Anti-Müllerian Hormone Level Determinants among Non-Polycystic-Ovary-Syndrome Women Undergoing In Vitro Fertilization: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Studied Parameters
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics for the Included Patients
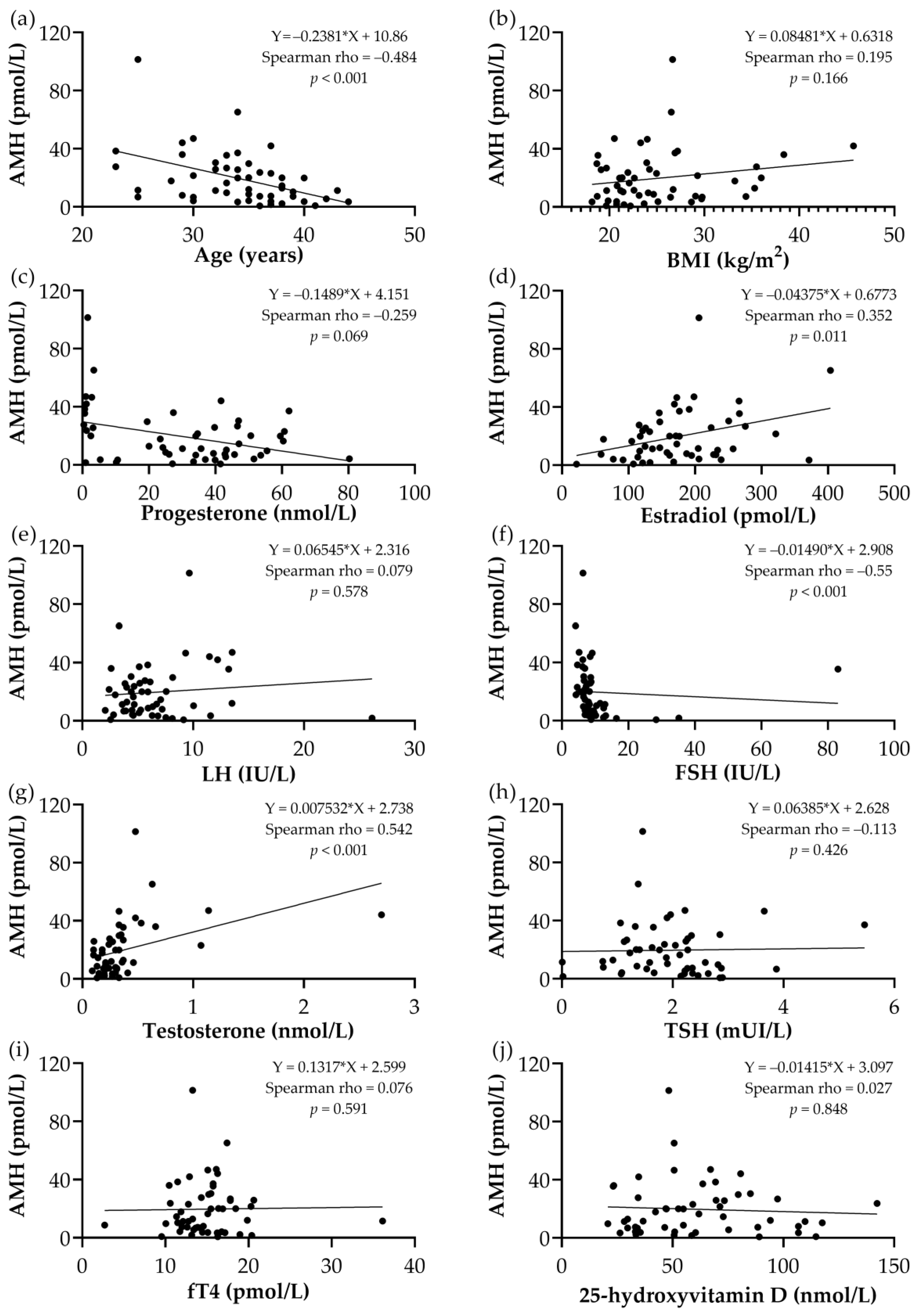
3.2. Correlation Analysis between AMH and the Studied Parameters
3.3. Regression Model Analysis for AMH
3.4. Comparative Analysis of the Studied Parameters Depending on the Presence of Autoimmune Thyroiditis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLennan, I.S.; Pankhurst, M.W. Anti-Müllerian Hormone Is a Gonadal Cytokine with Two Circulating Forms and Cryptic Actions. J. Endocrinol. 2015, 226, R45–R57. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.J.; Lambalk, C.B. A Systematic Review of Tests Predicting Ovarian Reserve and IVF Outcome. Hum. Reprod. Update 2006, 12, 685–718. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Choe, S.Y.; Cho, Y.J. Clinical Application of Serum Anti-Müllerian Hormone in Women. Clin. Exp. Reprod. Med. 2019, 46, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D. Predictors of IVF Outcome: Markers and Protocols. Women Health 2006, 2, 385–388. [Google Scholar] [CrossRef]
- Umarsingh, S.; Adam, J.K.; Krishna, S. The Relationship Between Anti-Müllerian Hormone (AMH) Levels and Pregnancy Outcomes in Patients Undergoing Assisted Reproductive Techniques (ART). Peerj 2020, 8, e10390. [Google Scholar] [CrossRef]
- Vijay, A.S.; Gopireddy, M.M.; Fyzullah, S.; Gollapalli, P.; Maheswari, M.; Rani, U.; Rajesh, S. Association between AMH Levels and Fertility/Reproductive Outcomes among Women Undergoing IVF: A Retrospective Study. J. Reprod. Infertil. 2022, 23, 54. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X.; Yang, H.; Feng, X.-J.; Lan, Y. Predictive Value of Anti-Mullerian Hormone for Pregnancy Outcomes Following Assisted Reproductive Techniques (ART) in Southwest China. Reprod. Health 2022, 19, 224. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, H.O.; Cha, S.W.; Park, C.W.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S. Age Specific Serum Anti-Müllerian Hormone Levels in 1,298 Korean Women with Regular Menstruation. Clin. Exp. Reprod. Med. 2011, 38, 93. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shi, L.; Feng, G.; Xiao, Z.; Chen, L.; Li, R.; Qiao, J. An Ovarian Reserve Assessment Model Based on Anti-Müllerian Hormone Levels, Follicle-Stimulating Hormone Levels, and Age: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e19096. [Google Scholar] [CrossRef]
- Iyer, S.; Kallathikumar, K.; Sinkar, P.; Velumani, A. Anti-Mullerian Hormone (AMH) and Age—An Indian Laboratory Retrospective Analysis. Asian J. Health Sci. 2019, 5, 7. [Google Scholar] [CrossRef]
- De Kat, A.C.; Van Der Schouw, Y.T.; Eijkemans, M.J.; Herber-Gast, G.C.; Visser, J.A.; Verschuren, W.M.; Broekmans, F.J. Back to the Basics of Ovarian Aging: A Population-Based Study on Longitudinal Anti-Müllerian Hormone Decline. BMC Med. 2016, 14, 151. [Google Scholar] [CrossRef]
- Giusti, M.; Mittica, M. Evaluation of Anti-Müllerian Hormone in Pre-Menopausal Women Stratified According to Thyroid Function, Autoimmunity and Age. Thyroid. Res. 2022, 15, 15. [Google Scholar] [CrossRef]
- Olszanecka-Glinianowicz, M.; Madej, P.; Owczarek, A.; Chudek, J.; Skałba, P. Circulating Anti-Müllerian Hormone Levels in Relation to Nutritional Status and Selected Adipokines Levels in Polycystic Ovary Syndrome. Clin. Endocrinol. 2015, 83, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Aghadavod, E.; Zarghami, N.; Farzadi, L.; Zare, M.; Movassaghpour, A.A.; Nouri, M. Evaluation of Relationship Between Serum Levels of Anti-Müllerian Hormone, Androgen, and Insulin Resistant with Retrieval Oocytes in Overweight Patients with Polycystic Ovary Syndrome. Adv. Biomed. Res. 2015, 4, 76. [Google Scholar] [CrossRef]
- Al-Taee, H.A.; Almadfai, Z.; Alkhafaji, Z.H. Effect of Obesity on Ovarian Reserve Parameters in Mid-Reproductive Age Women. F1000research 2012, 1, 43. [Google Scholar] [CrossRef][Green Version]
- Merhi, Z.O.; Seifer, D.B.; Weedon, J.; Adeyemi, O.; Holman, S.; Anastos, K.; Golub, E.T.; Young, M.; Karim, R.; Greenblatt, R.; et al. Circulating Vitamin D Correlates with Serum Antimüllerian Hormone Levels in Late-Reproductive-Aged Women: Women’s Interagency HIV Study. Fertil. Steril. 2012, 98, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Lata, I.; Tiwari, S.; Gupta, A.; Yadav, S.C.; Yadav, S. To Study the Vitamin D Levels in Infertile Females and Correlation of Vitamin D Deficiency With AMH Levels in Comparison to Fertile Females. J. Hum. Reprod. Sci. 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Moridi, I.; Chen, A.Y.; Tal, O.; Tal, R. The Association Between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef]
- Altuntaş, N.B.; Yildirim, S.B.; Güvey, H.; Erden, Ö. Is There a Relation Between Serum Vitamin D and Ovarian Reserve Markers in Infertile Women?: A Retrospective Cohort Study. J. Exp. Clin. Med. 2022, 39, 117–120. [Google Scholar] [CrossRef]
- Racoubian, E.; Aimagambetova, G.; Finan, R.R.; Almawi, W.Y. Age-Dependent Changes in Anti-Müllerian Hormone Levels in Lebanese Females: Correlation with Basal FSH and LH Levels and LH/FSH Ratio: A Cross-Sectional Study. BMC Women Health 2020, 20, 134. [Google Scholar] [CrossRef]
- Quresh, Z.; Almeida, F.A.; Jatale, R. Amh Correlation With Endogenous Hormones in Females: Indian Perspective. Glob. J. Res. Anal. 2022, 11, 19–23. [Google Scholar] [CrossRef]
- Abbas, E.M. Effect of Hypothyroidism on Ovarian Reserve Status in Iraqi Women: Hormonal Study. Bionatura 2023, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Qaisi, S.M. Effect of Anti Mullerian Hormone (AMH) on Hyperthyroidism with and without Polycystic Ovarian Syndrome (PCOS) in Female Patients. Iraqi J. Sci. 2021, 58, 1808–1814. [Google Scholar] [CrossRef]
- Eren, C.Y.; Azhari, A.; Gürsoy, Ö.Ö.; Godek, O. Prevalence of Premature Ovarian Failure in Patients with Autoimmune Thyroiditis. Health Care Acad. J. 2022, 9, 317–321. [Google Scholar] [CrossRef]
- Cotellessa, L. Role of Anti-Müllerian Hormone in the Central Regulation of Fertility. Semin. Reprod. Med. 2024, 42, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Pierre, A.; Rey, R.; Leclerc, A.; Arouche, N.; Hesters, L.; Catteau-Jonard, S.; Frydman, R.; Picard, J.Y.; Fanchin, R.; et al. Differential Regulation of Ovarian Anti-Müllerian Hormone (AMH) by Estradiol through α- and β-Estrogen Receptors. J. Clin. Endocrinol. Metab. 2012, 97, E1649–E1657. [Google Scholar] [CrossRef]
- Dilbaz, B.; Mert, S.A. Evaluation and Interpretation of AMH in Female Infertility. Düzce Tıp Fakültesi Derg. 2022, 24, 82–85. [Google Scholar] [CrossRef]
- Zhao, D.; Fan, J.; Wang, P.; Jiang, X.; Yao, J.; Li, X. Age-Specific Definition of Low Anti-Mullerian Hormone and Associated Pregnancy Outcome in Women Undergoing IVF Treatment. BMC Pregnancy Childbirth 2021, 21, 186. [Google Scholar] [CrossRef]
- Rodriguez-Purata, J.; Gingold, J.; Alonso-de-Mendieta, M.; Gomez-Cuesta, M.J.; Cervantes-Bravo, E. P-622 Age-specific reference intervals for anti-müllerian hormone in Mexico: A population-based study. implications for Latin America. Hum. Reprod. 2023, 38 (Suppl. 1), dead093.951. [Google Scholar] [CrossRef]
- Wang, L.-S.; Zhao, X.; Cai, X.; Fu, G. Age Specific Reference Intervals of Serum Anti-Müllerian Hormone Concentration of 1,253 Women with Healthy Females in Infertility Center. Clin. Exp. Obstet. Gynecol. 2019, 46, 526–530. [Google Scholar] [CrossRef]
- Bakr, E.H.; Ghoneim, H.M.; Tharwat, O. The Relationship between Serum Levels of Anti-Mullerian Hormone and Body Mass Index in Adolescents with Polycystic Ovary Syndrome. Crogr 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, Y.; Zhang, M.; Chen, Y.; Ye, J.; Han, Y.; Ma, D.; Zheng, X.; Yan, X.; Liu, C. Anti-Müllerian Hormone Was Independently Associated with Central Obesity but Not with General Obesity in Women with PCOS. Endocr. Connect. 2022, 11, e210243. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Huecas, L.; Piera-Jordán, C.Á.; Serrano De La Cruz-Delgado, V.; Zaragoza-Martí, A.; García-Velert, M.B.; Tordera-Terrades, C.; Sánchez-Sansegundo, M.; Martín-Manchado, L. Assessment of Nutritional Status and Its Influence on Ovarian Reserve: A Systematic Review. Nutrients 2023, 15, 2280. [Google Scholar] [CrossRef]
- Halder, A. Serum Anti-Müllerian Hormone: A Potential Biomarker for Polycystic Ovary Syndrome. Indian. J. Med. Res. 2023, 158, 397. [Google Scholar] [CrossRef] [PubMed]
- Im Hwang, Y.; Sung, N.Y.; Koo, H.S.; Cha, S.H.; Park, C.W.; Kim, J.Y.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S.; et al. Can High Serum Anti-Müllerian Hormone Levels Predict the Phenotypes of Polycystic Ovary Syndrome (PCOS) and Metabolic Disturbances in PCOS Patients? Clin. Exp. Reprod. Med. 2013, 40, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, D.; Liu, C.; Zhang, L. The Relationship between Insulin Resistance and Obesity and Serum Anti-Mullerian Hormone Level in Chinese Women with Polycystic Ovary Syndrome: A Retrospective, Single-Center Cohort Study. Int. J. Women Health. 2023, 15, 151–166. [Google Scholar] [CrossRef]
- Görkem, Ü.; Küçükler, F.K.; Toğrul, C.; Gülen, Ş. Obesity Does Not Compromise Ovarian Reserve Markers in Infertile Women. Geburtshilfe Frauenheilkd. 2019, 79, 79–85. [Google Scholar] [CrossRef]
- Kloos, J.; Coyne, K.D.; Weinerman, R.S. The Relationship Between anti-Müllerian Hormone, Body Mass Index and Weight Loss: A Review of the Literature. Clin. Obes. 2022, 12, e12559. [Google Scholar] [CrossRef]
- Jassam, H.M.S.; Fayez, S.S.; Abduljabar, M.K. Evaluation of Hormones Level in Infertility Women Because of Polycystic Ovary Syndrome. Int. J. Health Sci. 2022, 6, 9088–9094. [Google Scholar] [CrossRef]
- Rifatt, N.M.G.; Rahim, S.M. Relationship Between Serum Prostate Specific Antigen (PSA) in Women with Polycystic Ovary Syndrome and Some Reproductive Hormones in Kirkuk City. Tikrit J. Pure Sci. 2023, 22, 34–39. [Google Scholar] [CrossRef]
- Alamieer, W.S.A.; Shubber, L.A.; Azeez, N.S. Relationship Between Serum Antimullerian Hormone, Follicular Stimulating Hormone, and Luteal Hormone Levels with Ovarian Follicular Status on Day Three of Menstrual Cycle. Int. Res. J. Pharm. 2019, 9, 75–80. [Google Scholar] [CrossRef]
- Ishrat, S.; Deeba, F.; Anwary, S.A.; Banu, J. Correlation and Discordance of Anti-Mullerian Hormone with Follicle Stimulating Hormone in Infertile Women with Premature Ovarian Insufficiency and Diminished Ovarian Reserve. Int. J. Reprod. Contracept. Obstet. Gynecol. 2020, 10, 32. [Google Scholar] [CrossRef]
- Lin, L.C.; Li, C.-J.; Tsui, K. Serum Testosterone Levels Are Positively Associated with Serum Anti-Mullerian Hormone Levels in Infertile Women. Sci. Rep. 2021, 11, 6336. [Google Scholar] [CrossRef]
- Lv, P.P.; Jin, M.; Rao, J.P.; Chen, J.; Wang, L.Q.; Huang, C.C.; Yang, S.Q.; Yao, Q.P.; Feng, L.; Shen, J.M.; et al. Role of Anti-Müllerian Hormone and Testosterone in Follicular Growth: A Cross-Sectional Study. BMC Endocr. Disord. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Tulic, L.; Tulic, I.; Bila, J.; Nikolic, L.; Dotlic, J.; Lazarevic-Suntov, M.; Kalezic, I. Correlation of progesterone levels on the day of oocyte retrieval with basal hormonal status and the outcome of ART. Sci. Rep. 2020, 10, 22291. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, Z.; Bersinger, N.A.; McKinnon, B.D.; Schneider, S.; Mueller, M.D.; Von Wolff, M. Anti-Müllerian Hormone and Progesterone Levels Produced by Granulosa Cells Are Higher When Derived from Natural Cycle IVF Than from Conventional Gonadotropin-Stimulated IVF. Reprod. Biol. Endocrinol. 2015, 13, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, J.S.; Keefe, K.W.; Lanes, A.; Yanushpolsky, E. Premature progesterone elevation during the early and mid-follicular phases in fresh in vitro fertilization (IVF) cycles is associated with lower live birth, clinical pregnancy, and implantation rates. J. Assist. Reprod. Genet. 2023, 40, 1029–1035. [Google Scholar] [CrossRef]
- Kuroda, K.; Uchida, T.; Nagai, S.; Ozaki, R.; Yamaguchi, T.; Sato, Y.; Brosens, J.J.; Takeda, S. Elevated Serum Thyroid-Stimulating Hormone Is Associated with Decreased Anti-Müllerian Hormone in Infertile Women of Reproductive Age. J. Assist. Reprod. Genet. 2014, 32, 243–247. [Google Scholar] [CrossRef]
- Halici, M. Thyroid Hormones and Ovarian Reserve: A Comprehensive Study of Women Seeking Infertility Care. BMC Women Health. 2023, 23, 570. [Google Scholar] [CrossRef]
- Akin, E.O.; Aycan, Z. Evaluation of the Ovarian Reserve in Adolescents with Hashimoto’s Thyroiditis Using Serum Anti- Müllerian Hormone Levels. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 331. [Google Scholar] [CrossRef]
- Bahri, S.; Tehrani, F.R.; Amouzgar, A.; Rahmati, M.; Tohidi, M.; Vasheghani, M.; Azizi, F. Overtime Trend of Thyroid Hormones and Thyroid Autoimmunity and Ovarian Reserve: A Longitudinal Population Study with a 12-Year Follow Up. BMC Endocr. Disord. 2019, 19, 47. [Google Scholar] [CrossRef]
- Bucci, I.; Giuliani, C.; Dalmazi, G.D.; Formoso, G.; Napolitano, G. Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kitahara, Y.; Osuka, S.; Tsukui, Y.; Kobayashi, M.; Iwase, A. Effect of Hypothyroidism and Thyroid Autoimmunity on the Ovarian Reserve: A Systematic Review and Meta-analysis. Reprod. Med. Biol. 2021, 21, e12427. [Google Scholar] [CrossRef] [PubMed]
- van Heemst, D. The ageing thyroid: Implications for longevity and patient care. Nat. Rev. Endocrinol. 2024, 20, 5–15. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Wang, M.; Tang, M.; Liu, Y. Correlation Analysis between Ovarian Reserve and Thyroid Hormone Levels in Infertile Women of Reproductive Age. Front. Endocrinol. 2021, 12, 745199. [Google Scholar] [CrossRef] [PubMed]
- Kolcsár, M.; Berecki, B.; Gáll, Z. Relationship between Serum 25-Hydroxyvitamin D Levels and Hormonal Status in Infertile Women: A Retrospective Study. Diagnostics 2023, 13, 3024. [Google Scholar] [CrossRef]
- Garbedian, K.; Boggild, M.; Moody, J.; Liu, K.E. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 2013, 1, E77–E82. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Baird, D.D.; Weinberg, C.R.; Wilcox, A.J.; McConnaughey, D.R.; Steiner, A.Z. Pre-conception 25-hydroxyvitamin D (25(OH)D) and fecundability. Hum. Reprod. 2019, 34, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jukic, A.M.Z.; Song, H.; Zhang, L.; Yang, F.; Wu, S.; Yin, D.; Jiang, H. Serum Vitamin D Concentrations, Time to Pregnancy, and Pregnancy Outcomes among Preconception Couples: A Cohort Study in Shanghai, China. Nutrients. 2022, 14, 3058. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wan, Q.; Huang, J.; Han, T.; Qu, T.; Yu, L.L. Influence of Vitamin D supplementation on reproductive outcomes of infertile patients: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, P.; Van De Vijver, A.; Schutyser, V.; Milatovic, S.; Anckaert, E.; Schiettecatte, J.; Blockeel, C.; Camus, M.; Tournaye, H.; Polyzos, N.P. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum. Reprod. 2016, 32, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.; Ebrahimi, M.; Akbari-Asbagh, F. The effect of vitamin D status on ovarian reserve markers in infertile women: A prospective cross-sectional study. Int. J. Reprod. Biomed. IJRM 2020, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.A.; Houghton, L.A.; Jones, G.T.; Van Rij, A.M.; Morgan, K.; McLennan, I.S. The Level of Serum Anti-Müllerian Hormone Correlates with Vitamin D Status in Men and Women But Not in Boys. J. Clin. Endocrinol. Metab. 2012, 97, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.A.; Houghton, L.A.; Pankhurst, M.W.; Harper, M.J.; McLennan, I.S. Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients 2017, 9, 719. [Google Scholar] [CrossRef]
| Parameters (Unit of Measurement; Normal Range *) | Mean ± SD/Median (IQR) |
|---|---|
| Age (years) | 34.04 ± 4.95 |
| BMI (kg/m2) | 23.48 (IQR 6.34) |
| AMH (pmol/L) | 13.71 (IQR 20.61) |
| Progesterone (nmol/L; 1.59–63.60) | 33.76 (IQR 38.55) |
| Estradiol (pmol/L; 69.75–513.99) | 168.5 ± 75.05 |
| LH (IU/L; 2.3–10.0) | 5.51 (IQR 3.86) |
| FSH (IU/L; 1.1–9.5) | 8.06 (IQR 2.77) |
| Total testosterone (nmol/L; 0.18–2.80) | 0.28 (IQR 0.17) |
| TSH (mIU/L; 0.5–4.8) | 1.93 ± 0.92 |
| fT4 (pmol/L; 9.53–23.17) | 14.84 (IQR 4.0) |
| 25-OH vitamin D (nmol/L) | 60.93 ± 28.49 |
| Studied Parameter | Outcome | Absolute and Relative Frequency (n/N, %) |
|---|---|---|
| IVF | No IVF performed, only infertility consultation | 20/52 (38.5%) |
| Unsuccessful IVF | 14/52 (26.9%) | |
| Successful IVF | 18/52 (34.6%) | |
| Vitamin D status | Adequate vitamin D level (>50 nmol/L) | 32/52 (61.5%) |
| Deficient vitamin D (<50 nmol/L) | 20/52 (38.5%) | |
| Thyroid status | TSH < 2.5 mIU/L | 42/52 (80.8%) |
| TSH > 2.5 mIU/L | 10/52 (19.2%) | |
| Ovarian reserve | Adequate ovarian reserve (AMH > 7 pmol/L) | 37/52 (71.2%) |
| Low ovarian reserve (AMH < 7 pmol/L) | 15/52 (28.8%) | |
| Body weight | Normal weight | 19/52 (36.5%) |
| Overweight and obese | 33/52 (63.5%) | |
| Autoimmune thyroiditis | Present | 33/52 (63.5%) |
| Absent | 19/52 (36.5%) |
| Age | BMI | Progesterone | Estradiol | LH | FSH | Total Testosterone (nmol/L) | TSH | fT4 | 25-OH Vitamin D | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMH | Rho | −0.484 (−0.673 to −0.236) | 0.195 | −0.254 | 0.352 (0.079 to 0.575) | 0.079 | −0.550 (−0.719 to −0.318) | 0.542 (0.309 to 0.714) | −0.113 | 0.076 | 0.027 |
| p (2-tailed) | <0.001 * | 0.166 | 0.069 | 0.011 * | 0.578 | <0.001 * | <0.001 * | 0.426 | 0.591 | 0.848 | |
| Unstandardized Coefficients | Standardized Coefficient | t | p | ||
|---|---|---|---|---|---|
| B | SE | β | |||
| (Constant) | 8.494 | 3.543 | 2.397 | 0.021 * | |
| Age (years) | −0.243 | 0.066 | −0.459 | −3.678 | 0.001 * |
| BMI (kg/m2) | 0.048 | 0.064 | 0.107 | 0.753 | 0.456 |
| Progesterone (nmol/L) | −0.121 | 0.053 | −0.303 | −2.274 | 0.028 * |
| Estradiol (pmol/L) | 0.052 | 0.016 | 0.403 | 3.307 | 0.002 * |
| LH (IU/L) | 0.068 | 0.087 | 0.105 | 0.774 | 0.443 |
| FSH (IU/L) | −0.031 | 0.032 | −0.134 | −0.960 | 0.343 |
| Testosterone (nmol/L) | −0.027 | 0.031 | −0.108 | −0.886 | 0.381 |
| TSH (mIU/L) | 0.489 | 0.341 | 0.172 | 1.433 | 0.160 |
| fT4 (pmol/L) | −0.834 | 0.945 | −0.108 | −0.882 | 0.383 |
| 25-hydroxivitamin D (nmol/L) | −0.002 | 0.032 | −0.008 | −0.055 | 0.957 |
| AIT Present n = 33 | AIT Absent n = 19 | p | Test | |
|---|---|---|---|---|
| Age (years) mean ± SD | 34.67 ± 4.68 | 32.95 ± 5.33 | 0.231 | Independent-samples t-test |
| BMI (kg/m2) median (IQR) | 23.3 (5.51) | 24.65 (13.33) | 0.747 | Mann–Whitney |
| AMH (pmol/L) median (IQR) | 11.43 (21.64) | 14.57 (18.93) | 0.537 | Mann–Whitney |
| Progesterone (nmol/L) mean ± SD | 33.48 ± 19.27 | 23.64 ± 22.47 | 0.101 | Independent-samples t-test |
| Estradiol (pmol/L) mean ± SD | 182.8 ± 82.55 | 159.6 ± 59.01 | 0.287 | Independent-samples t-test |
| LH (IU/L) median (IQR) | 5.19 (3.43) | 5.91 (5.79) | 0.857 | Mann–Whitney |
| FSH (IU/L) median (IQR) | 8.03 (2.75) | 8.11 (3.6) | 0.676 | Mann–Whitney |
| Total testosterone (nmol/L) median (IQR) | 0.3 (0.15) | 0.26 (0.2) | 0.655 | Mann–Whitney |
| TSH (mIU/L) median (IQR) | 2.22 (1.01) | 1.4 (0.75) | 0.002 * | Mann–Whitney |
| fT4 (pmol/L) median (IQR) | 15.22 (3.48) | 13.29 (5.29) | 0.121 | Mann–Whitney |
| 25-hydroxyvitamin D (nmol/L) mean ± SD | 63.98 ± 29.64 | 55.63 ± 26.49 | 0.314 | Independent-samples t-test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolcsar, M.; Szabó, L.; Mihály, R.; Vass, E.R.; Gáll, Z. Anti-Müllerian Hormone Level Determinants among Non-Polycystic-Ovary-Syndrome Women Undergoing In Vitro Fertilization: A Retrospective Cross-Sectional Study. Medicina 2024, 60, 1387. https://doi.org/10.3390/medicina60091387
Kolcsar M, Szabó L, Mihály R, Vass ER, Gáll Z. Anti-Müllerian Hormone Level Determinants among Non-Polycystic-Ovary-Syndrome Women Undergoing In Vitro Fertilization: A Retrospective Cross-Sectional Study. Medicina. 2024; 60(9):1387. https://doi.org/10.3390/medicina60091387
Chicago/Turabian StyleKolcsar, Melinda, László Szabó, Renáta Mihály, Erzsébet Rozália Vass, and Zsolt Gáll. 2024. "Anti-Müllerian Hormone Level Determinants among Non-Polycystic-Ovary-Syndrome Women Undergoing In Vitro Fertilization: A Retrospective Cross-Sectional Study" Medicina 60, no. 9: 1387. https://doi.org/10.3390/medicina60091387
APA StyleKolcsar, M., Szabó, L., Mihály, R., Vass, E. R., & Gáll, Z. (2024). Anti-Müllerian Hormone Level Determinants among Non-Polycystic-Ovary-Syndrome Women Undergoing In Vitro Fertilization: A Retrospective Cross-Sectional Study. Medicina, 60(9), 1387. https://doi.org/10.3390/medicina60091387







