Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review
Abstract
1. Introduction
2. Nanotechnology: Innovative Synthesis Techniques and Health Applications
2.1. Bottom-Up Approaches
2.2. Green Synthesis Methods
2.3. Electrospun Nanofibrous Frameworks for Nerve Tissue Regeneration
3. Important Materials
3.1. Biodegradable Polymers
3.1.1. Polycaprolactone
3.1.2. Poly(Lactic-Co-Glycolic Acid) (PLGA)
3.1.3. Conducting Polymers
3.1.4. Natural Polymers
3.2. Therapeutic Opportunities: Neural Nerve Injuries and Tissue Engineering
3.2.1. Traumatic Brain Injury
3.2.2. Spinal Cord Injury
3.2.3. Peripheral Nerve Defect Repair
3.2.4. Drug Screening and Disease Modeling
3.3. Gelatin in Neuronal Protection
3.4. Self-Assembling Peptide Nanoparticles
4. Neural Regeneration
4.1. Neural Stem Cells
4.2. Axonal Growth and Neural Stem Migration
5. Challenges in Delivering Therapeutics to the Brain: Overcoming the Blood–Brain Barrier for Effective Neural Regeneration
6. Nanoparticles for BBB Modulation
Nanomaterial Strategies for Crossing the BBB
7. Combining BBB Modulation with Neural Regeneration
7.1. Neural Stem-Cell-Derived Exosomes
7.1.1. Understanding NSC Exosomes in Brain Injury Regeneration
7.1.2. Challenges and Opportunities
7.2. Neuromodulation-Based Stem Cell Therapy
Neuromodulation
7.3. Mesenchymal Stem Cells (MSCs)
Role of MSCs in Neuronal Regeneration
7.4. Electrical and Magnetic Stimulation
8. Applications, Case Studies, and Real-World Examples
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krsek, A.; Baticic, L. Nanotechnology-Driven Therapeutic Innovations in Neurodegenerative Disorders: A Focus on Alzheimer’s and Parkinson’s Disease. Future Pharmacol. 2024, 4, 352–379. [Google Scholar] [CrossRef]
- Vargas-Barona, A.; Bernáldez-Sarabia, J.; Castro-Ceseña, A.B. Lipid-polymer hybrid nanoparticles loaded with N-acetylcysteine for the modulation of neuroinflammatory biomarkers in human iPSC-derived PSEN2 (N141I) astrocytes as a model of Alzheimer’s disease. J. Mater. Chem. B 2024, 12, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Niri, A.D.; Zarchi, A.A.K.; Harati, P.G.; Salimi, A.; Mujokoro, B. Tissue engineering scaffolds in the treatment of brain disorders in geriatric patients. Artif. Organs 2019, 43, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.; Martinez, B. Biomaterial and tissue-engineering strategies for the treatment of brain neurodegeneration. Neural Regen. Res. 2022, 17, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Flores, F.; Garcia-Atutxa, I.; Santos, A.; Armendariz-Borunda, J. Toward a New Generation of Bio-Scaffolds for Neural Tissue Engineering: Challenges and Perspectives. Pharmaceutics 2023, 15, 1750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, N.; Chung, T.D.; Guo, Z.; Jamieson, J.J.; Liang, L.; Linville, R.M.; Pessell, A.F.; Wang, L.; Searson, P.C. The influence of physiological and pathological perturbations on blood-brain barrier function. Front. Neurosci. 2023, 17, 1289894. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood-brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Wang, F.; Sun, X.; Li, H.; Zhang, K.; Li, J. Recent advances in tissue repair of the blood-brain barrier after stroke. J. Tissue Eng. 2024, 15, 20417314241226551. [Google Scholar] [CrossRef]
- Tan, L.Y.; Cunliffe, G.; Hogan, M.P.; Yeo, X.Y.; Oh, C.; Jin, B.; Kang, J.; Park, J.; Kwon, M.-S.; Kim, M.; et al. Emergence of the brain-border immune niches and their contribution to the development of neurodegenerative diseases. Front. Immunol. 2024, 15, 1380063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zamproni, L.N.; Mundim, M.T.V.V.; Porcionatto, M.A. Neurorepair and Regeneration of the Brain: A Decade of Bioscaffolds and Engineered Microtissue. Front. Cell Dev Biol. 2021, 9, 649891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorick, C.M.; Breza, V.R.; Nowak, K.M.; Cheng, V.W.; Fisher, D.G.; Debski, A.C.; Hoch, M.R.; Demir, Z.E.; Tran, N.M.; Schwartz, M.R.; et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 2022, 191, 114583. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K. Non-Invasive Drug Delivery across the Blood-Brain Barrier: A Prospective Analysis. Pharmaceutics 2023, 15, 2599. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Kršek, A.; Batičić, L.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Miletić-Gršković, S.; Sotošek, V. Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery. Curr. Issues Mol. Biol. 2024, 46, 3794–3809. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Shen, Z.; Nieh, M.-P.; Li, Y. Decorating nanoparticle surface for targeted drug delivery: Opportunities and challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S. Top-Down and Bottom-Up Approaches for Synthesis of Nanoparticles. In Materials Research Foundations; Materials Research Forum: Millersville, PA, USA, 2023; pp. 92–130. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-Milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Maran, B.A.V.; Jeyachandran, S.; Kimura, M. A review on the electrospinning of polymer nanofibers and its biomedical applications. J. Compos. Sci. 2024, 8, 32. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Singh, J.P.; Kumar, M.; Sharma, A.; Pandey, G.; Chae, K.H.; Lee, S. Bottom-Up and Top-Down Approaches for MGO; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Hernández, A.R.R.; Cruz, A.G.; Campos-Delgado, J. Chemical Vapor Deposition Synthesis of Graphene on Copper Foils; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [PubMed]
- Oke, J.A.; Jen, T.-C. Atomic layer deposition thin film techniques and its bibliometric perspective. Int. J. Adv. Manuf. Technol. 2023, 126, 4811–4825. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green synthesis interventions of pharmaceutical industries for sustainable development. Curr. Res. Green Sustain. Chem. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; Arenas-Alatorre, J.Á. Green Synthesis of Nanoparticles: A Biological Approach; IntechOpen eBooks; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Jaison, J.P.; Balasubramanian, B.; Gangwar, J.; James, N.; Pappuswamy, M.; Anand, A.V.; Al-Dhabi, N.A.; Arasu, M.V.; Liu, W.-C.; Sebastian, J.K. Green Synthesis of Bioinspired Nanoparticles Mediated from Plant Extracts of Asteraceae Family for Potential Biological Applications. Antibiotics 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panagopoulos, G.N.; Megaloikonomos, P.D.; Mavrogenis, A.F. Current Trends and Future Perspectives for Peripheral Nerve Regeneration; Springer: Berlin/Heidelberg, Germany, 2019; pp. 411–423. [Google Scholar]
- O’brien, A.L.; West, J.M.; Saffari, T.M.; Nguyen, M.; Moore, A.M. Promoting nerve regeneration: Electrical stimulation, gene therapy, and beyond. Physiology 2022, 37, 302–310. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Qian, J.; Lin, Z.; Liu, Y.; Wang, Z.; Lin, Y.; Gong, C.; Ruan, R.; Zhang, J.; Yang, H. Functionalization strategies of electrospun nanofibrous scaffolds for nerve tissue engineering. Smart Mater. Med. 2021, 2, 260–279. [Google Scholar] [CrossRef]
- Ayaz, F.; Demir, D.; Bölgen, N. Electrospun nanofiber mats caged the mammalian macrophages on their surfaces and prevented their inflammatory responses independent of the fiber diameter. Sci. Rep. 2024, 14, 12339. [Google Scholar] [CrossRef]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. Part A 2007, 83A, 636–645. [Google Scholar] [CrossRef]
- Kim, I.-K.; Park, J.-H.; Kim, B.; Hwang, K.-C.; Song, B.-W. Recent advances in stem cell therapy for neurodegenerative disease: Three dimensional tracing and its emerging use. World J. Stem Cells 2021, 13, 1215–1230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, L.; Sun, Z.; Li, J.; Zhu, R.; Niu, B.; Tam, K.L.; Xiao, Q.; Li, J.; Wang, W.; Tsui, C.Y.; et al. Electrical stimulation at nanoscale topography boosts neural stem cell neurogenesis through the enhancement of autophagy signaling. Biomaterials 2021, 268, 120585. [Google Scholar] [CrossRef]
- Singh, V.K.; Haq, A.; Tiwari, M.; Saxena, A.K. Approach to management of nerve gaps in peripheral nerve injuries. Injury 2022, 53(4), 1308–1318. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Jiang, W.; Huang, Z.; Liu, T.; Feng, J. Fabrication of a highly conductive silk knitted composite scaffold by two-step electrostatic self-assembly for potential peripheral nerve regeneration. ACS Appl. Mater. Interfaces 2020, 12, 12317–12327. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, K.; Khorasani, M.T.; Rashidi, A.; Joupari, M.D. Preparation and characterization of graphene oxide aerogel/gelatin as a hybrid scaffold for application in nerve tissue engineering. Int. J. Polym. Mater. 2020, 12, 12317–12327. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Z.; Cai, Z.; Zhao, J.; Lu, M.; Liang, J.; Wang, F.; Qi, J.; Cui, W.; Deng, L. Bioinspired functional black phosphorus electrospun fibers achieving recruitment and biomineralization for staged bone regeneration. Small 2020, 16, 2005433. [Google Scholar] [CrossRef]
- Xi, K.; Gu, Y.; Tang, J.; Chen, H.; Xu, Y.; Wu, L.; Cai, F.; Deng, L.; Yang, H.; Shi, Q.; et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat. Commun. 2020, 11, 4504. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Zhu, Z.; Ni, B.; Yin, G.; Zhou, F.; Liu, J.; Guo, Q.; Guo, X. NgR expression in macrophages promotes nerve regeneration after spinal cord injury in rats. Arch. Orthop. Trauma Surg. 2010, 130, 945–951. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly(D,L-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.-S.; Park, S.B.; Kim, S.; Lee, M.; Park, S.-A.; Hwang, S.Y.; Koo, J.M.; Oh, D.X.; Park, J. Improved mechanical properties of biodegradable polycaprolactone nanocomposites prepared using cellulose nanocrystals. Cellulose 2023, 30, 11561–11574. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar] [CrossRef]
- Licciardello, M.; Traldi, C.; Bortolameazzi, M.; Testore, D.; Ciardelli, G.; Tonda-Turo, C. Aligned polycaprolactone/polyaniline electrospun nanofibers for directing neural stem cell differentiation and neuron arrangement. Front. Biomater. Sci. 2024, 3, 1362599. [Google Scholar] [CrossRef]
- Talebi, A.; Nasab, P.M.; Labbaf, S.; Roach, P. Polycaprolactone/Gelatin/Polypyrrole/Graphene Conductive Aligned Fibrous Scaffold with Ferulic Acid Encapsulation for Tissue Engineering Applications. Fibers Polym. 2023, 24, 2995–3006. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, D.; Wu, B.; Chen, X.; Li, X.; Li, Z.; Dai, F. Electrospun Piezoelectric Scaffold with External Mechanical Stimulation for Promoting Regeneration of Peripheral Nerve Injury. Biomacromolecules 2023, 24, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Sultana, T.; Joo, G.J.; Park, C.; Lee, B.-T. In vitro endothelial differentiation evaluation on polycaprolactone-methoxy polyethylene glycol electrospun membrane and fabrication of multilayered small-diameter hybrid vascular graft. J. Biomater. Appl. 2020, 34, 1395–1408. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar]
- Ghalei, S.; Li, J.; Douglass, M.; Garren, M.; Handa, H. Synergistic approach to develop antibacterial electrospun scaffolds using honey and S -Nitroso- N -acetyl penicillamine. ACS Biomater. Sci. Eng. 2021, 7, 517–526. [Google Scholar]
- Xu, X.; Ren, S.; Li, L.; Zhou, Y.; Peng, W.; Xu, Y. Biodegradable engineered fiber scaffolds fabricated by electrospinning for periodontal tissue regeneration. J. Biomater. Appl. 2020, 36, 55–75. [Google Scholar] [CrossRef]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.M.A.P.; Sandoval-Castellanos, A.M.; De Gregorio, C.; Contreras-Kallens, P.; Haycock, J.W. The Role of Schwann Cells in Peripheral Nerve Function, Injury, and Repair. In Cell Engineering and Regeneration; Reference Series in Biomedical Engineering; Gimble, J., Marolt Presen, D., Oreffo, R., Wolbank, S., Redl, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Hu, W.; Gu, J.; Deng, A.; Gu, X. Polyglycolic acid filaments guide Schwann cell migration in vitro and in vivo. Biotechnol. Lett. 2008, 30, 1937–1942. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhong, Q.; Ge, Y.; Guo, Z.; Tian, J.; Zhou, Y.; Ding, S.; Li, H.; Zhou, C. Dual drug loaded coaxial electrospun PLGA/PVP fiber for guided tissue regeneration under control of infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.; Santiago, M.D.; Irvin, J.A. Electrospun Conducting Polymers: Approaches and Applications. Materials 2022, 15, 8820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Azzawi, A.G.S.; Aziz, S.B.; Dannoun, E.M.A.; Iraqi, A.; Nofal, M.M.; Murad, A.R.; Hussein, A.M. A Mini Review on the Development of Conjugated Polymers: Steps towards the Commercialization of Organic Solar Cells. Polymers 2023, 15, 164. [Google Scholar] [CrossRef]
- Malik, A.H.; Habib, F.; Qazi, M.J.; Ganayee, M.A.; Ahmad, Z.; Yatoo, M.A. A short review article on conjugated polymers. J. Polym. Res. 2023, 30, 115. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Kaushik, G.; Khatua, C.; Ghosh, S.; Lahiri, D. Electrical Stimulation-Mediated Differentiation of Neural Cells on Conductive Carbon Nanofiller-Based Scaffold. Biomed. Mater. Devices 2023, 1, 301–318. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Vedakumari, S.W.; Jancy, S.J.V.; Prabakaran, L.; Raja Pravin, Y.; Senthil, R. A review on background, process and application of electrospun nanofibers for tissue regeneration. Proc. Inst. Mech. Engineers. Part H J. Eng. Med. 2023, 237, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Cui, S.; Mao, J.; Rouabhia, M.; Elkoun, S.; Zhang, Z. A biocompatible polypyrrole membrane for biomedical applications. RSC Adv. 2021, 11, 16996–17006. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Ji, Y.-R.; Hsu, Y.-H.; Liao, F.-C.; Kao, N.-T.; Huang, A.P.-H.; Young, T.-H. Gelatin-Based hydrogel for Three-Dimensional neuron culture application. ACS Omega 2023, 8, 45288–45300. [Google Scholar] [CrossRef]
- Huang, W.-H.; Ding, S.-L.; Zhao, X.-Y.; Li, K.; Guo, H.-T.; Zhang, M.-Z.; Gu, Q. Collagen for neural tissue engineering: Materials, strategies, and challenges. Mater. Today Bio 2023, 20, 100639. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Ucar, B.; Humpel, C. Collagen for brain repair: Therapeutic perspectives. Neural Regen. Res. 2018, 13, 595–598. [Google Scholar]
- Aijie, C.; Xuan, L.; Huimin, L.; Yanli, Z.; Yiyuan, K.; Yuqing, L.; Longquan, S. Nanoscaffolds in promoting regeneration of the peripheral nervous system. Nanomedicine 2018, 13, 1067–1085. [Google Scholar] [CrossRef]
- Yao, Y.; Cui, Y.; Zhao, Y.; Xiao, Z.; Li, X.; Han, S.; Chen, B.; Fang, Y.; Wang, P.; Pan, J.; et al. Efect of longitudinally oriented collagen conduit combined with nerve growth factor on nerve regeneration after dog sciatic nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.H.; Hsu, H.-P.; Spector, M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012, 33, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Skop, N.B.; Calderon, F.; Cho, C.H.; Gandhi, C.D.; Levison, S.W. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol. Cell. Ther. 2014, 2, 19. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Breuls, R.G.M.; Jiya, T.U.; Smit, T.H. Scaffold stiffness influences cell behavior: Opportunities for skeletal tissue engineering. Open Orthop. J. 2008, 2, 103. [Google Scholar] [CrossRef]
- Yiannakou, C.; Simitzi, C.; Manousaki, A.; Fotakis, C.; Ranella, A.; Stratakis, E. Cell patterning via laser micro/nano structured silicon surfaces. Biofabrication 2017, 9, 25024. [Google Scholar] [CrossRef]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-H.; Niu, C.-M.; Shi, J.-Q.; Wang, Y.-Y.; Yang, Y.-M.; Wang, H.-B. Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair. Neural Regen. Res. 2018, 13, 1455–1464. [Google Scholar]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.-Q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, M.; Zhang, H.-Q.; Li, G.-L.; Hua, Y.; Shen, Y.; Ji, X.-M.; Wu, C.-J.; An, H.; Ren, M. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regen. Res. 2019, 14, 1780. [Google Scholar] [PubMed]
- Liu, X.-Y.; Liang, J.; Wang, Y.; Zhong, L.; Zhao, C.-Y.; Wei, M.-G.; Wang, J.-J.; Sun, X.-Z.; Wang, K.-Q.; Duan, J.-H.; et al. Diffusion tensor imaging predicting neurological repair of spinal cord injury with transplanting collagen/chitosan scaffold binding bFGF. J. Mater. Sci. Mater. Med. 2019, 30, 123. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Cheng, S.; Han, S.; Shu, M.; Chen, B.; Chen, X.; Tang, F.; Wang, N.; Tu, Y.; et al. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials 2017, 137, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural Eng. 2020, 17, 46036. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Marquez, A.B.; O’seanain, C.; Fischer, H.; Blaeser, A.; Vogt, M.; Corallo, D.; Aveic, S. Exploring cancer cell behavior in vitro in three-dimensional multicellular bioprintable collagen-based hydrogels. Cancers 2019, 11, 180. [Google Scholar] [CrossRef]
- Curtin, C.; Nolan, J.; Conlon, R.; Deneweth, L.; Gallagher, C.; Tan, Y.; Cavanagh, B.; Asraf, A.; Harvey, H.; Miller-Delaney, S.; et al. A physiologically relevant 3D collagen-based scaffold–neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomater. 2018, 70, 84–97. [Google Scholar] [CrossRef]
- Kato-Negishi, M.; Morimoto, Y.; Onoe, H.; Takeuchi, S. Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv. Healthc. Mater. 2013, 2, 1564–1570. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar] [CrossRef]
- Biohaz, E.P.O.B.H.; Koutsoumanis, K.; Allende, A.; Bolton, D.J.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.M.; Hilbert, F.; et al. Potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non-ruminant farmed animals. EFSA J. 2020, 18, e06267. [Google Scholar]
- Wang, H. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, K.J.; Kalliainen, L.K. Collagen tube conduits in peripheral nerve repair: A retrospective analysis. Hand 2010, 5, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Claeys, K.G.; Schrading, S.; Rödler, J.V.; Altinova, H.; Schulz, J.B.; Weis, J.; Pallua, N.; van Neerven, S.G.A. Clinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide Neuromaix. Eur. J. Med. Res. 2017, 22, 34. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Lee, D.H.; Tamura, A.; Arisaka, Y.; Seo, J.-H.; Yui, N. Mechanically reinforced gelatin hydrogels by introducing slidable supramolecular cross-linkers. Polymers 2019, 11, 1787. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Bastiaens, A.; Xie, S.; Luttge, R. Nanogroove-enhanced hydrogel scaffolds for 3D neuronal cell culture: An easy access brain-on-chip model. Micromachines 2019, 10, 638. [Google Scholar] [CrossRef]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rütten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal differentiation from induced pluripotent stem cell-derived neurospheres by the application of oxidized alginate-gelatin-laminin hydrogels. Biomedicines 2021, 9, 261. [Google Scholar] [CrossRef]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Songchotikunpan, P.; Tattiyakul, J.; Supaphol, P. Extraction and electrospinning of gelatin from fish skin. Int. J. Biol. Macromol. 2008, 42, 247–255. [Google Scholar] [CrossRef]
- Hejčl, A.; Lesný, P.; Přádný, M.; Šedý, J.; Zámečník, J.; Jendelova, P.; Michalek, J.; Sykova, E. Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 6: 3D hydrogels with positive and negative surface charges and polyelectrolyte complexes in spinal cord injury repair. J. Mater. Sci. Mater. Med. 2009, 20, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Banker, G.; Goslin, K. Culturing Nerve Cells; MIT Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Ji, Y.-R.; Homaeigohar, S.; Wang, Y.-h.; Lin, C.; Su, T.-Y.; Cheng, C.-C.; Yang, S.-H.; Young, T.-H. Selective regulation of neurons, glial cells, and neural stem/precursor cells by poly (allylguanidine)-coated surfaces. ACS Appl. Mater. Interfaces 2019, 11, 48381–48392. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhu, M.; Wang, Y.; Luo, L.; Ye, Q.; Lee, B.H. Physical properties and cellular responses of gelatin methacryloyl bulk hydrogels and highly ordered porous hydrogels. Front. Soft Matter 2023, 2, 1101680. [Google Scholar] [CrossRef]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, W.; Zhang, X.; Zhou, Z.; Ding, Z.; Zhou, X.; Lu, Q.; Kaplan, D.L. Nerve Growth Factor-Laden Anisotropic silk nanofiber hydrogels to regulate Neuronal/Astroglial differentiation for scarless spinal cord repair. ACS Appl. Mater. Interfaces 2022, 14, 3701–3715. [Google Scholar] [CrossRef]
- De Martino, D.; Bravo-Cordero, J.J. Collagens in Cancer: Structural Regulators and Guardians of Cancer Progression. Cancer Res. 2023, 83, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Tonna, N.; Bianco, F.; Matteoli, M.; Cagnoli, C.; Antonucci, F.; Manfredi, A.; Mauro, N.; Ranucci, E.; Ferruti, P. A soluble biocompatible guanidine-containing polyamidoamine as promoter of primary brain cell adhesion and in vitro cell culturing. Sci. Technol. Adv. Mater. 2014, 15, 45007. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.; Li, S. The roles of neural stem cells in myelin regeneration and repair therapy after spinal cord injury. Stem Cell Res. Ther. 2024, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Moyon, S.; Holloman, M.; Salzer, J.L. Neural stem cells and oligodendrocyte progenitor cells compete for remyelination in the corpus callosum. Front. Cell. Neurosci. 2023, 17, 1114781. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Seki, T.; Imayoshi, I.; Tamamaki, N.; Hayashi, Y.; Tatebayashi, Y.; Hitoshi, S. Neural stem cells and neuro/gliogenesis in the central nervous system: Understanding the structural and functional plasticity of the developing, mature, and diseased brain. J. Physiol. Sci. 2016, 66, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, Q.; Yang, Y.; Chen, B.; Zhang, F.; Wang, Z.; Luan, Z. Identifying genes that affect differentiation of human neural stem cells and myelination of mature oligodendrocytes. Cell. Mol. Neurobiol. 2023, 43, 2337–2358. [Google Scholar] [CrossRef]
- Yu, C.; Xia, K.; Gong, Z.; Ying, L.; Shu, J.; Zhang, F.; Chen, Q.; Li, F.; Liang, C. The application of neural stem/progenitor cells for regenerative therapy of spinal cord injury. Curr. Stem Cell Res. Ther. 2019, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef] [PubMed]
- López-Muguruza, E.; Matute, C. Alterations of oligodendrocyte and myelin energy metabolism in multiple sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.W.; Olveda, G.E.; Bame, X.; Pereira, E.; Hill, R.A. Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice. Nat. Neurosci. 2023, 26, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Simons, M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron 2022, 110, 3549–3565. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Chell, J.M.; Le Merre, P.; Wu, Y.; Zamboni, M.; Bergenstråhle, J.; Stenudd, M.; Sopova, E.; Lundeberg, J.; Shupliakov, O.; et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science 2020, 370, eabb8795. [Google Scholar] [CrossRef]
- Marisca, R.; Hoche, T.; Agirre, E.; Hoodless, L.J.; Barkey, W.; Auer, F.; Castelo-Branco, G.; Czopka, T. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 2020, 23, 363–374. [Google Scholar] [CrossRef]
- Mezydlo, A.; Treiber, N.; Gavilanes, E.M.U.; Eichenseer, K.; Ancău, M.; Wens, A.; Carral, C.A.; Schifferer, M.; Snaidero, N.; Misgeld, T.; et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron 2023, 111, 1748–1759.e8. [Google Scholar] [CrossRef]
- Fang, M.; Tang, T.; Qiu, M.; Xu, X. Hedgehog signaling in CNS remyelination. Cells 2022, 11, 2260. [Google Scholar] [CrossRef]
- Caprariello, A.V.; Adams, D.J. The landscape of targets and lead molecules for remyelination. Nat. Chem. Biol. 2022, 18, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, Y.; Cai, Z.; Terekhova, M.; Swain, A.; Andhey, P.S.; Guimaraes, R.M.; Antonova, A.U.; Qiu, T.; Sviben, S.; et al. Transcriptomic atlas and interaction networks of brain cells in mouse CNS demyelination and remyelination. Cell Rep. 2023, 42, 112293. [Google Scholar] [CrossRef] [PubMed]
- Nazari, B.; Namjoo, Z.; Moradi, F.; Kazemi, M.; Ebrahimi-Barough, S.; Sadroddiny, E.; Ai, J. miR-219 overexpressing oligodendrocyte progenitor cells for treating compression spinal cord injury. Metab. Brain Dis. 2021, 36, 1069–1077. [Google Scholar] [CrossRef]
- Ngo, C.; Kothary, R. MicroRNAs in oligodendrocyte development and remyelination. J. Neurochem. 2022, 162, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-W. Advancing spinal cord injury treatment through stem cell therapy: A comprehensive review of cell types, challenges, and emerging technologies in regenerative medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhou, G.; Hu, X.; Han, S.; Gao, J. The combination of nanoscaffolds and stem cell transplantation: Paving a promising road for spinal cord injury regeneration. Biomed. Pharmacother. 2021, 143, 112233. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, K.; Fehlings, M.G. Incorporating combinatorial approaches to encourage targeted neural stem/progenitor cell integration following transplantation in spinal cord injury. Stem Cells Transl. Med. 2023, 12, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yao, D.; Chen, S.; Wang, J.; Pan, C.; Wu, D.; Liu, N.; Tang, Z. Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke. Cell Death Discov. 2023, 9, 215. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, M.; Guo, Q.; Liu, N.; Wang, Y.; Wu, T. Modulating axonal growth and neural stem cell migration with the use of uniaxially aligned nanofiber yarns welded with NGF-loaded microparticles. Mater. Today Adv. 2023, 17, 100343. [Google Scholar] [CrossRef]
- Chenghao, Y.; Wang, T.; Diao, H.; Liu, N.; Yi, Z.; Jiang, H.; Peng, Z.; Shan, Z.; Sun, Z.; Wu, T.; et al. Photothermal-triggered structural change of nanofiber scaffold integrating with graded mineralization to promote tendonebone healing. Adv. Fiber Mater. 2022, 4, 908–922. [Google Scholar]
- Morais, A.I.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of polymeric microparticles by electrospray: The impact of experimental parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav. Immun. 2015, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Friedman, A. Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 2012, 53 (Suppl. S6), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Jayant, R.D.; Sosa, D.; Kaushik, A.; Atluri, V.; Vashist, A.; Tomitaka, A.; Nair, M. Current status of non-viral gene therapy for CNS disorders. Expert Opin. Drug Deliv. 2016, 13, 1433–1445. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Recent progress of nanocarrier-based therapy for solid malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Vo, T.K.; Tran, N.-M.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Mohapatra, P.; Gopikrishnan, M.; Doss, C.G.P.; Chandrasekaran, N. How Precise are Nanomedicines in Overcoming the Blood-Brain Barrier? A Comprehensive Review of the Literature. Int. J. Nanomed. 2024, 19, 2441–2467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Suja, V.C. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2023, 8, e10367. [Google Scholar] [CrossRef]
- Mirón-Barroso, S.; Domènech, E.B.; Trigueros, S. Nanotechnology-based strategies to overcome current barriers in gene delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as drug delivery system: An updated review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional magnetite nanoparticles to enable delivery of siRNA for the potential treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Layek, B.; Singh, J. Design and validation of liposomal ApoE2 gene delivery system to evade blood-brain barrier for effective treatment of Alzheimer’s disease. Mol. Pharm. 2020, 18, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Kurawattimath, V.; Wilson, B.; Geetha, K.M. Nanoparticle-based drug delivery across the blood-brain barrier for treating malignant brain glioma. OpenNano 2023, 10, 100128. [Google Scholar] [CrossRef]
- Pawar, S.; Koneru, T.; McCord, E.; Tatiparti, K.; Sau, S.; Iyer, A.K. LDL receptors and their role in targeted therapy for glioma: A review. Drug Discov. Today 2021, 26, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Kapoor, B.; Jha, N.K.; Gupta, P.K.; Gupta, G.; Chellappan, D.K.; Devkota, H.P.; Prasher, P.; Ansari, S.; et al. Advances in designing of polymeric micelles for biomedical application in brain related diseases. Chem. Biol. Interact. 2022, 361, 109960. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Lakkaraju, A.K.; Frontzek, K. Toward therapy of human prion diseases. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 331–351. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as pharmaceutical excipients: Synthesis, properties, toxicity and biomedical applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef]
- Lee, C.C.; Mackay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Castro, N.J.; Shen, Y.-L.; Zhang, L.G. Chapter 16-Nanotechnology and 3D/4D Bioprinting for Neural Tissue Regeneration. In 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine, 2nd ed.; Zhang, L.G., Fisher, J.P., Leong, K.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 427–458. [Google Scholar] [CrossRef]
- Lu, Y.; Rivera-Rodriguez, A.; Tay, Z.W.; Hensley, D.; Fung, K.B.; Colson, C.; Saayujya, C.; Huynh, Q.; Kabuli, L.; Fellows, B.; et al. Combining magnetic particle imaging and magnetic fluid hyperthermia for localized and image-guided treatment. Int. J. Hyperth. 2020, 37, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Hanafy, N.A.N.; Li, B.; Mehanni, A.-H.E.; Taha, E.M.; Chen, W. An updated and comprehensive review on the potential health effects of curcumin-encapsulated micro/nanoparticles. Crit. Rev. Food Sci. Nutr. 2022, 1, 9731–9751. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y.; et al. Aptamer-conjugated gold nanoparticles targeting epidermal growth factor receptor variant III for the treatment of glioblastoma. Int. J. Nanomed. 2020, 15, 1363–1372. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef]
- Salameh, J.W.; Zhou, L.; Ward, S.M.; Chalarca, C.F.S.; Emrick, T.; Figueiredo, M.L. Polymer-mediated gene therapy: Recent advances and merging of delivery techniques. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1598. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yang, Y.; Liu, B.; He, J.; Nie, Z. Polymer-guided assembly of inorganic nanoparticles. Chem. Soc. Rev. 2020, 49, 465–508. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical applications of carbon nanomaterials: Fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) disorders: A present and future prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.D.; Tran, N.-M.; Van Vo, G. Lipid-based nanocarriers via nose-to-brain pathway for central nervous system disorders. Neurochem. Res. 2022, 47, 552–573. [Google Scholar] [CrossRef] [PubMed]
- Montegiove, N.; Calzoni, E.; Emiliani, C.; Cesaretti, A. Biopolymer nanoparticles for nose-to-brain drug delivery: A new promising approach for the treatment of neurological diseases. J. Funct. Biomater. 2022, 13, 125. [Google Scholar] [CrossRef]
- De Martini, L.B.; Sulmona, C.; Brambilla, L.; Rossi, D. Cell-penetrating peptides as valuable tools for nose-to-brain delivery of biological drugs. Cells 2023, 12, 1643. [Google Scholar] [CrossRef]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-responsive prodrug-based cancer nanomedicine. Ebiomedicine 2020, 56, 102821. [Google Scholar] [CrossRef]
- El-Boghdadly, K.; Pawa, A.; Chin, K.J. Local anesthetic systemic toxicity: Current perspectives. Local Reg. Anesth. 2018, 11, 35–44. [Google Scholar] [CrossRef]
- Zha, S.; Wong, K.; All, A.H. Intranasal delivery of functionalized polymeric nanomaterials to the brain. Adv. Healthc. Mater. 2022, 11, 2102610. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.A.; Erdő, F. Overcoming the blood–brain barrier challenges and tricks for CNS drug delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- D’agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef]
- Han, L.; Cai, Q.; Tian, D.; Kong, D.K.; Gou, X.; Chen, Z.; Strittmatter, S.M.; Wang, Z.; Sheth, K.N.; Zhou, J. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine 2016, 12, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Zhu, D.; Wang, Y.; Qing, G.; Zhang, Y.; Liu, X.; Liang, X.-J. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm. Sin. B 2021, 11, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Clinical Applications of Tumor-Targeted Systems. In New Nanomaterials and Techniques for Tumor-Targeted Systems; Huang, R., Wang, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 437–456. [Google Scholar] [CrossRef]
- Correia, A.; Monteiro, A.; Silva, R.; Moreira, J.; Lobo, J.S.; Silva, A. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood–brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Lan, M.; Yan, L.; Chen, X. Getting drugs to the brain: Advances and prospects of organic nanoparticle delivery systems for assisting drugs to cross the blood–brain barrier. J. Mater. Chem. B 2022, 10, 9314–9333. [Google Scholar] [CrossRef]
- Yadav, A.K.; Shukla, R.; Flora, S.J.S. Chapter 5-Nanomedical Drug Delivery for Neurodegenerative Disease. In Nanomedical Drug Delivery for Neurodegenerative Diseases; Yadav, A.K., Shukla, R., Flora, S.J.S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 67–79. [Google Scholar] [CrossRef]
- Furukawa, K.; Tanaka, M.; Oba, M. siRNA delivery using amphipathic cell-penetrating peptides into human hepatoma cells. Bioorganic Med. Chem. 2020, 28, 115402. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Smart strategies for therapeutic agent delivery into brain across the blood–brain barrier using receptor-mediated transcytosis. Chem. Pharm Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Toth, A.E.; Holst, M.R.; Nielsen, M.S. Vesicular transport machinery in brain endothelial cells: What we know and what we do not. Curr. Pharm. Des. 2020, 26, 1405–1416. [Google Scholar] [CrossRef]
- Smith, M.W.; Gumbleton, M. Endocytosis at the blood–brain barrier: From basic understanding to drug delivery strategies. J. Drug Target. 2006, 14, 191–214. [Google Scholar] [CrossRef]
- Luo, M.; Lee, L.K.C.; Peng, B.; Choi, C.H.J.; Tong, W.Y.; Voelcker, N.H. Delivering the promise of gene therapy with nanomedicines in treating central nervous system diseases. Adv. Sci. 2022, 9, 2201740. [Google Scholar] [CrossRef] [PubMed]
- Trompetero, A.; Gordillo, A.; del Pilar, M.C.; Cristina, V.M.; Cruz, R.H.B. Alzheimer’s disease and parkinson’s disease: A review of current treatment adopting a nanotechnology approach. Curr. Pharm. Des. 2018, 24, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Aljabali, A.A.; Gammoh, O.; Tambuwala, M.M. Advancements in neurotherapeutics: Nanoparticles overcoming the blood–brain barrier for precise CNS targeting. J. Nanopart. Res. 2024, 26, 123. [Google Scholar] [CrossRef]
- Li, K.; Ji, Q.; Liang, H.; Hua, Z.; Hang, X.; Zeng, L.; Han, H. Biomedical application of 2D nanomaterials in neuroscience. J. Nanobiotechnol. 2023, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [PubMed]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 52. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Chopp, M. Exosomes in stroke pathogenesis and therapy. J. Clin. Investig. 2016, 126, 1190–1197. [Google Scholar] [CrossRef]
- Xiong, Y.-Y.; Gong, Z.-T.; Tang, R.-J.; Yang, Y.-J. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics 2021, 11, 1046–1058. [Google Scholar] [CrossRef]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsubara, Y.; Uchikura, Y.; Sugiyama, T. Pathophysiology of preeclampsia: The role of exosomes. Int. J. Mol. Sci. 2021, 22, 2572. [Google Scholar] [CrossRef] [PubMed]
- Moeinabadi-Bidgoli, K.; Rezaee, M.; Rismanchi, H.; Mohammadi, M.M.; Babajani, A. Mesenchymal stem cell-derived antimicrobial peptides as potential anti-neoplastic agents: New insight into anticancer mechanisms of stem cells and exosomes. Front. Cell Dev. Biol. 2022, 10, 900418. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, L.; Wu, J.; Huang, T.; Zhang, Y.; Cao, J.; Ma, T.; Chen, J.; Zhang, C.; Zhang, X.; et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater. Sci. 2022, 10, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mu, J.; Zhang, Y.; Zhang, C.; Ma, T.; Chen, L.; Huang, T.; Wu, J.; Cao, J.; Feng, S.; et al. Stimulation by exosomes from hypoxia preconditioned human umbilical vein endothelial cells facilitates mesenchymal stem cells angiogenic function for spinal cord repair. ACS Nano 2022, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Song, Y.; Luo, Y.; Song, J.; Li, C.; Yang, S.; Guo, J.; Yu, J.; Zhang, X. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radic. Biol. Med. 2022, 192, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Pati, S.; Lee, J.-W. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35, 316–324. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of exosomes in central nervous system diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Bianchini, A.; Teng, K. Reticulocyte maturation and exosome release: Transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 1989, 74, 1844–1851. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R. Selective externalization of the transferrin receptor by sheep reticulocytes in vitro. Response to ligands and inhibitors of endocytosis. J. Biol. Chem. 1984, 259, 9776–9782. [Google Scholar] [CrossRef]
- Vashist, A.; Manickam, P.; Raymond, A.D.; Arias, A.Y.; Kolishetti, N.; Vashist, A.; Arias, E.; Nair, M. Recent advances in nanotherapeutics for neurological disorders. ACS Appl. Bio Mater. 2023, 6, 2614–2621. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, 58. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.M.; Nicaise, A.M.; Peruzzotti-Jametti, L.; Pluchino, S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020, 1729, 146615. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R.; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell communication by extracellular vesicles: Focus on microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef]
- Vogel, A.D.; Upadhya, R.; Shetty, A.K. Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. EBioMedicine 2018, 38, 273–282. [Google Scholar] [CrossRef]
- Bonilla, C.; Zurita, M. Cell-based therapies for traumatic brain injury: Therapeutic treatments and clinical trials. Biomedicines 2021, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Badner, A.; Reinhardt, E.K.; Nguyen, T.V.; Midani, N.; Marshall, A.T.; Lepe, C.A.; Echeverria, K.; Lepe, J.J.; Torrecampo, V.; Bertan, S.H.; et al. Freshly thawed cryobanked human neural stem cells engraft within endogenous neurogenic niches and restore cognitive function after chronic traumatic brain injury. J. Neurotrauma 2021, 38, 2731–2746. [Google Scholar] [CrossRef]
- Hering, C.; Shetty, A.K. Extracellular vesicles derived from neural stem cells, astrocytes, and microglia as therapeutics for easing TBI-induced brain dysfunction. Stem Cells Transl. Med. 2023, 12, 140–153. [Google Scholar] [CrossRef]
- Takahashi, S. Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological conditions of the neurovascular unit. Neuropathology 2020, 40, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Jiang, B.; Yu, S.; Xu, X. Reconstituting neurovascular unit based on the close relations between neural stem cells and endothelial cells: An effective method to explore neurogenesis and angiogenesis. Rev. Neurosci. 2020, 31, 143–159. [Google Scholar]
- Li, W.; Shan, B.; Cheng, X.; He, H.; Qin, J.; Zhao, H.; Tian, M.; Zhang, X.; Jin, G. circRNA Acbd6 promotes neural stem cell differentiation into cholinergic neurons via the miR-320-5p-Osbpl2 axis. J. Biol. Chem. 2022, 298, 101828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gao, Y.; Wang, F.; Cheng, X.; Zhao, T.; Zhao, Y.; Fan, M.; Zhu, L. Neural progenitor cells-secreted exosomal miR-210 induced by hypoxia influences cell viability. NeuroReport 2020, 31, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ding, L.; Chen, H.; Wang, Y.; Li, C.; Zhao, S.; Yang, X.; Ma, Y.; Zhu, J.; Qi, X.; et al. Neural stem cell-derived exosomes regulate neural stem cell differentiation through miR-9-Hes1 axis. Front. Cell Dev. Biol. 2021, 9, 601600. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019, 17, 96. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, J.; Zhu, L.; Peng, Y. Exosomal noncoding RNAs in Glioma: Biological functions and potential clinical applications. Mol. Cancer 2020, 19, 66. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Jeong, G.-B. A systematic review on the modifications of extracellular vesicles: A revolutionized tool of nano-biotechnology. J. Nanobiotechnol. 2021, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal stem cell-derived extracellular vesicles: Opportunities and challenges for clinical translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Hettich, B.F.; Bader, J.J.; Leroux, J.C. Encapsulation of hydrophilic compounds in small extracellular vesicles: Loading capacity and impact on vesicle functions. Adv. Healthc. Mater. 2022, 11, e2100047. [Google Scholar] [CrossRef]
- Chen, L.-K.; Zhu, Z.-H.; Jia, F.; Ahmed, W.; Zhang, G.-L.; Wang, H.; Lin, C.-Q.; Chen, W.-H. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen. Res. 2023, 18, 404–409. [Google Scholar] [CrossRef]
- Joshi, B.S.; Zuhorn, I.S. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood-brain barrier model. Eur. J. Neurosci. 2021, 53, 706–719. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes-beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef] [PubMed]
- EL Andaloussi, S.; Lakhal, S.; Mäger, I.; Wood, M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013, 65, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Cattaneo, E. Opinion: Neural stem cell therapy for neurological diseases: Dreams and reality. Nat. Rev. Neurosci. 2002, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-F.; Dong, Y.; Zhang, L.; Qi, J.; Yao, C.; Wang, Y.; Chai, R.; Liu, Y.; So, K.-F. Neuromodulation-Based stem cell therapy in brain Repair: Recent advances and future perspectives. Neurosci. Bull. 2021, 37, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Pistorius, S.S.; Hacker, M.L.; Waters, A.C.; Wang, J.; Provenza, N.R.; de Hemptinne, C.; Johnson, K.A.; Morrison, M.A.; Cernera, S. Advances in deep brain stimulation: From mechanisms to applications. J. Neurosci. 2023, 43, 7575–7586. [Google Scholar] [CrossRef]
- Miocinovic, S.; Somayajula, S.; Chitnis, S.; Vitek, J.L. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013, 70, 163–171. [Google Scholar] [CrossRef]
- Johnson, M.D.; Miocinovic, S.; McIntyre, C.C.; Vitek, J.L. Mechanisms and targets of deep brain stimulation in movement disorders. Neurother. J. Am. Soc. Exp. NeuroTher. 2008, 5, 294–308. [Google Scholar] [CrossRef]
- Hacker, M.L.; Rajamani, N.; Neudorfer, C.; Hollunder, B.; Oxenford, S.; Li, N.; Sternberg, A.L.; Davis, T.L.; Konrad, P.E.; Horn, A.; et al. Connectivity Profile for Subthalamic Nucleus Deep Brain Stimulation in Early Stage Parkinson Disease. Ann. Neurol. 2023, 94, 271–284. [Google Scholar] [CrossRef]
- Koirala, N.; Serrano, L.; Paschen, S.; Falk, D.; Anwar, A.R.; Kuravi, P.; Deuschl, G.; Groppa, S.; Muthuraman, M. Mapping of subthalamic nucleus using microelectrode recordings during deep brain stimulation. Sci. Rep. 2020, 10, 19241. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, E.L.; Sunde, N.; Mogensen, P.H.; Østergaard, K. MRI verified STN stimulation site--gait improvement and clinical outcome. Eur. J. Neurol. 2010, 17, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, D.E.; Tambirajoo, K.; Hasegawa, H.; Gimeno, H.; Kaminska, M.; Ashkan, K.; Selway, R.; Lin, J.-P. Probabilistic mapping of deep brain stimulation in childhood dystonia. Park. Relat. Disord. 2022, 105, 103–110. [Google Scholar] [CrossRef]
- Bower, K.L.; McIntyre, C.C. Deep brain stimulation of terminating axons. Brain Stimul. 2020, 13, 1863–1870. [Google Scholar] [CrossRef]
- Neumann, W.-J.; Steiner, L.A.; Milosevic, L. Neurophysiological mechanisms of deep brain stimulation across spatiotemporal resolutions. Brain 2023, 146, 4456–4468. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, L.; Goede, L.; Odekerken, V.J.J.; Wang, Q.; Li, N.; Neudorfer, C.; Rajamani, N.; Al-Fatly, B.; Reich, M.; Volkmann, J.; et al. Subthalamic and pallidal deep brain stimulation: Are we modulating the same network? Brain A J. Neurol. 2022, 145, 251–262. [Google Scholar] [CrossRef]
- Yu, Y.; Sanabria, D.E.; Wang, J.; Hendrix, C.M.; Zhang, J.; Nebeck, S.D.; Amundson, A.M.; Busby, Z.B.; Bauer, D.L.; Johnson, M.D.; et al. Parkinsonism Alters Beta Burst Dynamics across the Basal Ganglia-Motor Cortical Network. J. Neurosci. 2021, 41, 2274–2286. [Google Scholar] [CrossRef]
- Bijanki, K.R.; Pathak, Y.J.; Najera, R.A.; Storch, E.A.; Goodman, W.K.; Simpson, H.B.; Sheth, S.A. Defining functional brain networks underlying obsessive-compulsive disorder (OCD) using treatment-induced neuroimaging changes: A systematic review of the literature. J. Neurol. Neurosurg. Psychiatry 2021, 92, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef]
- Boutet, A.; Chow, C.T.; Narang, K.; Elias, G.J.B.; Neudorfer, C.; Germann, J.; Ranjan, M.; Loh, A.; Martin, A.J.; Kucharczyk, W.; et al. Improving Safety of MRI in Patients with Deep Brain Stimulation Devices. Radiology 2020, 296, 250–262. [Google Scholar] [CrossRef]
- Medtronic. MRI Guidelines for Medtronic Deep Brain Stimulation Systems. 2021. Available online: https://www.medtronic.com/content/dam/emanuals/neuro/CONTRIB_228155.pdf (accessed on 28 April 2024).
- Loh, A.; Gwun, D.; Chow, C.T.; Boutet, A.; Tasserie, J.; Germann, J.; Santyr, B.; Elias, G.; Yamamoto, K.; Sarica, C.; et al. Probing responses to deep brain stimulation with functional magnetic resonance imaging. Brain Stimul. 2022, 15, 683–694. [Google Scholar] [CrossRef]
- Miao, J.; Tantawi, M.; Koa, V.; Zhang, A.B.; Zhang, V.; Sharan, A.; Wu, C.; Matias, C.M. Use of Functional MRI in Deep Brain Stimulation in Parkinson’s Diseases: A Systematic Review. Front. Neurol. 2022, 13, 849918. [Google Scholar] [CrossRef]
- Boutet, A.; Madhavan, R.; Elias, G.J.B.; Joel, S.E.; Gramer, R.; Ranjan, M.; Paramanandam, V.; Xu, D.; Germann, J.; Loh, A.; et al. Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nat. Commun. 2021, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Filip, P.; Jech, R.; Fečíková, A.; Havránková, P.; Růžička, F.; Mueller, K.; Urgošík, D. Restoration of functional network state towards more physiological condition as the correlate of clinical effects of pallidal deep brain stimulation in dystonia. Brain Stimul. 2022, 15, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lai, Y.; Li, J.; He, N.; Li, D.; Yan, F.; Zhang, Y.; Zhang, C.; Sun, B.; Wei, H. BOLD frequency-dependent alterations in resting-state functional connectivity by pallidal deep brain stimulation in patients with Parkinson’s disease. J. Neurosurg. 2023, 139, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Allawala, A.; Bijanki, K.R.; Goodman, W.; Cohn, J.F.; Viswanathan, A.; Yoshor, D.; A Borton, D.; Pouratian, N.; Sheth, S.A. A Novel Framework for Network-Targeted Neuropsychiatric Deep Brain Stimulation. Neurosurgery 2021, 89, E116–E121. [Google Scholar] [CrossRef] [PubMed]
- Bohara, S.; Slepneva, N.; Norbu, T.; Motzkin, J.; Sugrue, L.; Lee, A.M.; Morrison, M. Patterns of fMRI connectivity during cycling stimulation associated with effective DBS for OCD. Brain Stimul. 2023, 16, 347. [Google Scholar] [CrossRef]
- Slepneva, N.; Frank, A.; Norbu, T.; Motzkin, J.; Sugrue, L.; Morrison, M.; Lee, A.M. BOLD fMRI response to therapeutic and nontherapeutic deep brain stimulation in obsessive-compulsive disorder. Brain Stimul. 2023, 16, 375. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef]
- Crowell, A.L.; Riva-Posse, P.; Holtzheimer, P.E.; Garlow, S.J.; Kelley, M.E.; Gross, R.E.; Denison, L.; Quinn, S.; Mayberg, H.S. Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Am. J. Psychiatry 2019, 176, 949–956. [Google Scholar] [CrossRef]
- Fricke, P.; Nickl, R.; Breun, M.; Volkmann, J.; Kirsch, D.; Ernestus, R.-I.; Steigerwald, F.; Matthies, C. Directional Leads for Deep Brain Stimulation: Technical Notes and Experiences. Stereotact. Funct. Neurosurg. 2021, 99, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.; Cagle, J.; Johnson, K.A.; Wong, J.K.; Hilliard, J.D.; Butson, C.R.; Okun, M.S.; de Hemptinne, C. Past, Present, and Future of Deep Brain Stimulation: Hardware, Software, Imaging, Physiology and Novel Approaches. Front. Neurol. 2022, 13, 825178. [Google Scholar] [CrossRef] [PubMed]
- Strelow, J.N.; Dembek, T.A.; Baldermann, J.C.; Andrade, P.; Jergas, H.; Visser-Vandewalle, V.; Barbe, M.T. Local field Potential-Guided contact selection using chronically implanted sensing devices for deep brain stimulation in Parkinson’s disease. Brain Sci. 2022, 12, 1726. [Google Scholar] [CrossRef] [PubMed]
- Cagle, J.N.; Wong, J.K.; Johnson, K.A.; Foote, K.D.; Okun, M.S.; de Hemptinne, C. Suppression and Rebound of Pallidal Beta Power: Observation Using a Chronic Sensing DBS Device. Front. Hum. Neurosci. 2021, 15, 749567. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.E.; Johnson, L.A.; Sanabria, D.E.; Wang, J.; Patriat, R.; Hill, M.; Marshall, E.; MacKinnon, C.D.; Cooper, S.E.; Schrock, L.E.; et al. Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson’s disease patients. Neurobiol. Dis. 2020, 139, 104819. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, N.C.; McDermott, H.J.; Lee, W.-L.; Xu, S.S.; Acevedo, N.; Begg, A.; Perera, T.; Thevathasan, W.; Bulluss, K.J. Electrically evoked and spontaneous neural activity in the subthalamic nucleus under general anesthesia. J. Neurosurg. 2021, 137, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, N.C.; McDermott, H.J.; Fallon, J.B.; Perera, T.; Brown, P.; Bulluss, K.J.; Thevathasan, W. Deep brain stimulation for Parkinson’s disease modulates high-frequency evoked and spontaneous neural activity. Neurobiol. Dis. 2019, 130, 104522. [Google Scholar] [CrossRef]
- Johnson, K.A.; Cagle, J.N.; Lopes, J.L.; Wong, J.K.; Okun, M.S.; Gunduz, A.; Shukla, A.W.; Hilliard, J.D.; Foote, K.D.; de Hemptinne, C. Globus pallidus internus deep brain stimulation evokes resonant neural activity in Parkinson’s disease. Brain Commun. 2023, 5, fcad025. [Google Scholar] [CrossRef]
- Xu, S.S.; Lee, W.-L.; Perera, T.; Sinclair, N.C.; Bulluss, K.J.; McDermott, H.J.; Thevathasan, W. Can brain signals and anatomy refine contact choice for deep brain stimulation in Parkinson’s disease? J. Neurol. Neurosurg. Psychiatry 2022, 93, 1338–1341. [Google Scholar] [CrossRef]
- Waters, A.C.; Veerakumar, A.; Choi, K.S.; Howell, B.; Tiruvadi, V.; Bijanki, K.R.; Crowell, A.; Riva-Posse, P.; Mayberg, H.S. Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum. Brain Mapp. 2018, 39, 4844–4856. [Google Scholar] [CrossRef]
- Saeedi, P.; Halabian, R.; Fooladi, A.A.I. A revealing review of mesenchymal stem cell therapy, clinical perspectives, and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J. Cell. Physiol. 2020, 235, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Fan, T.; Gao, S.; Jin, Y.; Zhang, M.; Ono, M. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res. Ther. 2021, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Kim, H.-S.; Shin, T.-H.; Kang, I.; Lee, J.Y.; Kim, J.-J.; Kang, H.K.; Seo, Y.; Lee, S.; Yu, K.-R.; et al. PGE2 maintains self-renewal of human adult stem cells via EP2-mediated autocrine signaling and its production is regulated by cell-to-cell contact. Sci. Rep. 2016, 6, 26298. [Google Scholar] [CrossRef]
- Ghorbani, F.; Movassaghpour, A.A.; Talebi, M.; Yousefi, M.; Abbaszadeh, H. Renoprotective effects of extracellular vesicles: A systematic review. Gene Rep. 2022, 26, 101491. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J. Cell. Physiol. 2020, 235, 9230–9240. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Malekpour, K.; Hazrati, A.; Zahar, M.; Markov, A.; Zekiy, A.O.; Navashenaq, J.G.; Roshangar, L.; Ahmadi, M. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Rev. Rep. 2022, 18, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, S.; Ejam, S.S.; Lafta, M.H.; Markov, A.; Yumashev, A.V.; Ahmadi, M. Mesenchymal stem cells and cancer therapy: Insights into targeting the tumour vasculature. Cancer Cell Int. 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Barczewska, M.; Maksymowicz, S.; Zdolińska-Malinowska, I.; Siwek, T.; Grudniak, M. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: An Original Study. Stem Cell Rev. Rep. 2020, 16, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Madigan, N.N.; Morris, J.; Jentoft, M.; Sorenson, E.J.; Butler, G.; Gastineau, D.; Dietz, A.; Windebank, A.J. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 2016, 87, 2230–2234. [Google Scholar] [CrossRef]
- Oh, K.W.; Moon, C.; Kim, H.Y.; Oh, S.I.; Park, J.; Lee, J.H.; Chang, I.Y.; Kim, K.S.; Kim, S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 2015, 4, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.V.P.; Larocca, T.F.; Souza, B.S.D.F.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; Martinez, A.C.D.O.M.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef]
- Volkman, R.; Offen, D. Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 2017, 35, 1867–1880. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Underlying Cause of Death 1999–2019 on CDC; CDC: Atlanta, GA, USA, 2020.
- Jalali, M.S.; Sarkaki, A.; Farbood, Y.; Azandeh, S.S.; Mansouri, E.; Ghasemi Dehcheshmeh, M.; Saki, G. Transplanted Wharton’s jelly mesenchymal stem cells improve memory and brain hippocampal electrophysiology in rat model of Parkinson’s disease. J. Chem. Neuroanat. 2020, 110, 101865. [Google Scholar] [CrossRef]
- Sun, Z.; Gu, P.; Xu, H.; Zhao, W.; Zhou, Y.; Zhou, L.; Zhang, Z.; Wang, W.; Han, R.; Chai, X.; et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Locomotor Function in Parkinson’s Disease Mouse Model Through Regulating Intestinal Microorganisms. Front. Cell Dev. Biol. 2022, 9, 808905. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-X.; Liang, F.-C.; Gu, P.; Xu, B.-L.; Xu, H.-J.; Wang, W.-T.; Hou, J.-Y.; Xie, D.-X.; Chai, X.-Q.; An, S.-J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Canesi, M.; Isalberti, M.; Isaias, I.U.; Montemurro, T.; Viganò, M.; Montelatici, E.; Boldrin, V.; Benti, R.; Cortelezzi, A.; et al. Autologous mesenchymal stem cell therapy for progressive supranuclear palsy: Translation into a phase I controlled, randomized clinical study. J. Transl. Med. 2014, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Canesi, M.; Giordano, R.; Lazzari, L.; Isalberti, M.; Isaias, I.U.; Benti, R.; Rampini, P.; Marotta, G.; Colombo, A.; Cereda, E.; et al. Finding a new therapeutic approach for no-option Parkinsonisms: Mesenchymal stromal cells for progressive supranuclear palsy. J. Transl. Med. 2016, 14, 127. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 459–509. [CrossRef]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M.; et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s & Dementia. Transl. Res. Clin. Interv. 2015, 1, 95–102. [Google Scholar]
- Sha, S.; Shen, X.; Cao, Y.; Qu, L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging 2021, 13, 15285–15306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Wu, Y.; Wang, Y.; Li, Y.; Xiang, C. Transplantation of Human Menstrual Blood-Derived Mesenchymal Stem Cells Alleviates Alzheimer’s Disease-Like Pathology in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 140. [Google Scholar] [CrossRef]
- Schrag, A.; Ben-Shlomo, Y.; Quinn, N.P. Prevalence of progressive supranuclear palsy and multiple system atrophy: A cross-sectional study. Lancet 1999, 354, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, T.Y.; Lee, Y.H.; Baik, K.; Jung, J.H.; Yoo, H.S.; Shim, C.J.; Eom, H.; Hong, J.-Y.; Kim, D.J.; et al. Phase I Trial of Intra-arterial Administration of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Patients with Multiple System Atrophy. Stem Cells Int. 2021, 2021, 9886877. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Wang, W.; Yuan, X.; Yu, H.; Zhao, M. Long-Term Clinical Efficacy of Human Umbilical Cord Blood Mononuclear Cell Transplantation by Lateral Atlanto-Occipital Space Puncture (Gong’s Puncture) for the Treatment of Multiple System Atrophy. Cell Transplant. 2022, 31, 9636897221136553. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Hwang, C.J.; Yun, H.-M.; Yeo, I.J.; Choi, D.-Y.; Park, P.-H.; Kim, H.S.; Lee, J.T.; Jung, Y.S.; Han, S.-B.; et al. Prevention of multiple system atrophy using human bone marrow-derived mesenchymal stem cells by reducing polyamine and cholesterol-induced neural damages. Stem Cell Res. Ther. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Dietz, A.B.; Zeller, A.D.; Gehrking, T.L.; Schmelzer, J.D.; Schmeichel, A.M.; Gehrking, J.A.; Suarez, M.D.; Sletten, D.M.; Pacheco, K.V.M.; et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology 2019, 93, e77–e87. [Google Scholar] [CrossRef]
- Na Kim, H.; Kim, D.Y.; Oh, S.H.; Kim, H.S.; Kim, K.S.; Lee, P.H. Feasibility and Efficacy of Intra-Arterial Administration of Mesenchymal Stem Cells in an Animal Model of Double Toxin-Induced Multiple System Atrophy. Stem Cells Transl. Med. 2017, 6, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J. Biol. Eng. 2023, 17, 44. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J.; et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 2022, 65, 291–302. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.-Y.; Chen, Z.-H.; Wang, N.-X.; Zhang, L.-M.; Chen, Y.-Y.; Mo, J.; Dong, J.-W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined With Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Xie, P.; Ling, H.; Pang, M.; He, L.; Zhuang, Z.; Zhang, G.; Chen, Z.; Weng, C.; Cheng, S.; Jiao, J.; et al. Umbilical cord mesenchymal stem cells promoting spinal cord injury repair visually monitored by AIE-TAT nanoparticles. Adv. Ther. 2022, 5, 2200076. [Google Scholar] [CrossRef]
- Omer, S.A.; McKnight, K.H.; Young, L.I.; Song, S. Stimulation strategies for electrical and magnetic modulation of cells and tissues. Cell Regen 2023, 12, 21. [Google Scholar] [CrossRef]
- Ito, H.; Bassett, C.A. Effect of weak, pulsing electromagnetic fields on neural regeneration in the rat. Clin. Orthop. Relat. Res. 1983, 181, 283–290. [Google Scholar] [CrossRef]
- De Pedro, J.A.; Pérez-Caballer, A.J.; Dominguez, J.; Collía, F.; Blanco, J.; Salvado, M. Pulsed electromagnetic fields induce peripheral nerve regeneration and endplate enzymatic changes. Bioelectromagnetics 2005, 26, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wen, X.; Ding, X.; Wang, Q.; Wang, S.; Yu, W. Advances in biotechnology and clinical therapy in the field of peripheral nerve regeneration based on magnetism. Front. Neurol. 2023, 14, 1079757. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 2003, 39, 163–173. [Google Scholar] [CrossRef]
- Lim, S.H.; Yee, G.T.; Khang, D. Nanoparticle-Based combinational strategies for overcoming the Blood-Brain barrier and Blood-Tumor Barrier. Int. J. Nanomed. 2024, 19, 2529–2552. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, C.; Garcia, J.B.; Palmi, M.; Sgaragli, G.; Benocci, A.; Meini, A.; Pessina, F.; Rossi, C.; Bonechi, C.; Pessina, G.P. The effect of exposure to high flux density static and pulsed magnetic fields on lymphocyte function. Bioelectromagnetics 2003, 24, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ben Yakir-Blumkin, M.; Loboda, Y.; Schächter, L.; Finberg, J.P. Neuroprotective effect of weak static magnetic fields in primary neuronal cultures. Neuroscience 2014, 278, 313–326. [Google Scholar] [CrossRef]
- Cao, L.; Wei, D.; Reid, B.; Zhao, S.; Pu, J.; Pan, T.; Yamoah, E.; Zhao, M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013, 14, 184–190. [Google Scholar] [CrossRef]
- Mann, A.; Steinecker-Frohnwieser, B.; Naghilou, A.; Millesi, F.; Supper, P.; Semmler, L.; Wolf, S.; Marinova, L.; Weigl, L.; Weiss, T.; et al. Nuclear Magnetic Resonance Treatment Accelerates the Regeneration of Dorsal Root Ganglion Neurons in vitro. Front. Cell. Neurosci. 2022, 16, 859545. [Google Scholar] [CrossRef]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of Mechanical Stimuli on Schwann Cell Biology. Front. Cell. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef]
- Xia, B.; Gao, J.; Li, S.; Huang, L.; Zhu, L.; Ma, T.; Zhao, L.; Yang, Y.; Luo, K.; Xiaowei, S.; et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 2020, 10, 8974–8995. [Google Scholar] [CrossRef] [PubMed]
- Turney, S.G.; Ahmed, M.; Chandrasekar, I.; Wysolmerski, R.B.; Goeckeler, Z.M.; Rioux, R.M.; Whitesides, G.M.; Bridgman, P.C.; Wu, B.; Decourt, B.; et al. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol. Biol. Cell 2016, 27, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Macias, M.Y.; Battocletti, J.H.; Sutton, C.H.; Pintar, F.A.; Maiman, D.J. Directed and enhanced neurite growth with pulsed magnetic field stimulation. Bioelectromagnetics 2000, 21, 272–286. [Google Scholar] [CrossRef]
- Eguchi, Y.; Ogiue-Ikeda, M.; Ueno, S. Control of orientation of rat Schwann cells using an 8-T static magnetic field. Neurosci. Lett. 2003, 351, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, M.; Koana, T.; Suzuki, Y.; Shimizu, H.; Nakagawa, M. Mutagenicity and co-mutagenicity of static magnetic fields detected by bacterial mutation assay. Mutat. Res. 1999, 427, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Huang, L.; Sun, Z.; Ma, T.; Zhu, S.; Quan, X.; Yang, Y.; Huang, J.; Luo, Z. Pulsed magnetic field promotes proliferation and neurotrophic genes expression in Schwann cells in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 2343–2353. [Google Scholar] [PubMed]
- Adams, C.F.; Dickson, A.W.; Kuiper, J.H.; Chari, D.M. Nanoengineering neural stem cells on biomimetic substrates using magnetofection technology. Nanoscale 2016, 8, 17869–17880. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, Y.; Wei, H.; Chen, W.; Liu, Y.; Yan, X.; Guo, L.; Liao, M.; Chen, B.; Chai, R.; et al. Superparamagnetic Iron Oxide Nanoparticles and Static Magnetic Field Regulate Neural Stem Cell Proliferation. Front. Cell. Neurosci. 2022, 15, 815280. [Google Scholar] [CrossRef]
- Santhosh, M.; Choi, J.H.; Choi, J.W. Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel. Nanomaterials 2019, 9, 1293. [Google Scholar] [CrossRef]
- Sirkkunan, D.S.; Muhamad, F.; Pingguan-Murphy, B. Application of “Magnetic Anchors” to Align Collagen Fibres for Axonal Guidance. Gels 2021, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Lacko, C.S.; Singh, I.; Wall, M.A.; Garcia, A.R.; Porvasnik, S.L.; Rinaldi, C.; Schmidt, C.E. Magnetic particle templating of hydrogels: Engineering naturally derived hydrogel scaffolds with 3D aligned microarchitecture for nerve repair. J. Neural Eng. 2020, 17, 016057. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Dinda, A.; Vishnubhatla, S.; Anwar, M.F.; Jain, S. A combinatorial approach to modulate microenvironment toward regeneration and repair after spinal cord injury in rats. Neurosci. Lett. 2021, 741, 135500. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, R.; Sasaki, Y.; Kawasaki, R.; Katagiri, K.; Sawada, S.I.; Mukai, S.A.; Akiyoshi, K. Magnetically Navigated Intracellular Delivery of Extracellular Vesicles Using Amphiphilic Nanogels. Bioconjug. Chem. 2019, 30, 2150–2155. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Ganguly, D.; Zuidema, J.M.; Cardinal, T.J.; Ziemba, A.M.; Kearns, K.R.; McCarthy, S.M.; Thompson, D.M.; Ramanath, G.; Borca-Tasciuc, D.-A.; et al. Injectable, magnetically orienting electrospun fiber conduits for neuron guidance. ACS Appl. Mater. Interfaces 2019, 11, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chang, J.J.; Yung, M.C.; Huang, W.C.; Chen, S.Y. Spontaneously Micropatterned Silk/Gelatin Scaffolds with Topographical, Biological, and Electrical Stimuli for Neuronal Regulation. ACS Biomater. Sci. Eng. 2020, 6, 1144–1153. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Jiao, Y.; Wu, C.; Guo, S.; Xiao, X.; Wei, X.; Wu, J.; Gao, P.; Wang, N.; et al. Biological Effects of a Three-Dimensionally Printed Ti6Al4V Scaffold Coated with Piezoelectric BaTiO3 Nanoparticles on Bone Formation. ACS Appl. Mater. Interfaces 2020, 12, 51885–51903. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Wang, S.; Kun, L.; Gao, J.; Chen, B.; Ye, Y.; Wang, F.; Tong, F.; Jiang, J.; et al. Control the Neural Stem Cell Fate with Biohybrid Piezoelectrical Magnetite Micromotors. Nano Lett. 2021, 21, 3518–3526. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Xiao, Z.; Liu, X.; Wu, C.; Wu, K.; Liu, A.; Wei, D.; Sun, J.; Zhou, L.; et al. Magnetoelectric Nanoparticles Incorporated Biomimetic Matrix for Wireless Electrical Stimulation and Nerve Regeneration. Adv. Healthc. Mater. 2021, 10, e2100695. [Google Scholar] [CrossRef]
- He, L.P.; Liu, P.Z.; Wen, Y.M.; Wu, J. Effect of temperature maintenance by forced-air warming blankets of different temperatures on changes in inflammatory factors in children undergoing congenital hip dislocation surgery. Chin. Med. J. 2020, 133, 1768–1773. [Google Scholar] [CrossRef]
- Wang, Z.; Baharani, A.; Wei, Z.; Truong, D.; Bi, X.; Wang, F.; Li, X.M.; Verge, V.M.K.; Zhang, Y. Low field magnetic stimulation promotes myelin repair and cognitive recovery in chronic cuprizone mouse model. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Mert, T.; Metin, T.O.; Sahin, E.; Yaman, S.; Sahin, M. Neuroprotective and anti-neuropathic actions of pulsed magnetic fields with low frequencies in rats with chronic peripheral neuropathic pain. Brain Res. Bull. 2021, 177, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mert, T.; Sahin, E.; Yaman, S.; Sahin, M. Pulsed magnetic field treatment ameliorates the progression of peripheral neuropathy by modulating the neuronal oxidative stress, apoptosis and angiogenesis in a rat model of experimental diabetes. Arch. Physiol. Biochem. 2022, 128, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, X.; Wei, W.; Wang, Y.; Dai, H. Mesoporous hollow Fe3O4 nanoparticles regulate the behavior of neuro-associated cells through induction of macrophage polarization in an alternating magnetic field. J. Mater. Chemistry. B 2022, 10, 5633–5643. [Google Scholar] [CrossRef]
- Hirai, T.; Nakamichi, N.; Yoneda, Y. Activator protein-1 complex expressed by magnetism in cultured rat hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 292, 200–207. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Lu, L.; Liu, Y. SPIONs mediated magnetic actuation promotes nerve regeneration by inducing and maintaining repair-supportive phenotypes in Schwann cells. J. Nanobiotechnol. 2022, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Darrow, D.P. Focused Ultrasound for Neuromodulation. Neurother. J. Am. Soc. Exp. NeuroTher. 2019, 16, 88–99. [Google Scholar] [CrossRef]
- Seo, Y.; Han, S.; Song, B.W.; Chang, J.W.; Na, Y.C.; Chang, W.S. Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood-Brain Barrier Modulation. Int. J. Mol. Sci. 2023, 24, 5712. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Mehta, R.I.; Haut, M.W.; Wang, P.; Ranjan, M.; Najib, U.; D’haese, P.-F.; Rezai, A.R. Ultrasound-mediated blood–brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer’s disease. Fluids Barriers CNS 2023, 20, 46. [Google Scholar] [CrossRef]
- Phan, T.; Fan, C.; Yeh, C. Application of ultrasound to enhancing stem cells associated therapies. Stem Cell Rev. Rep. 2023, 19, 1709–1725. [Google Scholar] [CrossRef]
- Vyas, N.; Manmi, K.; Wang, Q.; Jadhav, A.J.; Barigou, M.; Sammons, R.L.; Kuehne, S.A.; Walmsley, A.D. Which Parameters Affect Biofilm Removal with Acoustic Cavitation? A Review. Ultrasound Med. Biol. 2019, 45, 1044–1055. [Google Scholar] [CrossRef]
- Kong, C.; Chang, W.S. Preclinical Research on Focused Ultrasound-Mediated Blood–Brain Barrier Opening for Neurological Disorders: A review. Neurol. Int. 2023, 15, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Dhayalan, M.; Wang, W.; Riyaz, S.U.M.; Dinesh, R.A.; Shanmugam, J.; Irudayaraj, S.S.; Stalin, A.; Giri, J.; Mallik, S.; Hu, R. Advances in functional lipid nanoparticles: From drug delivery platforms to clinical applications. 3 Biotech 2024, 14, 57. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Omay, S.B.; Sheth, K.N.; Zhou, J. Nanoparticle-based drug delivery for the treatment of traumatic brain injury. Expert Opin. Drug Deliv. 2023, 20, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Akram, R.; Anwar, H.; Sajid, F.; Iman, T.; Han, H.S.; Raza, C.; De Aguilar, J.G. Adult neurogenesis: A real hope or a delusion? Neural Regen. Res. 2024, 19, 6–15. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Kharazmi, M.S.; Jafari, S.M. Natural bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega 2023, 8, 3667–3683. [Google Scholar] [CrossRef] [PubMed]
- Vassal, M.; Martins, F.; Monteiro, B.; Tambaro, S.; Martinez-Murillo, R.; Rebelo, S. Emerging pro-neurogenic therapeutic strategies for neurodegenerative diseases: A review of pre-clinical and Clinical research. Mol. Neurobiol. 2024, 5, 2200076. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef]
- Yang, Y.; Torbey, M.T. Angiogenesis and Blood-Brain Barrier Permeability in Vascular Remodeling after Stroke. Curr. Neuropharmacol. 2020, 18, 1250–1265. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, S.; Le, W. Nanotherapeutic and Stem Cell Therapeutic Strategies in Neurodegenerative Diseases: A Promising Therapeutic Approach. Int. J. Nanomed. 2023, 18, 611–626. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Akash, S.; Rashid, H.O.; Ray, T.K.; Rahaman, S.; Islam, M.; Anika, F.; Hosain, K.; Aovi, F.I.; et al. Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance. Biomed. Pharmacother. 2022, 153, 113305. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.; Yang, Y.; Nicol, C.J.B.; Wang, C. Gold nanoparticles in Neurological diseases: A review of Neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. [Google Scholar] [CrossRef]
- Sargazi, S.; Arshad, R.; Ghamari, R.; Rahdar, A.; Bakhshi, A.; Karkan, S.F.; Ajalli, N.; Bilal, M.; Díez-Pascual, A.M. siRNA-based nanotherapeutics as emerging modalities for immune-mediated diseases: A preliminary review. Cell Biol. Int. 2022, 46, 1320–1344. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, Y.; Burger, J.C.; Li, D.; Zhu, M.; Gong, S. Non-viral approaches for gene therapy and therapeutic genome editing across the blood–brain barrier. Med-X 2023, 1, 6. [Google Scholar] [CrossRef]
- Mengstie, M.A. Viral vectors for the in vivo delivery of CRISPR components: Advances and challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Q.; Qu, Y.; Yin, W.; Zhao, R.; Wang, X.; Yang, Y.; Guo, Z.N. Emerging strategies for nerve repair and regeneration in ischemic stroke: Neural stem cell therapy. Neural Regen. Res. 2024, 19, 2430–2443. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, X.; Chen, X.; Zhou, P.; Xu, F.; Liang, W. Nanotechnology shaping stem cell therapy: Recent advances, application, challenges, and future outlook. Biomed. Pharmacother. 2021, 137, 111236. [Google Scholar] [CrossRef] [PubMed]
- Nakabeppu, Y. Origins of Brain Insulin and Its Function. Adv. Exp. Med. Biol. 2019, 1128, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Franke, B.; De Witte, W.; Ruisch, I.H.; Haavik, J.; van Gils, V.; Jansen, W.J.; Vos, S.J.B.; Lind, L.; Buitelaar, J.K.; et al. Insulinopathies of the brain? Genetic overlap between somatic insulin-related and neuropsychiatric disorders. Transl. Psychiatry 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.M.; Hirschberg, P.R.; Sarkar, P.; Siegel, D.M.; Teegala, S.B.; Vail, G.M.; Routh, V.H. Insulin actions on hypothalamic glucose-sensing neurones. J. Neuroendocrinol. 2021, 33, e12937. [Google Scholar] [CrossRef]
- Shaughness, M.; Acs, D.; Brabazon, F.; Hockenbury, N.; Byrnes, K.R. Role of insulin in neurotrauma and Neurodegeneration: A review. Front. Neurosci. 2020, 14, 547175. [Google Scholar] [CrossRef]
- Quintana-Pajaro, L.; Padilla-Zambrano, H.S.; Ramos-Villegas, Y.; Lopez-Cepeda, D.; Andrade-Lopez, A.; Hoz, S.; Moscote-Salazar, L.R.; Joaquim, A.F.; Perdomo, W.A.F.; Janjua, T. Cerebral traumatic injury and glucose metabolism: A scoping review. Egypt. J. Neurosurg. 2023, 38, 62. [Google Scholar] [CrossRef]
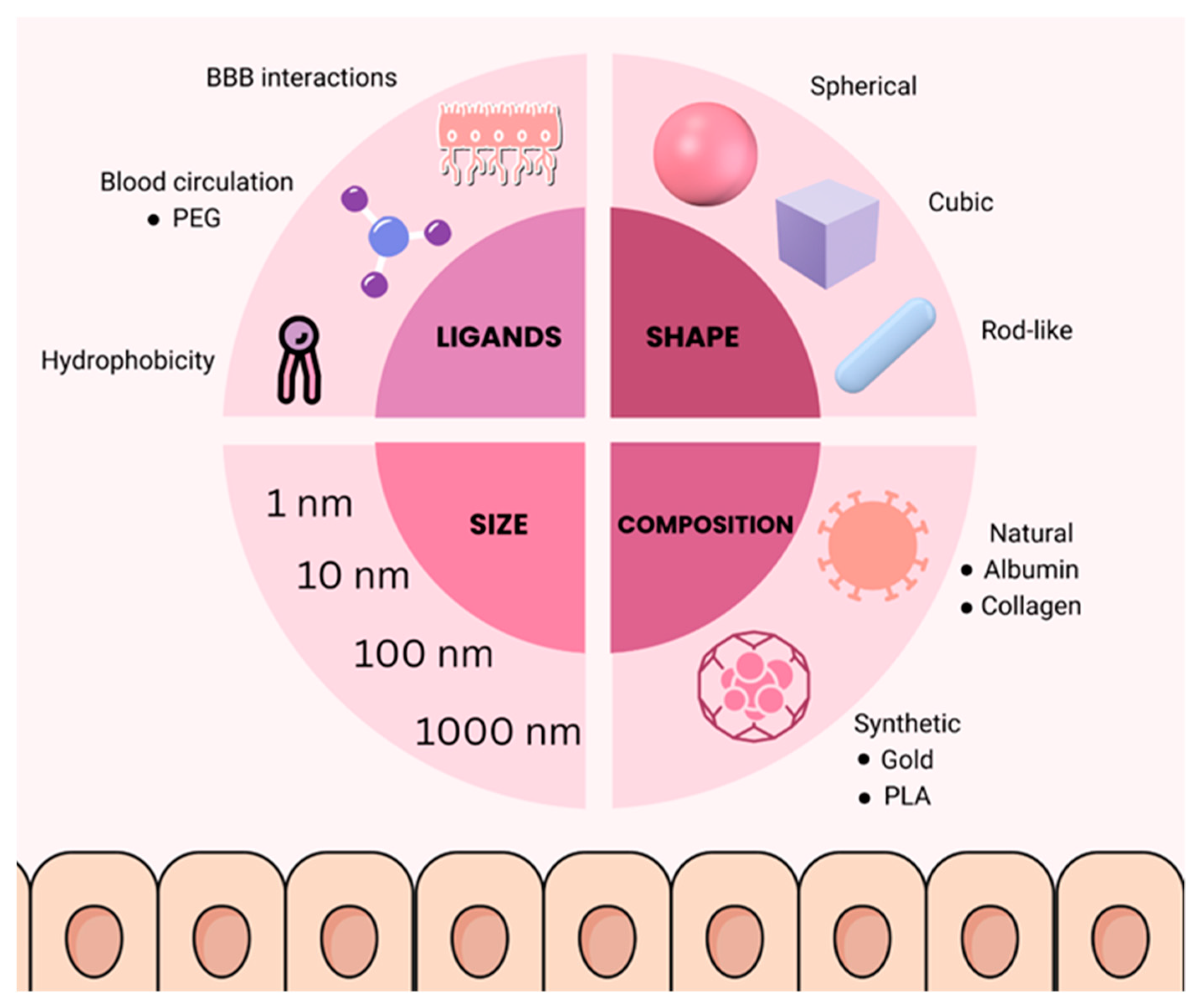
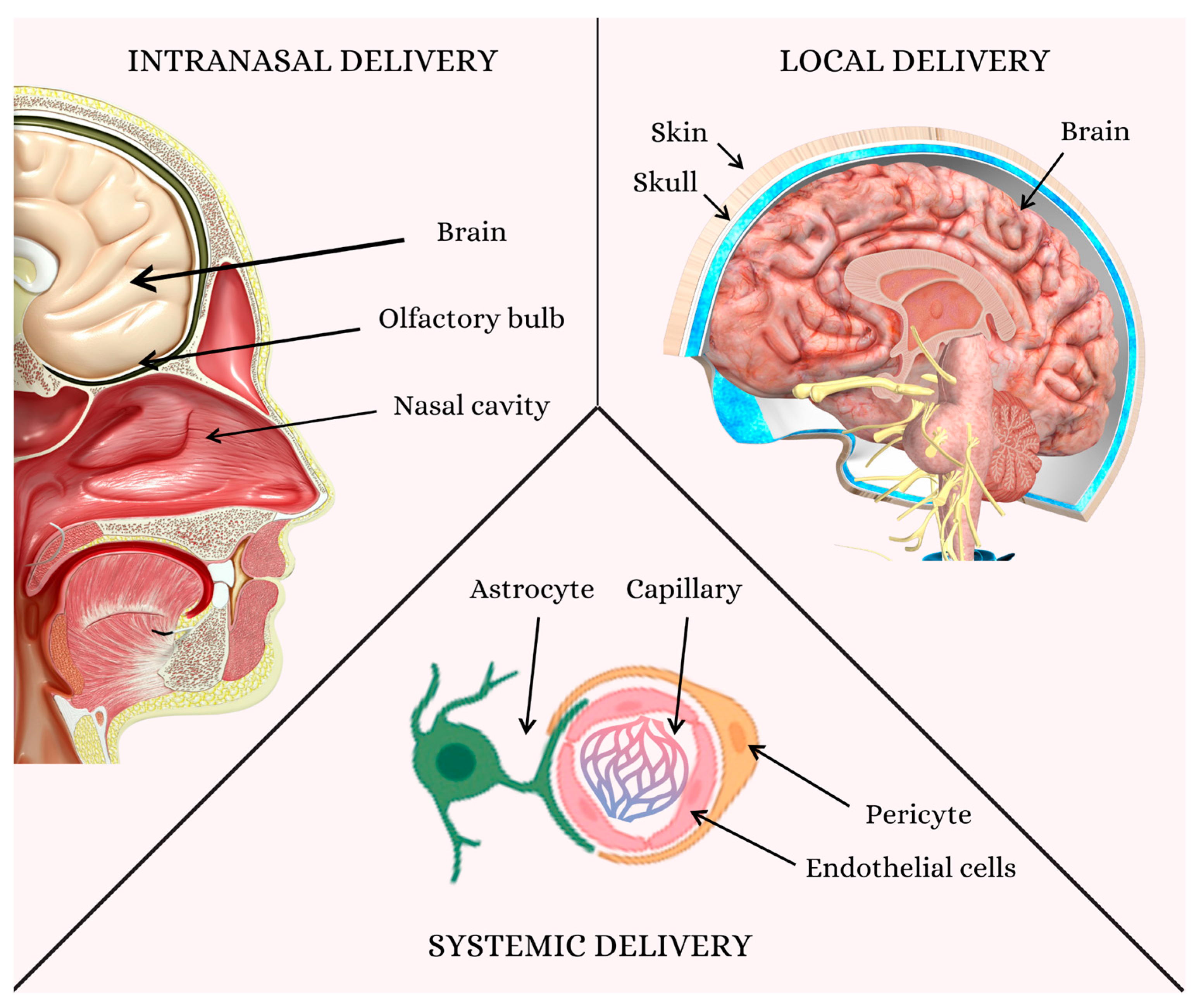
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krsek, A.; Jagodic, A.; Baticic, L. Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review. Medicina 2024, 60, 1384. https://doi.org/10.3390/medicina60091384
Krsek A, Jagodic A, Baticic L. Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review. Medicina. 2024; 60(9):1384. https://doi.org/10.3390/medicina60091384
Chicago/Turabian StyleKrsek, Antea, Ana Jagodic, and Lara Baticic. 2024. "Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review" Medicina 60, no. 9: 1384. https://doi.org/10.3390/medicina60091384
APA StyleKrsek, A., Jagodic, A., & Baticic, L. (2024). Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review. Medicina, 60(9), 1384. https://doi.org/10.3390/medicina60091384






