Abstract
Background and Subject: Hyponatraemia is a common electrolyte disorder. For patients with severe hyponatraemia, intensive care unit (ICU) admission may be required. This will enable close monitoring and allow safe management of sodium levels effectively. While severe hyponatraemia may be associated with significant symptoms, rapid overcorrection of hyponatraemia can lead to complications. We aimed to describe the management and outcomes of severe hyponatraemia in our ICU and identify risk factors for overcorrection. Materials and Methods: This was a retrospective single-centre cohort that included consecutive adults admitted to the ICU with serum sodium < 120 mmol/L between 1 January 2017 and 8 March 2023. Anonymised data were collected from electronic records. We included 181 patients (median age 67 years, 51% male). Results: Median admission serum sodium was 113 mmol/L (IQR: 108–117), with an average rate of improvement over the first 48 h of 10 mmol/L/day (IQR: 5–15 mmol/L). A total of 62 patients (34%) met the criteria for overcorrection at 48 h, and they were younger, presented with severe symptoms (seizures/arrythmias), and had lower admission sodium concentration. They were more likely to be treated with hypertonic saline infusions. Lower admission sodium was an independent risk factor for overcorrection within 48 h, whereas the presence of liver cirrhosis and fluid restriction was associated with normal correction. No difference was identified between the normal and overcorrected cohorts for ICU/hospital length of stay or mortality. Conclusions: In some patients with severe hyponatraemia, overcorrection is inevitable to avoid symptoms such as seizures and arrhythmias, and consequently, we highlight the key factors associated with overcorrection. Overall, we identified that overcorrection was common and concordant with the current literature.
1. Introduction
Hyponatraemia, characterised by a serum sodium concentration below 135 mmol/L, is a common electrolyte disturbance encountered in the intensive care unit (ICU) setting [1]. This condition can arise from various aetiologies, each presenting unique challenges in management and potential complications. Similarly, severe hyponatraemia (<120 mmol/L) can result from several factors, including (but not exclusively) fluid overload, syndrome of inappropriate antidiuretic hormone secretion (SIADH), adrenal insufficiency, diuretic use, cardiac/liver failure, and renal dysfunction [2]. There may be serious consequences of severe hyponatraemia, leading to altered mental status, seizures, coma, prolonged ICU length of stay, prolonged mechanical ventilation, and increased mortality [3,4].
The management of severe hyponatraemia involves careful assessment of the underlying cause and correction of sodium levels at a controlled rate to avoid osmotic demyelination syndrome (ODS), a rare consequence of rapid overcorrection that manifests as irreversible neurological damage [5]. Therapeutic strategies, according to internationally accepted guidelines, are variable and may include fluid restriction, diuretic therapy, and hypertonic saline in severe symptomatic cases, with close monitoring of serum sodium levels and neurological status [5,6,7]. Management of overcorrection can be achieved with the use of desmopressin (with or without enteral water intake or hypotonic intravenous fluid infusion); however, the exact impact of such a treatment is not entirely predictable, and current guidelines recommend seeking specialist endocrine input before initiating this treatment [5].
Overcorrection of hyponatraemia, according to European guidelines, is defined as a rise in serum sodium concentration exceeding 10 mmol/L within 24 h, more than 18 mmol/L within the first 48 h, or more than 8 mmol/L/day after the initial 24 h of admission [5,8]. Rapid correction of severe hyponatraemia poses a significant risk for developing ODS [5,8]. Thus, a cautious approach to sodium correction is paramount, guided by frequent monitoring and adherence to established protocols.
Hyponatraemia in the ICU presents multifactorial challenges in diagnosis and management, emphasising the importance of a systematic approach to prevent both the deleterious effects of low sodium levels and the potential harm of rapid correction. However, detailed large datasets from ICU cohorts are lacking, particularly the management strategies utilised when managing patients with severe hyponatraemia. Consequently, this report aims to provide a comprehensive overview of patient characteristics, symptomology, ICU management strategies, the rate of corrections, complications, and outcomes of patients admitted with severe hyponatraemia.
2. Materials and Methods
2.1. Study Population
This single-centre retrospective observational study of adult patients admitted to the general ICU with severe hyponatraemia (<120 mmol/L) between 23 December 2016 and 8 March 2023 for up to the initial seven days of admission.
2.2. Data Collection
The ICU and hospital clinical notes system (MetaVision (iMDsoft, Tel Aviv, Israel)) and CHARTS (custom software for University Hospital Southampton NHS Trust, version 35) were reviewed and yielded all relevant information. Reason for hospital/ICU admission, date of admission/discharge, baseline demographics, past medical history, drug history, and social history were reported. Patients, their families, emergency/ward notes, previous admissions, outpatient records, and general practitioner records are routinely utilised to populate the dataset that was retrospectively reviewed for this study. Alcohol excess disorder was not defined specifically within this report and relied upon patient/family declaration, general practitioner, or previous admission reports.
Symptoms were recorded as confusion, arrhythmia, seizures, weakness, nausea, or vomiting. Admission Glasgow Coma Score (GCS) was also reported. Daily electrolyte panels are sent for all patients with 4–6 hourly arterial blood gas analysis for real-time electrolyte monitoring. Admission types of blood, including thyroid function, random cortisol, serum and urine osmolarities, and urinary sodium, were sent.
Treatments for hyponatraemia included intravenous saline solution infusion, stratified by hypertonic (1.8% or 2.7%) or isotonic (0.9%), hypertonic fluid bolus, sodium chloride oral tablets, vaptans, and fluid restriction. Desmopressin use was also recorded to evaluate overcorrection management.
2.3. Outcomes and Definitions
The primary outcome was the prevalence of overcorrection within 48 h of ICU admission (defined as below). The secondary outcomes were hospital survival, ICU survival, ICU length of stay, hospital length of stay, and prevalence of ODS.
Overcorrection was defined according to international clinical practice/consensus guidelines and included an increase in serum sodium of more than (a) 10 mmol/L in the first 24 h, (b) 8 mmol/L in the second 24 h period, or (c) 18 mmol/L within the initial 48 h [5,6].
2.4. Ethical Approval
This study is part of a large study investigating the outcome of critically ill patients in the ICU (CRIT-CO) study. This study was sponsored by University Hospital Southampton NHS Foundation Trust (RHM CRI 0370), and ethical approval was obtained from the NHS Health Research Authority, HRA, UK (IRAS 232922) on 26 November 2018. This study was also registered as part of a quality improvement project at University Hospital Southampton NHS Trust (ZAUD 7281). All identifiable patient data are anonymised, and due to the retrospective observational nature of the study, the consent has been waived. This study is compliant with local ethical standards, and no identifiable patient data are presented here.
2.5. Statistical Analysis
We tested for normality using the Shapiro–Wilks test, and as our dataset was non-normally distributed, we reported continuous variables as median (inter-quartile range, (IQR)). Baseline characteristics are described by median with IQR for continuous variables and counts with percentages for categorical variables. Kruskal–Wallis test and Fischer’s exact test for continuous and binary outcomes, respectively. Logistic regression models were constructed to analyse predictors of overcorrection at 48 h. Variables were included within the multivariable models based on clinical rationality and an univariable significance threshold of p < 0.25. Subsequent backward selection was performed using the Akaike Information Criterion to produce a final model. All analyses were performed using R (version 4.2.2) and regression analysis using the MASS package [9].
3. Results
3.1. Baseline Characteristics and Overcorrection Incidence
We included 181 patients admitted with severe hyponatraemia (<120 mmol/L) with a median age of 67 years (IQR 52, 77), and 51% were male. The majority (n = 129, 71.3%) were initially admitted to the hospital for clinical presentations not related to hyponatraemia. The comorbidities included hypertension (52%), diabetes mellitus (22%), congestive cardiac failure (15%), ischaemic heart disease (14%), liver cirrhosis (14%), and history of excess alcohol use (28%). The medications on admission included proton pump inhibitors (PPI) (47%), diuretics (33%), antidepressants or antipsychotics (20%), and corticosteroids (14%). The common presenting symptoms were confusion (51%), lethargy (44%), weakness (28%), nausea (27%), and vomiting (24%). Severe symptoms, including seizures and arrythmias, occurred in 14% and 5%, respectively. The median admission sodium, serum osmolality, and urinary sodium were 113 mmol/L (IQR 108, 117), 240 mOsm/kg (IQR 231, 256), and 32 mEq/L (IQR 15, 61), respectively. The treatment offered while in ICU included 0.9% saline infusion (73%), any hypertonic saline infusion (44%), hypertonic saline boluses (29%), and fluid restriction (47%) (Table 1).

Table 1.
Baseline characteristics table.
We sub-classified the cohort into two groups based on the patients meeting any of the 48 h overcorrection criteria presented in the methods section [5,6]. A breakdown of the number of patients meeting each overcorrection criterion is available in the Supplementary Materials (Table S2). Overcorrection within 48 h in a combined definition was seen in 62 (34%) patients. Proportionally, the overcorrected group was younger (66 vs. 69 years) and had fewer patients with liver cirrhosis (8.1% vs. 17%). Moreover, on initial presentation, the presence of more severe symptoms, including arrhythmias and seizures, was more common in the overcorrection group (3.4% vs. 8.1% and 10% vs. 23%, respectively). Admission serum sodium and the minimum serum sodium measured within the first week of ICU admission are highly concordant. Furthermore, overcorrected patients exhibit lower admission and minimum sodium values than normal corrected patients (111 mmol/L vs. 114 mmol/L). Overcorrected patients were more likely to receive any hypertonic saline infusion (47% vs. 43%) and were less likely to be treated with fluid restriction or salt tablets (37% vs. 52% and 19% vs. 25%, respectively). We report a low incidence of the use of vaptan medication within our cohort (3.3%) without a significant difference between the normal and overcorrected cohorts.
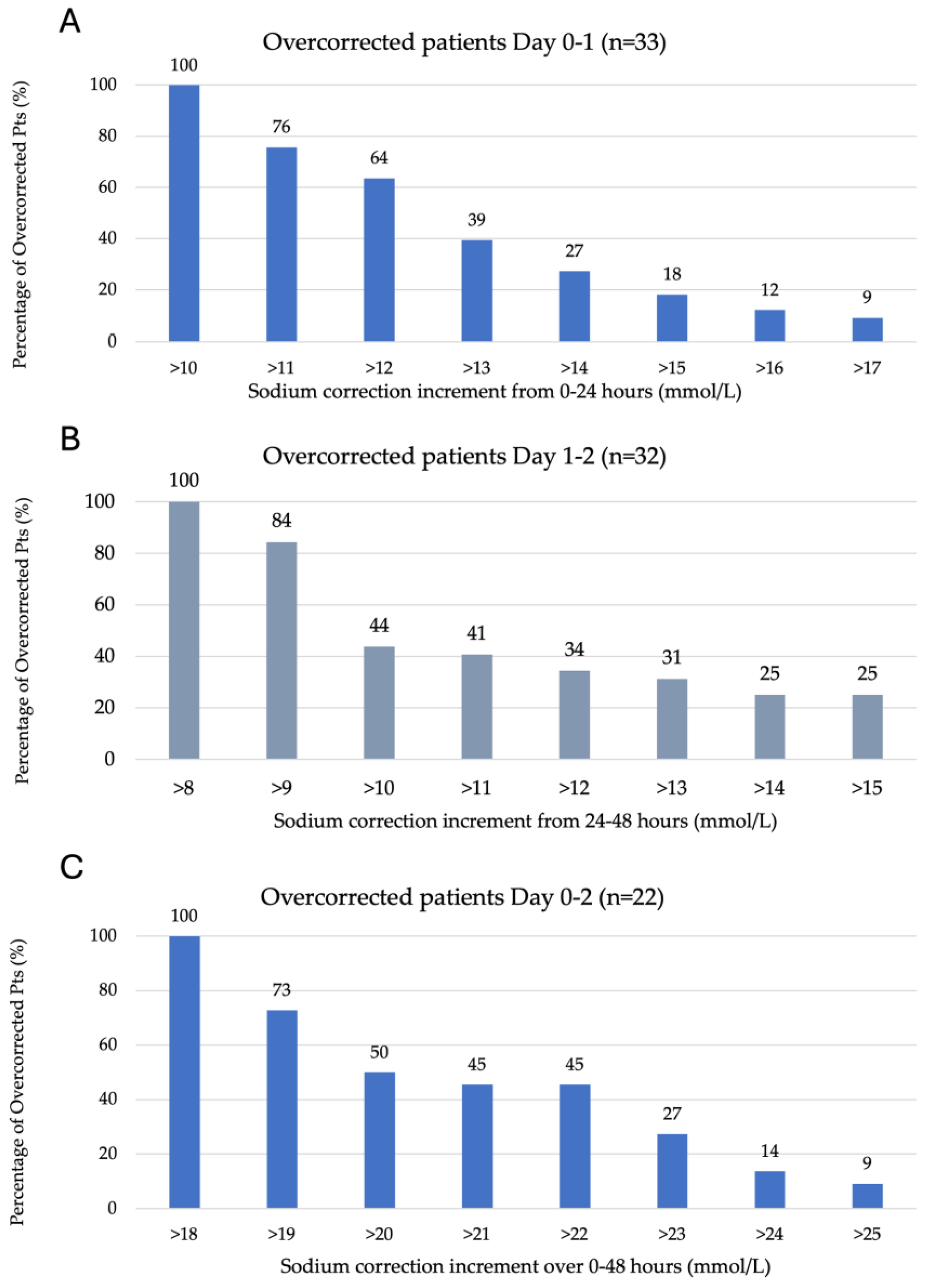
Figure 1A–C represents the degree of overcorrection seen according to each of the overcorrection criteria outlined in the methods section. A total of 36% of 33 patients overcorrected between 0 and 24 h of ICU admission had a sodium increase between 11 and 12 mmol/L, with the remaining 64% experiencing an increase of >12 mmol/L on day one. A total of 56% of 32 patients overcorrected between 24 and 48 h of ICU admission experienced a sodium increase of 9–10 mmol/L, with the remaining 44% having a rise of >10 mmol/L and 25% of patients experiencing a rise of >15 mmol/L. A total of 50% of 22 patients overcorrected from 0 to 48 h experienced a sodium increase of 19–20 mmol/L, with the remaining having a sodium increase >20 mmol/L.
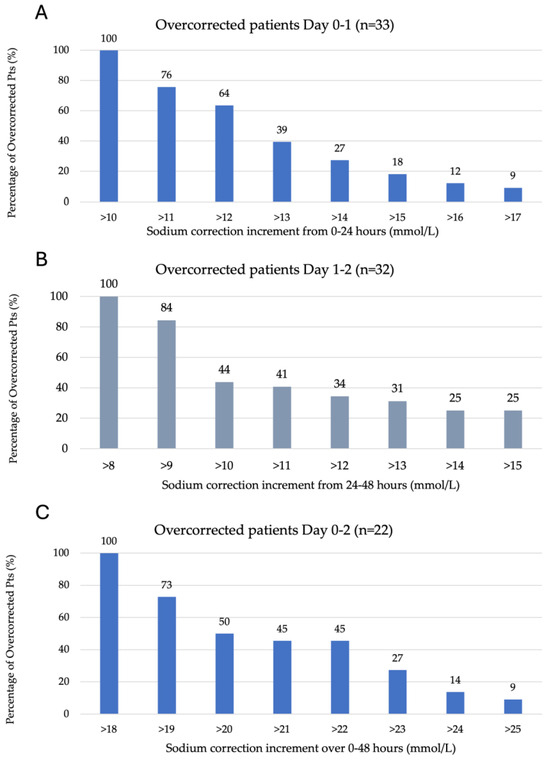
Figure 1.
Serum sodium kinetics over the initial 48 h of ICU admission. (A) Overcorrection by day one (0–24 h), defined as >10 mmol/L (n = 33). (B) Overcorrection on day two (24–48 h) is defined as >8 mmol/L (n = 32). (C) Overcorrection from admission to day two (0–48 h) is defined as >18 mmol/L (n = 22).
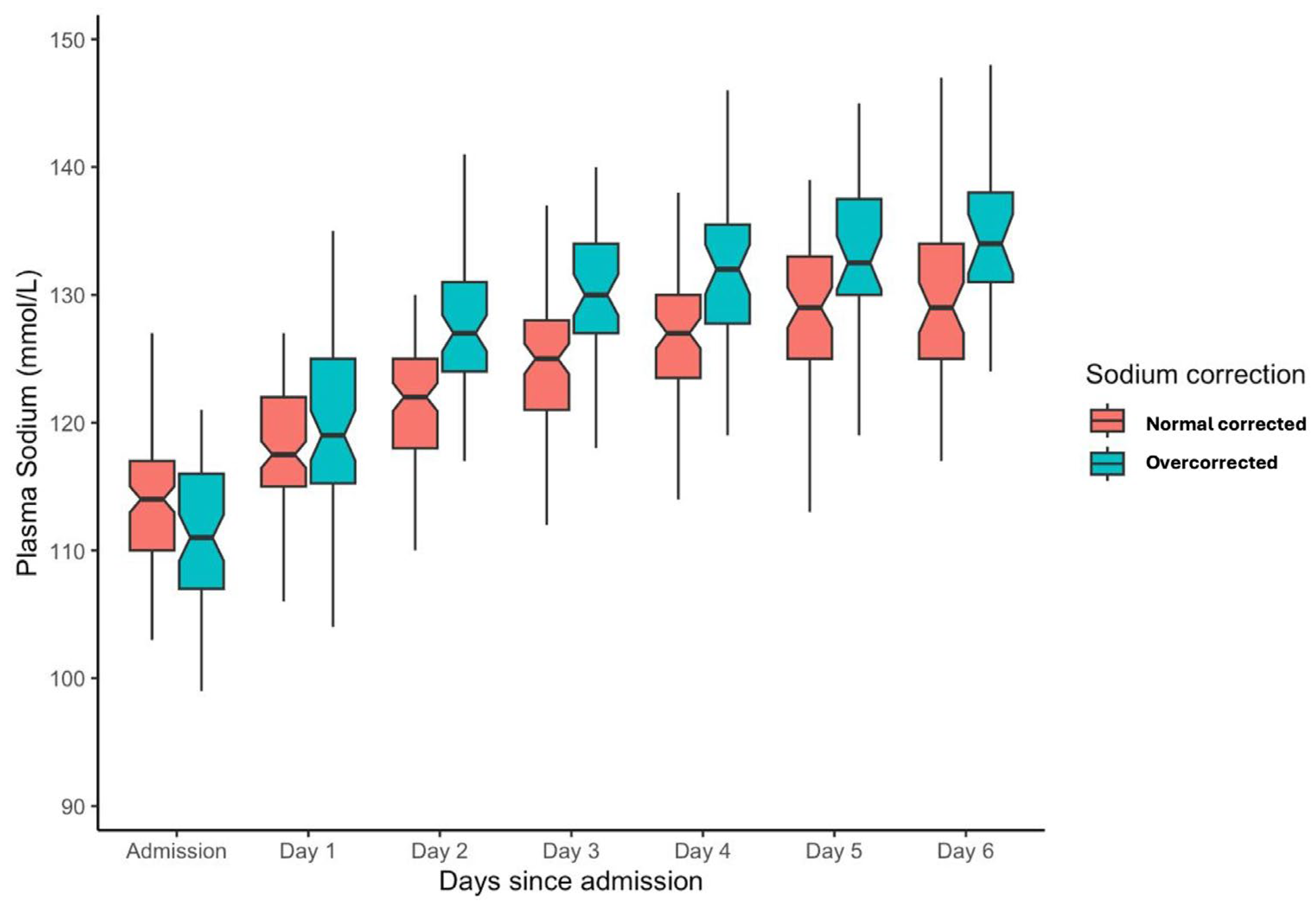
The sodium correction rate for the entire population over the initial 48 h of admission was a median of 10 mmol/L (IQR: 5–15 mmol/L). For the overcorrection cohort, the median serum sodium increment from 0 to 48 h was 17 mmol/L (IQR: 15–20 mmol/L), and for normal correction 7 mmol/L (IQR: 1–11 mmol/L). From admission to one week, serum sodium levels improved from a median of 113 (IQR 108, 117) to 131 (IQR 126, 135). Stratification into overcorrection or normal correction cohorts is depicted in Figure 2.
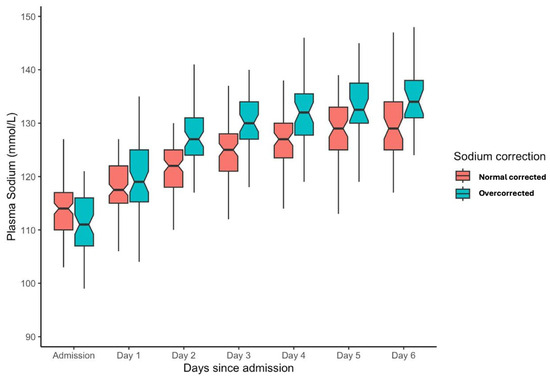
Figure 2.
Box plot for serum sodium level from admission for the initial week of ICU admission stratified into overcorrection and normal correction cohorts.
3.2. Risk Factors for Overcorrection at 48 h
Factors associated with overcorrection at 48 h from univariable analysis were performed (Table S3). The multivariable model found lower admission sodium value was an independent risk associated with overcorrection at 48 h, whereas liver cirrhosis and use of fluid restriction were independently associated with normal correction within 48 h (Table 2).

Table 2.
Multivariable analysis was deduced from the backward selection using the Akaike Information Criterion method.
3.3. Length of Stay
ICU length of stay for the entire cohort was a median of 3 days (IQR 2–4). While there was no difference in the ICU length of stay between these two groups, there was a trend towards shorter overall hospital stay for patients who had overcorrection of sodium levels at 48 h [8 days (IQR 6–17) versus 12 days (IQR 7–22), p = 0.084] (Table 3).

Table 3.
ICU and hospital length of stay.
3.4. Survival and ODS
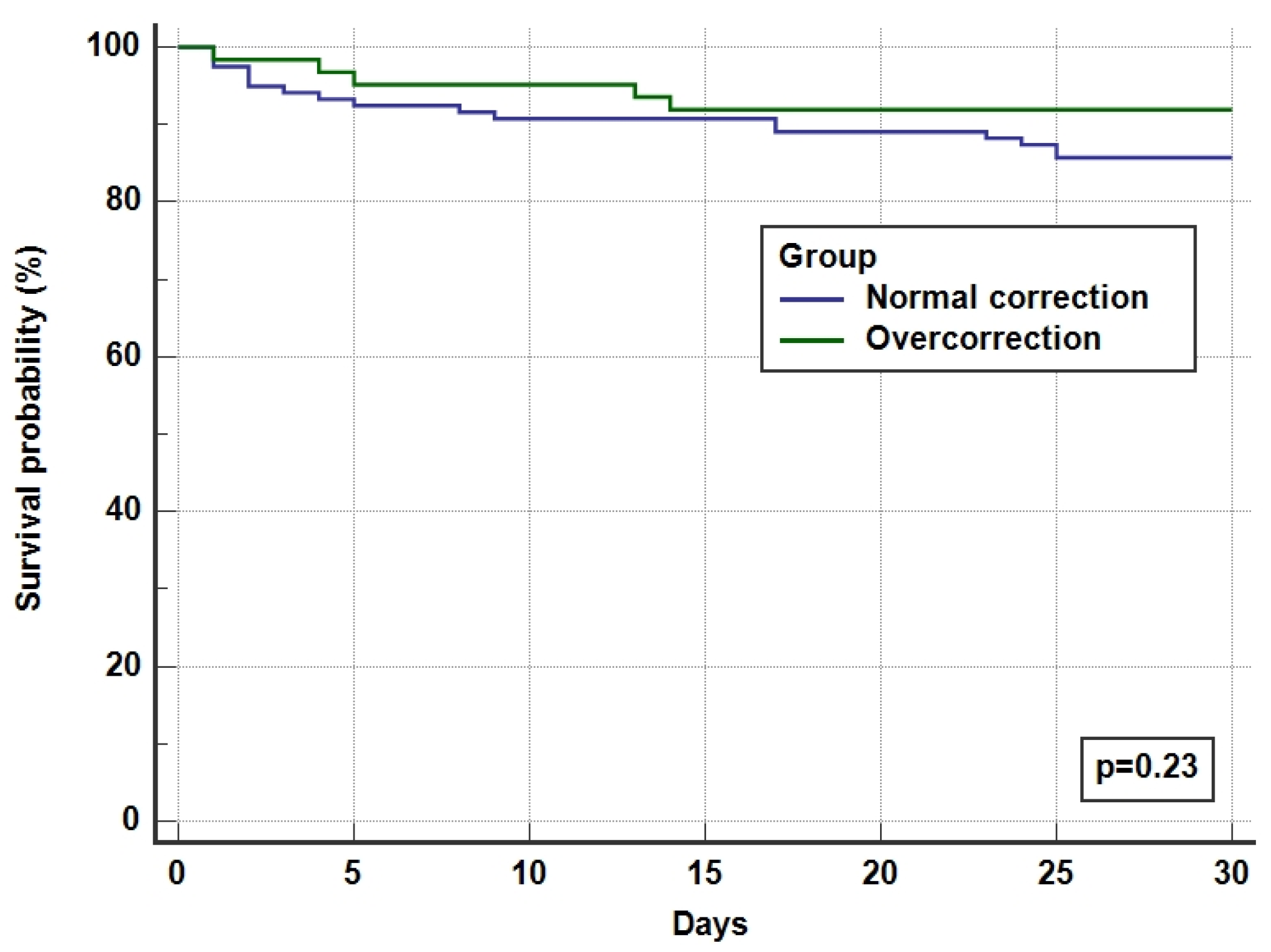
The overall ICU and hospital survival for the whole cohort was 92% and 87%, respectively, with no difference between overcorrected and normal corrected groups (Table 4 and Figure 3). There was one incidence (0.55%) of ODS in the overcorrection group who had recovered after a period of neurorehabilitation.

Table 4.
ICU and hospital survival.
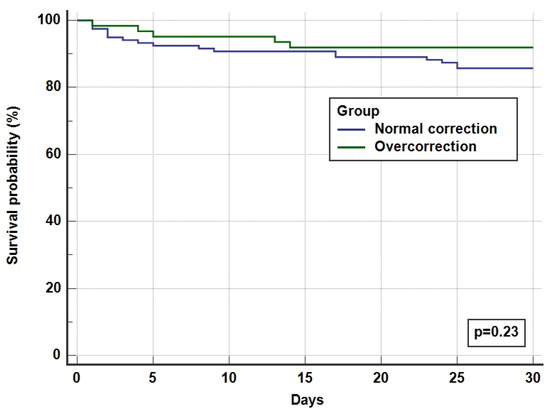
Figure 3.
Kaplan Meier survival curves for 30-day hospital survival.
4. Discussion
In this retrospective cohort study of severe hyponatraemia in the ICU, we reviewed clinical data to determine the incidence and risk factors for overcorrection within 48 h. We also reviewed associations between overcorrection, length of stay, and mortality. We also determined the incidence of ODS in our cohort. We found that 34% of patients met the criteria for overcorrection at 48 h, and lower admission sodium value was an independent risk factor for overcorrection. Furthermore, pre-admission liver cirrhosis and the use of fluid restriction were independent risk factors for normal correction. We identified no statistically significant difference in ICU or hospital length of stay or mortality, although a non-significant trend towards longer hospital length of stay in the normally corrected cohort was noted. ODS was present in 0.55% of severe hyponatremic patients and occurred following overcorrection within 48 h in a patient with risk factors for ODS. To the best of our knowledge, this is the largest UK observation of severe hyponatraemia in ICU patients.
The correction rate of hyponatraemia is based upon several factors, including neurohormonal composition, degree of critical illness, treatment choice, the chronicity of hyponatraemia, and aetiology [10,11,12]. The overcorrection incidence of hyponatraemia is variable within the published literature, occurring in 15–48% of patients [13,14], with the exact figure being dependent upon variable definitions of hyponatraemia and overcorrection. Studies where overcorrection was defined using a higher threshold (>12 mmol/L/day) revealed a lower incidence of overcorrection (14–20.8%) [13,15,16,17], whereas a lower threshold definition (>8 mmol/L/day) revealed a higher incidence (41–44%) [18,19]. Furthermore, where the hyponatraemia definition was set to a higher value (>125 to >130 mmol/L), the incidence of overcorrection was lower (14–19.6%) [16,17]. We found that lower admission sodium was an independent risk factor for overcorrection, a result concordant with other reports [13,16,18], which might explain why studies including more severe hyponatraemic patients report a higher incidence of overcorrection. Study designs similar to this report for both overcorrection and hyponatraemia definitions reveal an incidence between 27.9 and 44.9% [18,20,21], which is in keeping with our findings.
We identified that known liver cirrhosis was independently associated with a normal sodium correction rate. This association is possibly related to the large extravascular fluid volumes that occur in severe liver disease, resulting in a more treatment-resistant state or possibly less use of hypertonic saline with a more fluid-restrictive strategy. George et al. identified that the Charlson Comorbidity Index score (which contains liver disease) was independently associated with overcorrection; however, no breakdown into the score constituents was performed [18].
With regard to treatments utilised for sodium correction, the use of fluid restriction was associated with the normal correction. This is intuitive when one considers that fluid restriction manipulates the ADH response, causing a reduction in water retention in the renal tubules. This process, in comparison to directly administering intravenous sodium chloride, is slower at improving serum sodium concentrations [22,23].
European and American guidelines advocate the use of hypertonic saline infusions/boluses in hyponatraemia with severe symptoms [5,24]. Although efficacious, the effect of hypertonic saline on serum sodium concentration is unpredictable and risks overcorrection [8,25]. We identified that overcorrected patients were more likely to present with severe symptoms (seizures/arrythmias) and were more likely to be treated with 2.7% saline infusions. Concordant with our results, a large cohort study (n = 1490) identified that the use of hypertonic saline was associated with overcorrection in a univariate analysis (p < 0.01), although this was not significant in multivariable models [18]. Some studies have demonstrated a clinical benefit of using desmopressin alongside hypertonic saline infusions to facilitate a safe and controlled rise in serum sodium concentration [26,27]; however, a recent randomised controlled trial demonstrated no meaningful differences in sodium correction rate, symptom control, length of stay, ODS, or mortality compared to placebo [28].
ODS is a rare yet devastating consequence of hyponatraemia overcorrection and may result in severe neurological disability and death in 33–55% of cases [29]. The exact incidence of ODS is not known, although observational studies of hyponatraemic patients demonstrate an incidence of 0.2–2% [4,13,18,21,30]. This value is not dissimilar to our findings of 0.55%. Risk factors for ODS include malnutrition, chronic alcoholism and liver cirrhosis, hypokalaemia, hypophosphatemia, and central nervous system hypoxia [5,31,32,33]. The patient we identified who developed ODS within our overcorrection cohort presented with such risk factors.
While our study revealed no difference in ICU length of stay, there was a trend towards prolonged hospital length of stay with normal sodium correction (p = 0.087). In a small cohort study of 67 patients, Giordano et al. showed significantly reduced length of stay in the overcorrected cohort vs. normal corrected patients (3.8 days ± 0.4 vs. 10.7 days ± 0.7) [14]. Similarly, Geoghegan et al. evaluated 412 hyponatraemic patients, highlighting a significant increase in hospital length of stay in under-corrected patients (0–5 mmol/L/day) in multivariable analysis [21]. Other studies of a similar cohort size to this report demonstrate non-superiority of normal correction [17,34]. Given the known deleterious effects of prolonged hospital stays both on patients and healthcare providers, such associations should be investigated further, particularly in those with less risk of developing ODS [35].
We demonstrated no significant mortality difference between the over- and normal corrected cohorts in our report, concordant with another study of a similar size [17]. However, a smaller cohort study conducted in the United Kingdom showed a large discrepancy in hospital mortality between overcorrected, normally corrected, and non-responding patients in favour of overcorrection (2.1% vs. 4.5% vs. 20%, respectively) [20]. Larger studies have further demonstrated this mortality benefit [14,18,19,36]. This survival benefit may be explained by less prolonged exposure to detrimentally lower serum sodium concentrations; however, it is important to consider that poorly responsive or unresponsive hyponatraemia may, in fact, be a consequence of a more severe and resistant disease state, which may confound both lengths of stay and mortality outcomes.
It is well established that severe hyponatraemia is associated with poor patient outcomes, regardless of aetiology [4,37]. However, the current guidelines for correction rate in hyponatraemia are designed to prevent neurological complications and ODS [5,24]. Given the presented data and summary of the literature, such complications are rare, associated with clear risk factors, and, in some reports, are not associated with overcorrection at all [19]. In fact, reduced mortality, improved survival, and reduced length of stay should call into question whether overcorrection is preferable in selective groups of patients with minimal risk of ODS. Perhaps a more personalised target should be applied. Ayus and Moritz (2023) suggested that the current sodium correction targets are restrictive and prohibit the use of effective hypertonic saline treatment. They, therefore, propose a more liberal target of 15–20 mmol/L/day; however, at present, no research has been conducted to prove both the efficacy and safety of this target [38]. Sterns et al., in a recent report, reiterate the potential risk of ODS in severe hyponatraemic patients with overcorrection. The authors note the possibility of underreporting given that magnetic resonance imaging (MRI) is not 100% sensitive for ODS diagnosis and correct coding is not always achieved, meaning true ODS patients are potentially missed in retrospective analyses [39]. Furthermore, in reports where ODS is identified in patients without an observed overcorrection, there is a risk that they may have already experienced an overcorrection event prior to hospital attendance. Overall, this highlights the need for a randomised trial evaluating different sodium correction targets before different treatment targets are advocated.
The main strength of our study was its comprehensive patient capture over six years of clinical practice. The patients are representative of hyponatraemia patients within an ICU setting, and the treatment options utilised are concordant with current applicable guidelines. We also utilised an overcorrection definition that was in accordance with European guidelines and inclusive of all patients overcorrected within 48 h. However, there are several limitations of this observational study that are worthy of note. This is pragmatic, real-world clinical practice data. Although treatment guidelines both locally and nationally were followed within this cohort, with a small population size from only a single centre, there is a risk of incongruency between the local practice used in our unit and elsewhere. Additionally, given the rarity of ODS within this population, it is difficult to perform any meaningful statistical analysis. This study was performed as a retrospective data analysis, meaning key clinical information has not been captured, including volume status on arrival to the hospital/ICU, a clear working diagnosis for hyponatraemia, and acute or chronic hyponatraemia status. There were additionally some missing data for serum and urine osmolarities, urine sodium, cortisol, and TSH levels; however, this has been documented clearly in the Supplementary Materials (Table S1).
5. Conclusions
This retrospective single-centre cohort study has identified that overcorrection of severe hyponatraemia is common and is more likely to occur in younger patients without liver cirrhosis, presenting with a lower admission serum sodium concentration and severe symptoms, and treated with 2.7% saline infusions. However, in multivariate analysis, only the admission of lower sodium levels was associated with overcorrection. ODS is rare and occurs in less than 1% of our cohort. Correction rates for hyponatraemia are designed to prevent ODS; however, with well-defined risk factors for ODS and the risk of prolonged length of stay and mortality with inadequate hyponatraemia management, future research should be directed towards determining whether a more liberal target for select severe hyponatraemia patients is safe and effective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina60091412/s1, Table S1:Missing data from baseline characteristics; Table S2: number of patients meeting each criteria of overcorrection within 48 h definition; Table S3: Univariate analysis for risk of overcorrection within 48 h.
Author Contributions
Conceptualization, A.D. and T.R.; methodology, T.R., M.B., A.J.R.W. and A.D.; formal analysis, T.R., M.B. and A.D.; data curation, T.R., B.-A.P., N.P., A.S., S.V., D.H. and R.B.; writing—original draft preparation, T.R.; writing—review and editing, T.R., A.J.R.W. and A.D.; visualization, T.R.; supervision, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki as part of the wider CRIT-CO study investigating the outcomes of patients with critical illness in our ICU. The CRIT-CO study is sponsored by the University Hospital Southampton NHS Foundation Trust (RHM CRI 0370), and ethical approval was obtained from the NHS Health Research Authority (IRAS 232922) on 26 November 2018.
Informed Consent Statement
The requirement for informed consent was waived due to the retrospective observational nature of this study.
Data Availability Statement
The study data are available upon request.
Acknowledgments
We acknowledge ICU trainees who helped with data collection for this study: Tarek Al Smadi, Elsie Hunter, and Eleanor Taylor.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeVita, M.V.; Gardenswartz, M.H.; Konecky, A.; Zabetakis, P.M. Incidence and etiology of hyponatremia in an intensive care unit. Clin. Nephrol. 1990, 34, 163–166. [Google Scholar]
- Sterns, R.H. Disorders of Plasma Sodium—Causes, Consequences, and Correction. N. Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Hyponatremia. N. Engl. J. Med. 2000, 342, 1581–1589. [Google Scholar] [CrossRef]
- Seethapathy, H.; Zhao, S.; Ouyang, T.; Passos, C.; Sarang, A.; Cheung, P.W.; Waikar, S.S.; Steele, D.J.R.; Kalim, S.; Allegretti, A.S.; et al. Severe Hyponatremia Correction, Mortality, and Central Pontine Myelinolysis. NEJM Evid. 2023, 2. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 2014, 29 (Suppl. 2), i1–i39. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Barth, J.; Levy, M. Society for Endocrinology Endocrine Emergency Medicine Guidance: Emergency management of severe symptomatic hyponatraemia in adult patients. Endocr. Connect. 2016, 5, G4–G6. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Zietse, R. Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines. J. Am. Soc. Nephrol. 2017, 28, 1340–1349. [Google Scholar] [CrossRef]
- Mohmand, H.K.; Issa, D.; Ahmad, Z.; Cappuccio, J.D.; Kouides, R.W.; Sterns, R.H. Hypertonic Saline for Hyponatremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 1110–1117. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Rafat, C.; Flamant, M.; Gaudry, S.; Vidal-Petiot, E.; Ricard, J.D.; Dreyfuss, D. Hyponatremia in the intensive care unit: How to avoid a Zugzwang situation? Ann. Intensive Care 2015, 5, 39. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Tucker, B.M.; Madias, N.E. Diagnosis and Management of Hyponatremia: A Review. JAMA 2022, 328, 280–291. [Google Scholar] [CrossRef]
- Sterns, R.H.; Hix, J.K.; Silver, S.M. Management of Hyponatremia in the ICU. Chest 2013, 144, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Wong, R.; Hamblin, P.S.; Zajac, J.; Grossmann, M. Patients Presenting with Severe Hypotonic Hyponatremia: Etiological Factors, Assessment, and Outcomes. Hosp. Pract. 2009, 37, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Ciarambino, T.; Priore, E.L.; Castellino, P.; Malatino, L.; Cataliotti, A.; Paolisso, G.; Adinolfi, L.E. Serum sodium correction rate and the outcome in severe hyponatremia. Am. J. Emerg. Med. 2017, 35, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yoon, S.; Kim, E.J.; Seo, J.W.; Koo, J.-R.; Oh, Y.K.; Jo, Y.H.; Kim, S.; Baek, S.H. Risk factors for overcorrection of severe hyponatremia: A post hoc analysis of the SALSA trial. Kidney Res. Clin. Pract. 2022, 41, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Aratani, S.; Hara, M.; Nagahama, M.; Taki, F.; Futatsuyama, M.; Tsuruoka, S.; Komatsu, Y. A low initial serum sodium level is associated with an increased risk of overcorrection in patients with chronic profound hyponatremia: A retrospective cohort analysis. BMC Nephrol. 2017, 18, 316. [Google Scholar] [CrossRef]
- Pham, C.T.; Kassab, H.S.; Johnston, J.P. Evaluation of Serum Sodium Correction Rates for Management of Hyponatremia in Hospitalized Patients. Ann. Pharmacother. 2022, 56, 131–138. [Google Scholar] [CrossRef]
- George, J.C.; Zafar, W.; Bucaloiu, I.D.; Chang, A.R. Risk Factors and Outcomes of Rapid Correction of Severe Hyponatremia. Clin. J. Am. Soc. Nephrol. 2018, 13, 984–992. [Google Scholar] [CrossRef]
- Kinoshita, T.; Mlodzinski, E.; Xiao, Q.; Sherak, R.; Raines, N.H.; Celi, L.A. Effects of correction rate for severe hyponatremia in the intensive care unit on patient outcomes. J. Crit. Care 2023, 77, 154325. [Google Scholar] [CrossRef]
- Arshad, M.F.; Iqbal, A.; Weeks, J.; Fonseca, I.; Munir, A.; Bennet, W. Hypertonic saline for severe symptomatic hyponatraemia: Real-world findings from the UK. Endocr. Connect. 2022, 11, e220007. [Google Scholar] [CrossRef]
- Geoghegan, P.; Harrison, A.M.; Thongprayoon, C.; Kashyap, R.; Ahmed, A.; Dong, Y.; Rabinstein, A.A.; Kashani, K.B.; Gajic, O. Sodium Correction Practice and Clinical Outcomes in Profound Hyponatremia. Mayo Clin. Proc. 2015, 90, 1348–1355. [Google Scholar] [CrossRef]
- Krisanapan, P.; Vongsanim, S.; Pin-On, P.; Ruengorn, C.; Noppakun, K. Efficacy of Furosemide, Oral Sodium Chloride, and Fluid Restriction for Treatment of Syndrome of Inappropriate Antidiuresis (SIAD): An Open-label Randomized Controlled Study (The EFFUSE-FLUID Trial). Am. J. Kidney Dis. 2020, 76, 203–212. [Google Scholar] [CrossRef]
- Garrahy, A.; Galloway, I.; Hannon, A.M.; Dineen, R.; O’kelly, P.; Tormey, W.P.; O’reilly, M.W.; Williams, D.J.; Sherlock, M.; Thompson, C.J. Fluid Restriction Therapy for Chronic SIAD; Results of a Prospective Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2020, 105, e4360–e4369. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G.; Goldsmith, S.R.; Greenberg, A.; Korzelius, C.; Schrier, R.W.; Sterns, R.H.; Thompson, C.J. Diagnosis, Evaluation, and Treatment of Hyponatremia: Expert Panel Recommendations. Am. J. Med. 2013, 126, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Achinger, S.G.; Ayus, J.C. Treatment of Hyponatremic Encephalopathy in the Critically ill. Crit. Care Med. 2017, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.K.; Marino, K.K.; DeGrado, J.R.; Szumita, P.M.; Dube, K.M. Evaluation of Desmopressin in Critically Ill Patients with Hyponatremia Requiring 3% Hypertonic Saline. Am. J. Med. Sci. 2021, 361, 711–717. [Google Scholar] [CrossRef]
- Jingushi, N.; Tsuzuki, S.; Fujii, K.; Uenishi, N.; Iwata, M.; Terasawa, T. Association of intranasal desmopressin therapy with overcorrection of severe hyponatremia: A retrospective, propensity score-based, single-center cohort study. J. Crit. Care 2021, 64, 53–61. [Google Scholar] [CrossRef]
- Pakchotanon, K.; Kanjanasuphak, N.; Chuasuwan, A.; Gojaseni, P.; Chittinandana, A. Safety and efficacy of proactive versus reactive administration of desmopressin in severe symptomatic hyponatremia: A randomized controlled trial. Sci. Rep. 2024, 14, 7487. [Google Scholar] [CrossRef]
- Lambeck, J.; Hieber, M.; Dreßing, A.; Niesen, W.-D. Central Pontine Myelinosis and Osmotic Demyelination Syndrome. Dtsch. Aerzteblatt Online 2019, 116, 600–606. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, T.E.; Shin, S.; Topf, J.; Kwan, J.L.; Weinerman, A.; Tang, T.; Raissi, A.; Koppula, R.; Razak, F.; Verma, A.A.; et al. Osmotic Demyelination Syndrome in Patients Hospitalized with Hyponatremia. NEJM Evid. 2023, 2. [Google Scholar] [CrossRef]
- Ambati, R.; Kho, L.K.; Prentice, D.; Thompson, A. Osmotic demyelination syndrome: Novel risk factors and proposed pathophysiology. Intern. Med. J. 2023, 53, 1154–1162. [Google Scholar] [CrossRef]
- Ayus, J.C.; Arieff, A.I. Chronic Hyponatremic Encephalopathy in Postmenopausal Women. JAMA 1999, 281, 2299–2304. [Google Scholar] [CrossRef]
- Ayus, J.C.; Krothapalli, R.K.; Arieff, A.I. Treatment of Symptomatic Hyponatremia and Its Relation to Brain Damage. N. Engl. J. Med. 1987, 317, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Pelouto, A.; Refardt, J.C.; Christ-Crain, M.; Zandbergen, A.A.M.; Hoorn, E.J. Overcorrection and undercorrection with fixed dosing of bolus hypertonic saline for symptomatic hyponatremia. Eur. J. Endocrinol. 2023, 188, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Murray, P.; Robin, J.; Wilkinson, P.; Fluck, D.; Fry, C.H. Evaluation of the Association of Length of Stay in Hospital and Outcomes. Int. J. Qual. Health Care 2021, 34. [Google Scholar] [CrossRef]
- Darmon, M.; Pichon, M.; Schwebel, C.; Ruckly, S.; Adrie, C.; Haouache, H.; Azoulay, E.; Bouadma, L.; Clec’h, C.; Garrouste-Orgeas, M.; et al. Influence of Early Dysnatremia Correction on Survival of Critically ill Patients. Shock 2014, 41, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gu, S.; Parikh, A.; Radhakrishnan, J. Prevalence of Hyponatremia and Association with Mortality: Results from NHANES. Am. J. Med. 2013, 126, 1127–1137.e1. [Google Scholar] [CrossRef] [PubMed]
- Ayus, J.C.; Moritz, M.L. Hyponatremia Treatment Guidelines—Have They Gone Too Far? NEJM Evid. 2023, 2. [Google Scholar] [CrossRef]
- Sterns, R.H.; Rondon-Berrios, H.; Adrogué, H.J.; Berl, T.; Burst, V.; Cohen, D.M.; Christ-Crain, M.; Cuesta, M.; Decaux, G.; Emmett, M.; et al. Treatment Guidelines for Hyponatremia: Stay the Course. Clin. J. Am. Soc. Nephrol. 2023, 19, 129–135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).