Evaluating the Effectiveness of Proton Beam Therapy Compared to Conventional Radiotherapy in Non-Metastatic Rectal Cancer: A Systematic Review of Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Protocol and Registration
2.2. Literature Search
2.3. Inclusion and Exclusion Criteria
2.4. Literature Screening
2.5. Outcomes
2.6. Data Extraction
2.7. Quality and Risk of Bias Assessment
2.8. Data Synthesis
2.9. Subgroup Analysis
3. Results
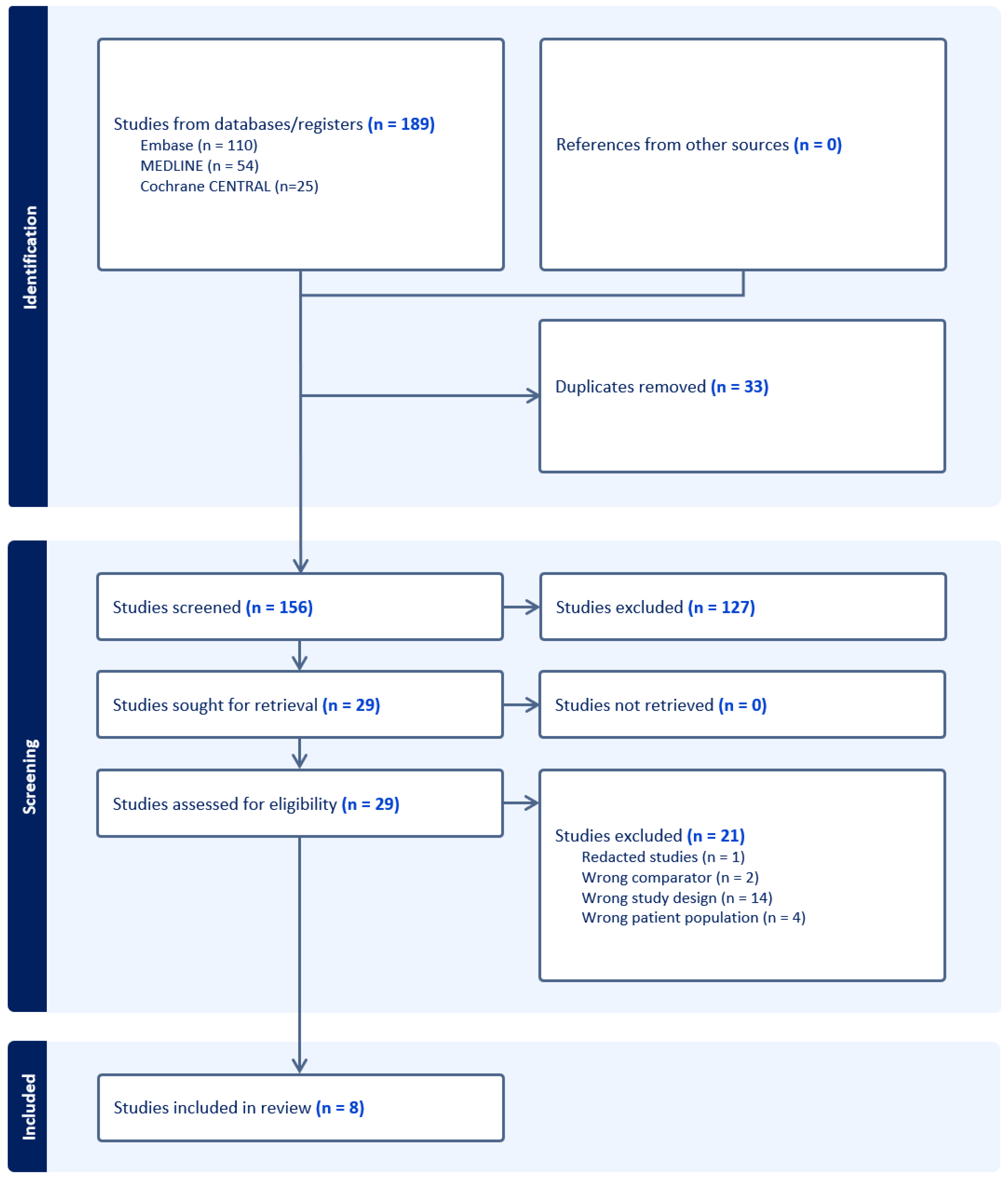
3.1. The Results of the Literature Search
3.2. Overview of Included Studies
3.3. Adverse Treatment Outcomes
3.3.1. Acute Toxicity
3.3.2. Late Toxicity
3.3.3. Radiation Exposure from Dosimetric Analyses
Pelvis
Sacrum
Bowel
Bladder
Other Structures
Models for Tumour Control
3.4. Oncological Outcomes
3.4.1. Local and Distant Recurrence
3.4.2. Overall Survival
3.4.3. Progression-Free Survival
3.5. Results from Quality and Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Query
- exp Rectal Neoplasms/
- ((rectal or rectum or recti or pararect* or anal or anus or perianal) adj5 (adenoma* or cancer* or carcinoma* or tumo* or malign* or neoplas* or mass)).tw,kf.
- 1 or 2
- Proton Therapy/
- exp Radiotherapy, Computer-Assisted/
- PBT.tw,kf.
- XRT.tw,kf.
- photon*.tw,kf.
- proton*.tw,kf.
- 4 or 6 or 9
- 5 or 7 or 8
- 3 and 10 and 11
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Bhudia, J.; Glynne-Jones, R. The Evolving Neoadjuvant Treatment Paradigm for Patients with Locoregional mismatch Repair Proficient Rectal Cancer. Curr. Treat. Options Oncol. 2022, 23, 453–473. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Nam, B.-H.; Kim, K.-P.; Kim, J.E.; Park, S.J.; Park, Y.S.; Park, J.O.; Kim, S.Y.; Kim, J.H.; Ahn, J.B.; et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): An open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014, 15, 1245–1253. [Google Scholar] [CrossRef]
- Martling, A.; Holm, T.; Johansson, H.; Rutqvist, L.; Cedermark, B. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: Long-term follow-up of a population-based study. Cancer 2001, 92, 896–902. [Google Scholar] [CrossRef]
- Figueiredo, N.; Panteleimonitis, S.; Popeskou, S.; Cunha, J.F.; Qureshi, T.; Beets, G.L.; Heald, R.J.; Parvaiz, A. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur. J. Surg. Oncol. 2018, 44, 484–489. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.R.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro Jr, U.; e Sousa Jr, A.H.S.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–718. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Fok, M.; Toh, S.; Easow, J.; Fowler, H.; Clifford, R.; Parsons, J.; Vimalachandran, D. Proton beam therapy in rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2021, 38, 101638. [Google Scholar] [CrossRef] [PubMed]
- Brændengen, M.; Tveit, K.M.; Bruheim, K.; Cvancarova, M.; Berglund; Glimelius, B. Late Patient-Reported Toxicity after Preoperative Radiotherapy or Chemoradiotherapy in Nonresectable Rectal Cancer: Results From a Randomized Phase III Study. Int. J. Radiat. Oncol. 2011, 81, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, R.; Zhang, Z.; Li, T.; Li, F.; Liu, H.; Li, G. Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: A systematic review and meta-analysis of randomized controlled trials. Color. Dis. 2016, 18, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.; Calais, G.; Daban, A.; Berger, C.; Radosevic-Jelic, L.; Maingon, P.; Bardet, E.; Pierart, M.; Briffaux, A.; Group, E.R. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: Assessment of acute toxicity and treatment compliance: Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur. J. Cancer 2004, 40, 219–224. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.-M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.J.; Rashid, A.; Shim, J.S.C.; West, J.; Humes, D.J.; Grainge, M.J. Long-term adverse effects and healthcare burden of rectal cancer radiotherapy: Systematic review and meta-analysis. ANZ J. Surg. 2023, 93, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R. A review of proton therapy—Current status and future directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Gaito, S.; Aznar, M.; Burnet, N.; Crellin, A.; France, A.; Indelicato, D.; Kirkby, K.; Pan, S.; Whitfield, G.; Smith, E. Assessing Equity of Access to Proton Beam Therapy: A Literature Review. Clin. Oncol. 2023, 35, e528–e536. [Google Scholar] [CrossRef]
- Vitti, E.T.; Parsons, J.L. The Radiobiological Effects of Proton Beam Therapy: Impact on DNA Damage and Repair. Cancers 2019, 11, 946. [Google Scholar] [CrossRef]
- Levin, W.; Kooy, H.; Loeffler, J.S.; Delaney, T.F. Proton beam therapy. Br. J. Cancer 2005, 93, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Jiang, L.; Cui, X.; Zhang, J.; Yu, J. Proton beam therapy for cancer in the era of precision medicine. J. Hematol. Oncol. 2018, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Barsky, A.R.; Reddy, V.K.; Plastaras, J.P.; Ben-Josef, E.; Metz, J.M.; Wojcieszynski, A.P. Proton beam re-irradiation for gastrointestinal malignancies: A systematic review. J. Gastrointest. Oncol. 2020, 11, 187–202. [Google Scholar] [CrossRef]
- Leeman, J.E.; Romesser, P.B.; Zhou, Y.; McBride, S.; Riaz, N.; Sherman, E.; Cohen, M.A.; Cahlon, O.; Lee, N. Proton therapy for head and neck cancer: Expanding the therapeutic window. Lancet Oncol. 2017, 18, e254–e265. [Google Scholar] [CrossRef] [PubMed]
- Vaios, E.J.; Wo, J.Y. Proton beam radiotherapy for anal and rectal cancers. J. Gastrointest. Oncol. 2020, 11, 176–186. [Google Scholar] [CrossRef]
- Rombi, B.; Ares, C.; Hug, E.B.; Schneider, R.; Goitein, G.; Staab, A.; Albertini, F.; Bolsi, A.; Lomax, A.J.; Timmermann, B. Spot-Scanning Proton Radiation Therapy for Pediatric Chordoma and Chondrosarcoma: Clinical Outcome of 26 Patients Treated at Paul Scherrer Institute. Int. J. Radiat. Oncol. 2013, 86, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; van Rossum, P.S.; Chu, Y.; Hobbs, B.P.; Grassberger, C.; Hong, T.S.; Liao, Z.; Yang, J.; Zhang, X.; Netherton, T.; et al. Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Comprehensive Analysis of Randomized Phase 2B Trial of Proton Beam Therapy Versus Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. 2024, 118, 368–377. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lin, K.-C.; Chen, W.-M.; Shia, B.-C.; Wu, S.-Y. Comparing the oncologic outcomes of proton therapy and intensity-modulated radiation therapy for head and neck squamous cell carcinoma. Radiother. Oncol. 2024, 190, 109971. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The Prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel Cd Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Colaco, R.J.; Nichols, R.C.; Huh, S.; Getman, N.; Ho, M.W.; Li, Z.; Morris, C.G.; Mendenhall, W.M.; Mendenhall, N.P.; Hoppe, B.S. Protons offer reduced bone marrow, small bowel, and urinary bladder exposure for patients receiving neoadjuvant radiotherapy for resectable rectal cancer. J. Gastrointest. Oncol. 2014, 5, 388. [Google Scholar]
- Isacsson, U.; Montelius, A.; Jung, B.; Glimelius, B. Comparative treatment planning between proton and X-ray therapy in locally advanced rectal cancer. Radiother. Oncol. 1996, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, C.J.; Jørgensen, J.B.; Petersen, J.B.; Jensen, L.N.; Iversen, L.H.; Pedersen, B.G.; Spindler, K.-L.G. Pelvic insufficiency fractures, dose volume parameters and plan optimization after radiotherapy for rectal cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Ludmir, E.B.; Polamraju, P.; Williamson, T.; Melkun, M.M.; Herman, J.D.; Krishnan, S.; Koay, E.J.; Koong, A.C.; Minsky, B.D.; et al. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin. Transl. Radiat. Oncol. 2019, 19, 59–65. [Google Scholar] [CrossRef]
- Pedone, C.; Sorcini, B.; Staff, C.; Färlin, J.; Fokstuen, T.; Frödin, J.-E.; Nilsson, P.J.; Martling, A.; Valdman, A. Preoperative short-course radiation therapy with PROtons compared to photons in high-risk RECTal cancer (PRORECT): Initial dosimetric experience. Clin. Transl. Radiat. Oncol. 2023, 39, 100562. [Google Scholar] [CrossRef]
- Radu, C.; Norrlid, O.; Brændengen, M.; Hansson, K.; Isacsson, U.; Glimelius, B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013, 52, 528–537. [Google Scholar] [CrossRef]
- Rønde, H.S.; Kallehauge, J.F.; Kronborg, C.J.S.; Nyvang, L.; Rekstad, B.L.; Hanekamp, B.A.; Appelt, A.L.; Guren, M.G.; Spindler, K.L.S. Intensity modulated proton therapy planning study for organ at risk sparing in rectal cancer re-irradiation. Acta Oncol. 2021, 60, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.A.; Wagner, D.M.; Conradi, L.-C.; Hennies, S.; Ghadimi, M.; Hess, C.F.; Christiansen, H. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: A planning study with clinical implications. Radiother. Oncol. 2012, 102, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.T.; Both, S.; Sharkoski, T.; Goldrath, K.; Tochner, Z.; Apisarnthanarax, S.; Metz, J.M.; Plastaras, J.P. Proton Reirradiation of Recurrent Rectal Cancer: Dosimetric Comparison, Toxicities, and Preliminary Outcomes. Int. J. Part. Ther. 2014, 1, 2–13. [Google Scholar] [CrossRef]
- Jeans, E.B.; Jethwa, K.R.; Harmsen, W.S.; Neben-Wittich, M.; Ashman, J.B.; Merrell, K.W.; Giffey, B.; Ito, S.; Kazemba, B.; Beltran, C.; et al. Clinical Implementation of Preoperative Short-Course Pencil Beam Scanning Proton Therapy for Patients with Rectal Cancer. Adv. Radiat. Oncol. 2020, 5, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ofuya, M.; McParland, L.; Murray, L.; Brown, S.; Sebag-Montefiore, D.; Hall, E. Systematic review of methodology used in clinical studies evaluating the benefits of proton beam therapy. Clin. Transl. Radiat. Oncol. 2019, 19, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.K.; Patel, S.; Herman, J.M.; Wild, A.; Nagda, S.N.; Altoos, T.; Tunceroglu, A.; Azad, N.; Gearheart, S.; Moss, R.A.; et al. Intensity-Modulated Radiation Therapy for Rectal Carcinoma Can Reduce Treatment Breaks and Emergency Department Visits. Int. J. Surg. Oncol. 2012, 2012, 891067. [Google Scholar] [CrossRef]
- Samuelian, J.M.; Callister, M.D.; Ashman, J.B.; Young-Fadok, T.M.; Borad, M.J.; Gunderson, L.L. Reduced Acute Bowel Toxicity in Patients Treated With Intensity-Modulated Radiotherapy for Rectal Cancer. Int. J. Radiat. Oncol. 2012, 82, 1981–1987. [Google Scholar] [CrossRef]
- Sipaviciute, A.; Sileika, E.; Burneckis, A.; Dulskas, A. Late gastrointestinal toxicity after radiotherapy for rectal cancer: A systematic review. Int. J. Color. Dis. 2020, 35, 977–983. [Google Scholar] [CrossRef]
- Koroulakis, A.; Molitoris, J.; Kaiser, A.; Hanna, N.; Bafford, A.; Jiang, Y.; Bentzen, S.; Regine, W.F. Reirradiation for Rectal Cancer Using Pencil Beam Scanning Proton Therapy: A Single Institutional Experience. Adv. Radiat. Oncol. 2021, 6, 100595. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Ishikawa, H.; Murakami, M.; Nakamura, M.; Shimizu, S.; Enomoto, T.; Oda, T.; Mizumoto, M.; Nakai, K.; Okumura, T.; et al. Proton Beam Therapy for Local Recurrence of Rectal Cancer. Anticancer Res 2021, 41, 3589–3595. [Google Scholar] [CrossRef]
- Jankowski, M.; Bała, D.; Las-Jankowska, M.; Wysocki, W.; Nowikiewicz, T.; Zegarski, W. Overall treatment outcome—Analysis of long-term results of rectal cancer treatment on the basis of a new parameter. Arch. Med. Sci. 2020, 16, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, Y.; Suzuki, M.; Seto, I.; Azami, Y.; Machida, M.; Takayama, K.; Sulaiman, N.S.; Nakasato, T.; Kikuchi, Y.; Murakami, M.; et al. Proton beam reirradiation for locally recurrent rectal cancer patients with prior pelvic irradiation. J. Radiat. Res. 2024, 65, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tambas, M.; van der Laan, H.P.; Steenbakkers, R.J.; Doyen, J.; Timmermann, B.; Orlandi, E.; Hoyer, M.; Haustermans, K.; Georg, P.; Burnet, N.G.; et al. Current practice in proton therapy delivery in adult cancer patients across Europe. Radiother. Oncol. 2022, 167, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Raben, D.; Crawford, E.D.; Flaig, T.W.; Kessler, E.R.; Lam, E.T.; Maroni, P.; Pugh, T.J. Patient characterization and usage trends of proton beam therapy for localized prostate cancer in the United States: A study of the National Cancer Database. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 438–446. [Google Scholar] [CrossRef]
- Willmann, J.; Leiser, D.; Weber, D.C. Oncological Outcomes, Long-Term Toxicities, Quality of Life and Sexual Health after Pencil-Beam Scanning Proton Therapy in Patients with Low-Grade Glioma. Cancers 2023, 15, 5287. [Google Scholar] [CrossRef]
- Khong, J.; Tee, H.; Gorayski, P.; Le, H.; Penniment, M.; Jessop, S.; Hansford, J.; Penfold, M.; Green, J.; Skelton, K.; et al. Proton beam therapy in paediatric cancer: Anticipating the opening of the Australian Bragg Centre for Proton Therapy and Research. J. Med. Imaging Radiat. Oncol. 2023. [Google Scholar]

| Author | Year | Country of Publication | Study Design | Total Patients (n) | Age (Median; Range) | Sex (M:F) | Tumour Grade | Tumour Stage (UICC-TMN) | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Colaco et al. [34] | 2014 | USA | Comparative dosimetric study | 8 | NR | NR | T2-T3 | NR | PBT, 3DCRT, IMRT |
| Isacsson et al. [35] | 1996 | Sweden | Comparative dosimetric study | 6 | 60 (47–79) | 4:2 | T4 | III | PBT, X-ray, Mixed |
| Kronborg et al. [36] | 2020 | Denmark | Comparative dosimetric study | 9 | 69 (35–81) | 5:4 | T3-T4 | IIa-III | PBT, 3DCRT, IMRT, VMAT |
| Moningi et al. [37] | 2019 | USA | Comparative dosimetric study and retrospective single-arm non-randomised trial | 15 | 74 (55–91) | 8:7 | NR | NR | PBT, 3DCRTPBT reirridiation |
| Pedone et al. [38] | 2023 | Sweden | Comparative dosimetric study | 20 | 57 (36–73) | 12:8 | T2-T4 | IIb-III | PBT, VMAT |
| Radu et al. [39] | 2013 | Sweden | Comparative dosimetric study | 7 | 64 (52–75) | 6:1 | T4 | III | PBT, VMAT |
| Rønde et al. [40] | 2021 | Denmark, Norway | Comparative dosimetric study | 15 | NR | 8:7 | NR | NR | PBT, 3DCRT, |
| Wolff et al. [41] | 2012 | Germany | Retrospective comparative dosimetric study | 25 | 62.5 (44–73) | 15:10 | T2-T4 | IIa-III | PBT, 3DCRT, IMRT, RapidArc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, K.; Marchant, J.N.; Le, K.D.R. Evaluating the Effectiveness of Proton Beam Therapy Compared to Conventional Radiotherapy in Non-Metastatic Rectal Cancer: A Systematic Review of Clinical Outcomes. Medicina 2024, 60, 1426. https://doi.org/10.3390/medicina60091426
Le K, Marchant JN, Le KDR. Evaluating the Effectiveness of Proton Beam Therapy Compared to Conventional Radiotherapy in Non-Metastatic Rectal Cancer: A Systematic Review of Clinical Outcomes. Medicina. 2024; 60(9):1426. https://doi.org/10.3390/medicina60091426
Chicago/Turabian StyleLe, Kelvin, James Norton Marchant, and Khang Duy Ricky Le. 2024. "Evaluating the Effectiveness of Proton Beam Therapy Compared to Conventional Radiotherapy in Non-Metastatic Rectal Cancer: A Systematic Review of Clinical Outcomes" Medicina 60, no. 9: 1426. https://doi.org/10.3390/medicina60091426





