Registry. Resuscitation 2016, 105, 188–195. [Google Scholar] [CrossRef]
- Gräsner, J.-T.; Herlitz, J.; Tjelmeland, I.B.M.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of Cardiac Arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef]
- Perkins, G.D.; Graesner, J.-T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive Summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Greif, R.; Böttiger, B.W.; Burkart, R.; Cimpoesu, D.; Georgiou, M.; Yeung, J.; Lippert, F.; S Lockey, A.; Olasveengen, T.M.; et al. European Resuscitation Council Guidelines 2021: Systems Saving Lives. Resuscitation 2021, 161, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical Pathophysiology of Hypoxic Ischemic Brain Injury after Cardiac Arrest: A “Two-Hit” Model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Brassard, P.; Labrecque, L.; Smirl, J.D.; Tymko, M.M.; Caldwell, H.G.; Hoiland, R.L.; Lucas, S.J.E.; Denault, A.Y.; Couture, E.J.; Ainslie, P.N. Losing the Dogmatic View of Cerebral Autoregulation. Physiol. Rep. 2021, 9, e14982. [Google Scholar] [CrossRef]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Moerman, A.; De Hert, S. Why and How to Assess Cerebral Autoregulation? Best. Pract. Res. Clin. Anaesthesiol. 2019, 33, 211–220. [Google Scholar] [CrossRef]
- Czosnyka, M.; Miller, C. Monitoring of Cerebral Autoregulation. Neurocrit. Care 2014, 21, 95–102. [Google Scholar] [CrossRef]
- Depreitere, B.; Citerio, G.; Smith, M.; Adelson, P.D.; Aries, M.J.; Bleck, T.P.; Bouzat, P.; Chesnut, R.; De Sloovere, V.; Diringer, M.; et al. Cerebrovascular Autoregulation Monitoring in the Management of Adult Severe Traumatic Brain Injury: A Delphi Consensus of Clinicians. Neurocrit. Care 2021, 34, 731–738. [Google Scholar] [CrossRef]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the Cerebral Circulation: Bedside Assessment and Clinical Implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef]
- Aaslid, R.; Lindegaard, K.F.; Sorteberg, W.; Nornes, H. Cerebral Autoregulation Dynamics in Humans. Stroke 1989, 20, 45–52. [Google Scholar] [CrossRef]
- Tiecks, F.P.; Lam, A.M.; Aaslid, R.; Newell, D.W. Comparison of Static and Dynamic Cerebral Autoregulation Measurements. Stroke 1995, 26, 1014–1019. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Menon, D.K.; Pickard, J.D. Monitoring of Cerebral Autoregulation in Head-Injured Patients. Stroke 1996, 27, 1829–1834. [Google Scholar] [CrossRef]
- Olsen, M.H.; Riberholt, C.G.; Plovsing, R.R.; Møller, K.; Berg, R.M.G. Reliability of the Mean Flow Index (Mx) for Assessing Cerebral Autoregulation in Healthy Volunteers. Physiol. Rep. 2021, 9, e14923. [Google Scholar] [CrossRef]
- Claassen, J.A.; Meel-van den Abeelen, A.S.; Simpson, D.M.; Panerai, R.B. Transfer Function Analysis of Dynamic Cerebral Autoregulation: A White Paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow Metab. 2016, 36, 665–680. [Google Scholar] [CrossRef]
- Moerman, A.; De Hert, S. Recent Advances in Cerebral Oximetry. Assessment of Cerebral Autoregulation with near-Infrared Spectroscopy: Myth or Reality? F1000Res 2017, 6, 1615. [Google Scholar] [CrossRef]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using Near-Infrared Spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef]
- Steiner, L.A.; Pfister, D.; Strebel, S.P.; Radolovich, D.; Smielewski, P.; Czosnyka, M. Near-Infrared Spectroscopy Can Monitor Dynamic Cerebral Autoregulation in Adults. Neurocrit. Care 2009, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Carrera, E.; Brady, K.M.; Kirkpatrick, P.J.; Pickard, J.D.; Smielewski, P. Continuous Assessment of Cerebral Autoregulation With Near-Infrared Spectroscopy in Adults After Subarachnoid Hemorrhage. Stroke 2010, 41, 1963–1968. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Laing, R.J.; Menon, D.; Pickard, J.D. Continuous Assessment of the Cerebral Vasomotor Reactivity in Head Injury. Neurosurgery 1997, 41, 11–19. [Google Scholar] [CrossRef]
- Jaeger, M.; Schuhmann, M.U.; Soehle, M.; Meixensberger, J. Continuous Assessment of Cerebrovascular Autoregulation after Traumatic Brain Injury Using Brain Tissue Oxygen Pressure Reactivity*. Crit. Care Med. 2006, 34, 1783–1788. [Google Scholar] [CrossRef]
- Jaeger, M.; Soehle, M.; Schuhmann, M.U.; Meixensberger, J. Clinical Significance of Impaired Cerebrovascular Autoregulation After Severe Aneurysmal Subarachnoid Hemorrhage. Stroke 2012, 43, 2097–2101. [Google Scholar] [CrossRef]
- van den Brule, J.M.D.; van der Hoeven, J.G.; Hoedemaekers, C.W.E. Cerebral Perfusion and Cerebral Autoregulation after Cardiac Arrest. Biomed. Res. Int. 2018, 2018, 4143636. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Open Med. 2009, 3, 172–181. [Google Scholar] [CrossRef]
- Ameloot, K.; Genbrugge, C.; Meex, I.; Jans, F.; Boer, W.; Vander Laenen, M.; Ferdinande, B.; Mullens, W.; Dupont, M.; Dens, J.; et al. An Observational Near-Infrared Spectroscopy Study on Cerebral Autoregulation in Post-Cardiac Arrest Patients: Time to Drop ‘One-Size-Fits-All’ Hemodynamic Targets? Resuscitation 2015, 90, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Kudoh, I. Cerebral Autoregulation Is Impaired in Patients Resuscitated after Cardiac Arrest. Acta Anaesthesiol. Scand. 1996, 40, 1149–1153. [Google Scholar] [CrossRef]
- Sundgreen, C.; Larsen, F.S.; Herzog, T.M.; Knudsen, G.M.; Boesgaard, S.; Aldershvile, J. Autoregulation of Cerebral Blood Flow in Patients Resuscitated From Cardiac Arrest. Stroke 2001, 32, 128–132. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Gooderham, P.; Menon, D.K.; Brasher, P.M.A.; Foster, D.; Cardim, D.; Czosnyka, M.; Smielewski, P.; Gupta, A.K.; Ainslie, P.N.; et al. The Burden of Brain Hypoxia and Optimal Mean Arterial Pressure in Patients With Hypoxic Ischemic Brain Injury After Cardiac Arrest*. Crit. Care Med. 2019, 47, 960–969. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Smielewski, P.; Bhate, T.D.; Brasher, P.M.; Foster, D.; Menon, D.K.; Gupta, A.K.; Czosnyka, M.; Henderson, W.R.; Gin, K.; et al. Using the Relationship between Brain Tissue Regional Saturation of Oxygen and Mean Arterial Pressure to Determine the Optimal Mean Arterial Pressure in Patients Following Cardiac Arrest: A Pilot Proof-of-Concept Study. Resuscitation 2016, 106, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Bindra, J.; Chuan, A.; Jaeger, M.; Aneman, A. Are Changes in Cerebrovascular Autoregulation Following Cardiac Arrest Associated with Neurological Outcome? Results of a Pilot Study. Resuscitation 2015, 96, 192–198. [Google Scholar] [CrossRef] [PubMed]
- van den Brule, J.M.D.; van Kaam, C.R.; van der Hoeven, J.G.; Claassen, J.A.H.R.; Hoedemaekers, C.W.E. Influence of Induced Blood Pressure Variability on the Assessment of Cerebral Autoregulation in Patients after Cardiac Arrest. Biomed. Res. Int. 2018, 2018, 8153241. [Google Scholar] [CrossRef] [PubMed]
- Laurikkala, J.; Aneman, A.; Peng, A.; Reinikainen, M.; Pham, P.; Jakkula, P.; Hästbacka, J.; Wilkman, E.; Loisa, P.; Toppila, J.; et al. Association of Deranged Cerebrovascular Reactivity with Brain Injury Following Cardiac Arrest: A Post-Hoc Analysis of the COMACARE Trial. Crit. Care 2021, 25, 350. [Google Scholar] [CrossRef]
- Bindra, J.; Pham, P.; Aneman, A.; Chuan, A.; Jaeger, M. Non-Invasive Monitoring of Dynamic Cerebrovascular Autoregulation Using Near Infrared Spectroscopy and the Finometer Photoplethysmograph. Neurocrit. Care 2016, 24, 442–447. [Google Scholar] [CrossRef]
- Calviello, L.A.; Cardim, D.; Czosnyka, M.; Preller, J.; Smielewski, P.; Siyal, A.; Damian, M.S. Feasibility of Non-Invasive Neuromonitoring in General Intensive Care Patients Using a Multi-Parameter Transcranial Doppler Approach. J. Clin. Monit. Comput. 2022, 36, 1805–1815. [Google Scholar] [CrossRef]
- Balu, R.; Rajagopalan, S.; Baghshomali, S.; Kirschen, M.; Amurthur, A.; Kofke, W.A.; Abella, B.S. Cerebrovascular Pressure Reactivity and Intracranial Pressure Are Associated with Neurologic Outcome after Hypoxic-Ischemic Brain Injury. Resuscitation 2021, 164, 114–121. [Google Scholar] [CrossRef]
- Hazenberg, L.; Aries, M.; Beqiri, E.; Mess, W.; van Mook, W.; Delnoij, T.; Zeiler, F.; van Kuijk, S.; Tas, J. Are NIRS-Derived Cerebral Autoregulation and ABPopt Values Different between Hemispheres in Hypoxic-Ischemic Brain Injury Patients Following Cardiac Arrest? J. Clin. Monit. Comput. 2023, 37, 1427–1430. [Google Scholar] [CrossRef]
- Griesdale, D.E.G.; Sekhon, M.S.; Wood, M.D.; Cardim, D.; Brasher, P.M.A.; McCredie, V.; Sirounis, D.; Foster, D.; Krasnogolova, Y.; Smielewski, P.; et al. Near-Infrared Spectroscopy to Assess Cerebral Autoregulation and Optimal Mean Arterial Pressure in Patients With Hypoxic-Ischemic Brain Injury: A Prospective Multicenter Feasibility Study. Crit. Care Explor. 2020, 2, e0217. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lara, L.; Geocadin, R.; Zorrilla-Vaca, A.; Healy, R.; Radzik, B.R.; Palmisano, C.; Mirski, M.; Ziai, W.C.; Hogue, C. Validation of Near-Infrared Spectroscopy for Monitoring Cerebral Autoregulation in Comatose Patients. Neurocrit. Care 2017, 27, 362–369. [Google Scholar] [CrossRef]
- Crippa, I.A.; Vincent, J.-L.; Zama Cavicchi, F.; Pozzebon, S.; Annoni, F.; Cotoia, A.; Njimi, H.; Gaspard, N.; Creteur, J.; Taccone, F.S. Cerebral Autoregulation in Anoxic Brain Injury Patients Treated with Targeted Temperature Management. J. Intensive Care 2021, 9, 67. [Google Scholar] [CrossRef]
- Tachino, J.; Nonomiya, Y.; Taniuchi, S.; Shintani, A.; Nakao, S.; Takegawa, R.; Hirose, T.; Sakai, T.; Ohnishi, M.; Shimazu, T.; et al. Association between Time-Dependent Changes in Cerebrovascular Autoregulation after Cardiac Arrest and Outcomes: A Prospective Cohort Study. J. Cereb. Blood Flow Metab. 2023, 43, 1942–1950. [Google Scholar] [CrossRef]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103, i3–i13. [Google Scholar] [CrossRef]
- Author, A.; Tetičkovič, E.; Menih, M.; Magdič, J.; Prispel, Č.; Avtor, I. TCD monitoring of Cerebral Blood Flow. Acta Med.-Biotech. 2009, 2, 11–18. [Google Scholar] [CrossRef]
- Larsen, F.S.; Olsen, K.S.; Hansen, B.A.; Paulson, O.B.; Knudsen, G.M. Transcranial Doppler Is Valid for Determination of the Lower Limit of Cerebral Blood Flow Autoregulation. Stroke 1994, 25, 1985–1988. [Google Scholar] [CrossRef]
- Purkayastha, S.; Sorond, F. Transcranial Doppler Ultrasound: Technique and Application. Semin. Neurol. 2013, 32, 411–420. [Google Scholar] [CrossRef]
- Panerai, R.B. Transcranial Doppler for Evaluation of Cerebral Autoregulation. Clin. Auton. Res. 2009, 19, 197–211. [Google Scholar] [CrossRef]
- Vitt, J.R.; Loper, N.E.; Mainali, S. Multimodal and Autoregulation Monitoring in the Neurointensive Care Unit. Front. Neurol. 2023, 14, 1155986. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.C.; Lam, M.Y.; Llwyd, O.; Salinet, A.S.M.; Bor-Seng-Shu, E.; Panerai, R.B.; Robinson, T.G. Cerebral Autoregulation and Response to Intravenous Thrombolysis for Acute Ischemic Stroke. Sci. Rep. 2020, 10, 10554. [Google Scholar] [CrossRef]
- Rasulo, F.A.; Girardini, A.; Lavinio, A.; De Peri, E.; Stefini, R.; Cenzato, M.; Nodari, I.; Latronico, N. Are Optimal Cerebral Perfusion Pressure and Cerebrovascular Autoregulation Related to Long-Term Outcome in Patients With Aneurysmal Subarachnoid Hemorrhage? J. Neurosurg. Anesthesiol. 2012, 24, 3–8. [Google Scholar] [CrossRef]
- Johnson, U.; Engquist, H.; Lewén, A.; Howells, T.; Nilsson, P.; Ronne-Engström, E.; Rostami, E.; Enblad, P. Increased Risk of Critical CBF Levels in SAH Patients with Actual CPP below Calculated Optimal CPP. Acta Neurochir. (Wien.) 2017, 159, 1065–1071. [Google Scholar] [CrossRef]
- Reinhard, M.; Neunhoeffer, F.; Gerds, T.A.; Niesen, W.-D.; Buttler, K.-J.; Timmer, J.; Schmidt, B.; Czosnyka, M.; Weiller, C.; Hetzel, A. Secondary Decline of Cerebral Autoregulation Is Associated with Worse Outcome after Intracerebral Hemorrhage. Intensive Care Med. 2010, 36, 264–271. [Google Scholar] [CrossRef]
- Sarwal, A.; Robba, C.; Venegas, C.; Ziai, W.; Czosnyka, M.; Sharma, D. Are We Ready for Clinical Therapy Based on Cerebral Autoregulation? A Pro-Con Debate. Neurocrit. Care 2023, 39, 269–283. [Google Scholar] [CrossRef]
- Lee, J.K.; Brady, K.M.; Chung, S.-E.; Jennings, J.M.; Whitaker, E.E.; Aganga, D.; Easley, R.B.; Heitmiller, K.; Jamrogowicz, J.L.; Larson, A.C.; et al. A Pilot Study of Cerebrovascular Reactivity Autoregulation after Pediatric Cardiac Arrest. Resuscitation 2014, 85, 1387–1393. [Google Scholar] [CrossRef]
- Kirschen, M.P.; Majmudar, T.; Beaulieu, F.; Burnett, R.; Shaik, M.; Morgan, R.W.; Baker, W.; Ko, T.; Balu, R.; Agarwal, K.; et al. Deviations from NIRS-Derived Optimal Blood Pressure Are Associated with Worse Outcomes after Pediatric Cardiac Arrest. Resuscitation 2021, 168, 110–118. [Google Scholar] [CrossRef]
- Kurz, J.E.; Smith, C.M.; Wainwright, M.S. Thermoregulate, Autoregulate and Ventilate. Curr. Opin. Pediatr. 2017, 29, 259–265. [Google Scholar] [CrossRef]
- Zipfel, J.; Hegele, D.; Hockel, K.; Kerscher, S.R.; Heimberg, E.; Czosnyka, M.; Neunhoeffer, F.; Schuhmann, M.U. Monitoring of Cerebrovascular Pressure Reactivity in Children May Predict Neurologic Outcome after Hypoxic-Ischemic Brain Injury. Child’s Nerv. Syst. 2022, 38, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.E.; Maa, T.; Chung, M.G.; O’Brien, N.F. Cerebral Blood Flow Velocity and Autoregulation in Paediatric Patients Following a Global Hypoxic-Ischaemic Insult. Resuscitation 2018, 126, 191–196. [Google Scholar] [CrossRef]
- Lee, J.K.; Brady, K.M.; Mytar, J.O.; Kibler, K.K.; Carter, E.L.; Hirsch, K.G.; Hogue, C.W.; Easley, R.B.; Jordan, L.C.; Smielewski, P.; et al. Cerebral Blood Flow and Cerebrovascular Autoregulation in a Swine Model of Pediatric Cardiac Arrest and Hypothermia*. Crit. Care Med. 2011, 39, 2337–2345. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Brady, K.M.; Koehler, R.; Smielewski, P.; Czosnyka, M.; Donnelly, J.; Lee, J.K. Comparison of Wavelet and Correlation Indices of Cerebral Autoregulation in a Pediatric Swine Model of Cardiac Arrest. Sci. Rep. 2020, 10, 5926. [Google Scholar] [CrossRef]
- Lee, J.K.; Yang, Z.-J.; Wang, B.; Larson, A.C.; Jamrogowicz, J.L.; Kulikowicz, E.; Kibler, K.K.; Mytar, J.O.; Carter, E.L.; Burman, H.T.; et al. Noninvasive Autoregulation Monitoring in a Swine Model of Pediatric Cardiac Arrest. Anesth. Analg. 2012, 114, 825–836. [Google Scholar] [CrossRef]
- Berg, R.M.G.; Plovsing, R.R. Effects of Short-Term Mechanical Hyperventilation on Cerebral Blood Flow and Dynamic Cerebral Autoregulation in Critically Ill Patients with Sepsis. Scand. J. Clin. Lab. Investig. 2016, 76, 226–233. [Google Scholar] [CrossRef]
- de Azevedo, D.S.; Salinet, A.S.M.; de Lima Oliveira, M.; Teixeira, M.J.; Bor-Seng-Shu, E.; de Carvalho Nogueira, R. Cerebral Hemodynamics in Sepsis Assessed by Transcranial Doppler: A Systematic Review and Meta-Analysis. J. Clin. Monit. Comput. 2017, 31, 1123–1132. [Google Scholar] [CrossRef]
- Crippa, I.A.; Subirà, C.; Vincent, J.-L.; Fernandez, R.F.; Hernandez, S.C.; Cavicchi, F.Z.; Creteur, J.; Taccone, F.S. Impaired Cerebral Autoregulation Is Associated with Brain Dysfunction in Patients with Sepsis. Crit. Care 2018, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Al-Kawaz, M.; Cho, S.-M.; Gottesman, R.F.; Suarez, J.I.; Rivera-Lara, L. Impact of Cerebral Autoregulation Monitoring in Cerebrovascular Disease: A Systematic Review. Neurocrit. Care 2022, 36, 1053–1070. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Czosnyka, M.; Smielewski, P.; Varsos, G.V.; Kasprowicz, M.; Brady, K.M.; Pickard, J.D.; Kirkpatrick, P.J. Monitoring Cerebral Autoregulation After Subarachnoid Hemorrhage. In Intracranial Pressure and Brain Monitoring XV; Springer: Cham, Switzerland, 2016; pp. 199–203. [Google Scholar]
- Budohoski, K.P.; Czosnyka, M.; Smielewski, P.; Kasprowicz, M.; Helmy, A.; Bulters, D.; Pickard, J.D.; Kirkpatrick, P.J. Impairment of Cerebral Autoregulation Predicts Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Stroke 2012, 43, 3230–3237. [Google Scholar] [CrossRef]
- Soehle, M.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P.J. Continuous Assessment of Cerebral Autoregulation in Subarachnoid Hemorrhage. Anesth. Analg. 2004, 98, 1133–1139. [Google Scholar] [CrossRef]
- Brown, C.H.; Neufeld, K.J.; Tian, J.; Probert, J.; LaFlam, A.; Max, L.; Hori, D.; Nomura, Y.; Mandal, K.; Brady, K.; et al. Effect of Targeting Mean Arterial Pressure During Cardiopulmonary Bypass by Monitoring Cerebral Autoregulation on Postsurgical Delirium Among Older Patients. JAMA Surg. 2019, 154, 819. [Google Scholar] [CrossRef]
- Liu, X.; Donnelly, J.; Brady, K.M.; Akiyoshi, K.; Bush, B.; Koehler, R.C.; Lee, J.K.; Hogue, C.W.; Czosnyka, M.; Smielewski, P.; et al. Comparison of Different Metrics of Cerebral Autoregulation in Association with Major Morbidity and Mortality after Cardiac Surgery. Br. J. Anaesth. 2022, 129, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nomura, Y.; Whitman, G.; Sussman, M.; Schena, S.; Kilic, A.; Choi, C.W.; Akiyoshi, K.; Neufeld, K.J.; Lawton, J.; et al. Cerebral Autoregulation in the Operating Room and Intensive Care Unit after Cardiac Surgery. Br. J. Anaesth. 2021, 126, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Rikhraj, K.J.K.; Wood, M.D.; Hoiland, R.L.; Thiara, S.; Griesdale, D.E.G.; Sekhon, M.S. Determining Optimal Mean Arterial Pressure After Cardiac Arrest: A Systematic Review. Neurocrit. Care 2021, 34, 621–634. [Google Scholar] [CrossRef]
- Longhitano, Y.; Iannuzzi, F.; Bonatti, G.; Zanza, C.; Messina, A.; Godoy, D.; Dabrowski, W.; Xiuyun, L.; Czosnyka, M.; Pelosi, P.; et al. Cerebral Autoregulation in Non-Brain Injured Patients: A Systematic Review. Front. Neurol. 2021, 12, 732176. [Google Scholar] [CrossRef] [PubMed]
- Caldas, J.R.; Haunton, V.J.; Panerai, R.B.; Hajjar, L.A.; Robinson, T.G. Cerebral Autoregulation in Cardiopulmonary Bypass Surgery: A Systematic Review. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 494–503. [Google Scholar] [CrossRef]
- Santos, D.P.D.A.D.; Thirumala, P.D.; Reddy, G.; Barros, D.F.D.; Faria, V.N.R.; Shandal, V.; Kurtz, P. Risk of Perioperative Stroke and Cerebral Autoregulation Monitoring: A Systematic Review. Arq. Neuropsiquiatr. 2022, 80, 1196–1203. [Google Scholar] [CrossRef]
- Rivera-Lara, L.; Zorrilla-Vaca, A.; Geocadin, R.; Ziai, W.; Healy, R.; Thompson, R.; Smielewski, P.; Czosnyka, M.; Hogue, C.W. Predictors of Outcome With Cerebral Autoregulation Monitoring. Crit. Care Med. 2017, 45, 695–704. [Google Scholar] [CrossRef]
- de-Lima-Oliveira, M.; Salinet, A.S.M.; Nogueira, R.C.; de Azevedo, D.S.; Paiva, W.S.; Teixeira, M.J.; Bor-Seng-Shu, E. Intracranial Hypertension and Cerebral Autoregulation: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 113, 110–124. [Google Scholar] [CrossRef]
| Study | Type of Study | Main Finding | Setting | Study Population | Monitoring Method | Maneuver | Main Limitation | Autoregulation Indices |
|---|---|---|---|---|---|---|---|---|
| Ameloot K et al. [29] | Prospective observational study | CBFA disturbed in one-third CA patients, correlated with worse outcomes. Time spent under optimal MAP is negatively associated with survival | Tertiary care hospital | 51 patients after CA | NIRS, invasive blood pressure monitoring | / | In a small sample size, COX predicted optimal MAP as an average value, insufficient data on drugs used | COx |
| Nishizawa H et al. [30] | Prospective interventional study | CBFA impaired in patients after CA | Department of Anesthesiology, University school of medicine | Eight patients after CA | A catheter was inserted percutaneously into the right internal jugular vein, the tip positioned in the jugular bulb for venous blood gas measurement. Arterial pressure measurement and arterial blood gas samples were obtained | MAP changed to a value of 30% lower or higher than baseline by infusing trimethaphan or methoxamine | Small sample size | Arterial-jugular bulb venous oxygen content difference (AVDO2) calculated at each MAP level. After that, 1/AVDO2, cerebral blood flow indices (CBFI) were calculated. The changes in CBFI and oxygen saturation of jugular venous blood seen after the decrease or increase in MAP indicates impairment of CBFA |
| Sundgreen C et al. [31] | Case-control interventional study | CBFA is absent or right-shifted in the majority of patients after CA | University Hospital ICU | 18 patients after CA in and out of hospital, 6 healthy control subjects | TCD, invasive blood pressure monitoring | A stepwise rise in MAP by use of norepinephrine infusion | Small sample size | MAP was plotted against the mean Fv, and a possible lower limit of autoregulation was identified |
| Sekhon MS et al. [32] | Prospective interventional study | A linear relationship between increased MAP and PBTO2 in HIBI patients. Perfusion within proximity of optimal MAP may be associated with improved PBTO2 | Quaternary ICU | Ten patients after CA | Multimodal: brain tissue oxygenation, intracranial pressure, jugular venous continuous oximetry, NIRS | / | Small sample size, definition of brain hypoxia from the traumatic brain injury literature, patient cohort with pulseless circulatory arrest | PRx |
| Sekhon MS et al. [33] | Proof-of-concept feasibility study | Feasibility of determining optimal MAP using cerebral oximetry in patients after CA | General hospital ICU | 20 patients after CA | NIRS, invasive blood pressure monitoring | / | Proof of concept study, no granular data on carbon dioxide | COx |
| Pham P et al. [34] | Case-control study | Early impairment of CBFA after CA independently associated with mortality at three months follow-ups | General hospital ICU | 23 patients after CA, 28 healthy volunteers | NIRS, invasive blood pressure monitoring, fino-meter for noninvasive blood pressure monitoring in healthy volunteers | Blood pressure changes induced in healthy volunteers through bed-tilt positional changes, Valsalva maneuvers and short immersion of one hand in ice-cold water | Small sample size, intermittent measurement | TOx |
| Van den Brule JMD et al. [35] | Prospective observational study | Changes in experimental settings strongly influence the results of estimation of CBFA | University Hospital ICU | 13 patients after CA | TCD, invasive blood pressure monitoring | Repeated changes in the position of the bed from horizontal to a maximum of 30 degrees Trendelenburg and 30 degrees anti-Trendelenburg | Small sample size, changes in quality of the recorded signal, the influence of bed position on venous outflow | CBFA was calculated in the time domain (Mx) and frequency domain (transfer function analysis) |
| Laurikkala J et al. [36] | Post-hoc analysis of multicenter srandomised controlled pilot study | Impaired cerebrovascular reactivity is common after CA, especially in patients with chronic hypertension. Decreased upper MAP bound and a narrower MAP range for maintained cerebrovascular reactivity associated with poor outcome, severe brain injury assessed by NfL | Six ICUs in Finland and one ICU in Denmark | 120 patients after CA | NIRS, invasive blood pressure monitoring | / | Limitations of NIRS and TOx to monitor cerebrovascular reactivity | TOx |
| Bindra J et al. [37] | Prospective observational study | Dynamic CBFA can be continuously assessed noninvasively | General hospital ICU | Diverse cohort, including 1 patient after CA | NIRS, invasive blood pressure monitoring, finometer for noninvasive blood pressure monitoring | / | Small sample size, finometer being operator depended | CBFA as a correlation coefficient between invasive arterial blood pressure and rSO2 (iTOx) or noninvasive arterial blood pressure and rSO2 (nTOx) |
| Calviello LA et al. [38] | Prospective study | Multiparameter TCD neuromonitoring increases outcome predictive power. TCD-based indices can be applied to general intensive care monitoring | University Hospital ICU | Diverse cohort, including 14 patients after CA | TCD and ABP were continuously monitored non-invasively using a finger probe or a pressure monitoring kit | / | Small sample size, preliminary results, patients not separated by condition | Mx |
| Balu R et al. [39] | Retrospective observational study | Cerebrovascular pressure reactivity and ICP appear to be associated with neurologic outcomes in patients with HIBI | NCCU at University Hospital | Diverse cohort, including 32 patients after CA | ICP monitoring and interstitial brain tissue oxygen measurements through quad-lumen bolt invasive blood pressure monitoring | / | Single-center retrospective design, selection and indication biases, monitoring only the frontal lobe, temporal trends not analyzed, not using cerebral performance score as an outcome | PRx |
| Hazenberg L et al. [40] | Prospective observational study | No differences between left and right-sided NIRS recordings or CBFA estimation in HIBI patients | ICU University Medical Center | 11 patients after CA | NIRS, invasive blood pressure monitoring | / | Small sample size, short monitoring time, possibility of local pathology | COx |
| Griesdale DEG et al. [41] | Prospective multicenter cohort study | It is feasible to recruit and collect high-frequency physiological data in patients after CA. Time below optimal MAP and duration of dysfunctional CBFA not associated with an unfavorable neurologic outcome | ICUs in three teaching hospitals | 59 patients after CA | NIRS, invasive blood pressure monitoring | / | Underpowered analyses, inability to control for potential confounds | COx |
| Rivera-Lara L et al. [42] | Prospective observational cohort study | Monitoring CBFA with NIRS-derived COx is correlated and agrees well with previously validated TCD-based methods | NCCU at the teaching hospital | Diverse cohort, including one patient after CA | NIRS, TCD, invasive blood pressure monitoring | / | Small sample size, heterogeneous lesions in a population with acute coma | COx, Mx |
| Crippa IA et al. [43] | Retrospective analysis of prospectively collected data | CBFA is frequently altered in CA patients treated by targeted temperature management. Altered CBFA during normothermia was independently associated with poor outcome | Department of intensive care at University hospital | 50 patients after CA | TCD, invasive blood pressure monitoring | / | Cerebrovascular resistance or absolute CBF values were not measured directly MAP in relation to individual CBFA curves not investigated | Mx |
| Tachino J et al. [44] | A prospective, observational cohort study | Mortality increased significantly with longer non-CBFA time within 96 h after the return of spontaneous circulation | Trauma and Acute Critical Care Center | 100 consecutive patients after CA | NIRS, invasive blood pressure monitoring | / | Observational research and differences in patient characteristics and clinical protocol did not account for the potential influence of targeted temperature control and CO2 on cerebral circulation | COx |
| Monitoring Method | Number of Research |
|---|---|
| NIRS | 8 |
| TCD | 4 |
| ICP and interstitial brain tissue oxygen measurements through quad-lumen bolt | 1 |
| Internal jugular vein catheter | 1 |
| Multimodal | 2 |
| Mathematical Model | Number of Research |
|---|---|
| COx | 5 |
| TOx | |
| Mx | 3 |
| PRx | 2 |
| Multimodal | 1 |
| Other | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovčič, R.; Rakusa, M.; Markota, A. Monitoring of Cerebral Blood Flow Autoregulation after Cardiac Arrest. Medicina 2024, 60, 1381. https://doi.org/10.3390/medicina60091381
Petrovčič R, Rakusa M, Markota A. Monitoring of Cerebral Blood Flow Autoregulation after Cardiac Arrest. Medicina. 2024; 60(9):1381. https://doi.org/10.3390/medicina60091381
Chicago/Turabian StylePetrovčič, Rok, Martin Rakusa, and Andrej Markota. 2024. "Monitoring of Cerebral Blood Flow Autoregulation after Cardiac Arrest" Medicina 60, no. 9: 1381. https://doi.org/10.3390/medicina60091381





