Current Challenges in Coronary Bifurcation Interventions
Abstract
:1. Introduction
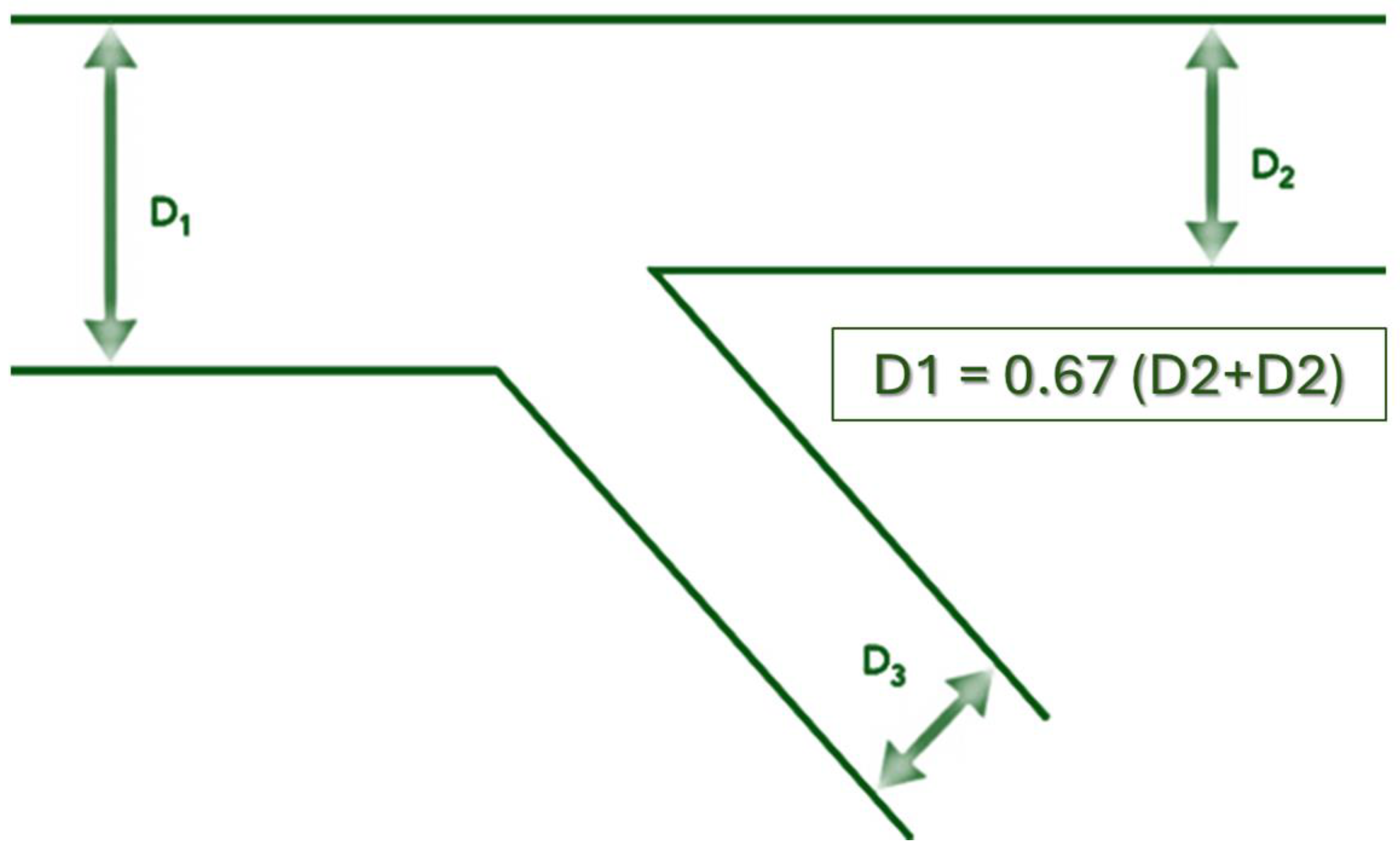
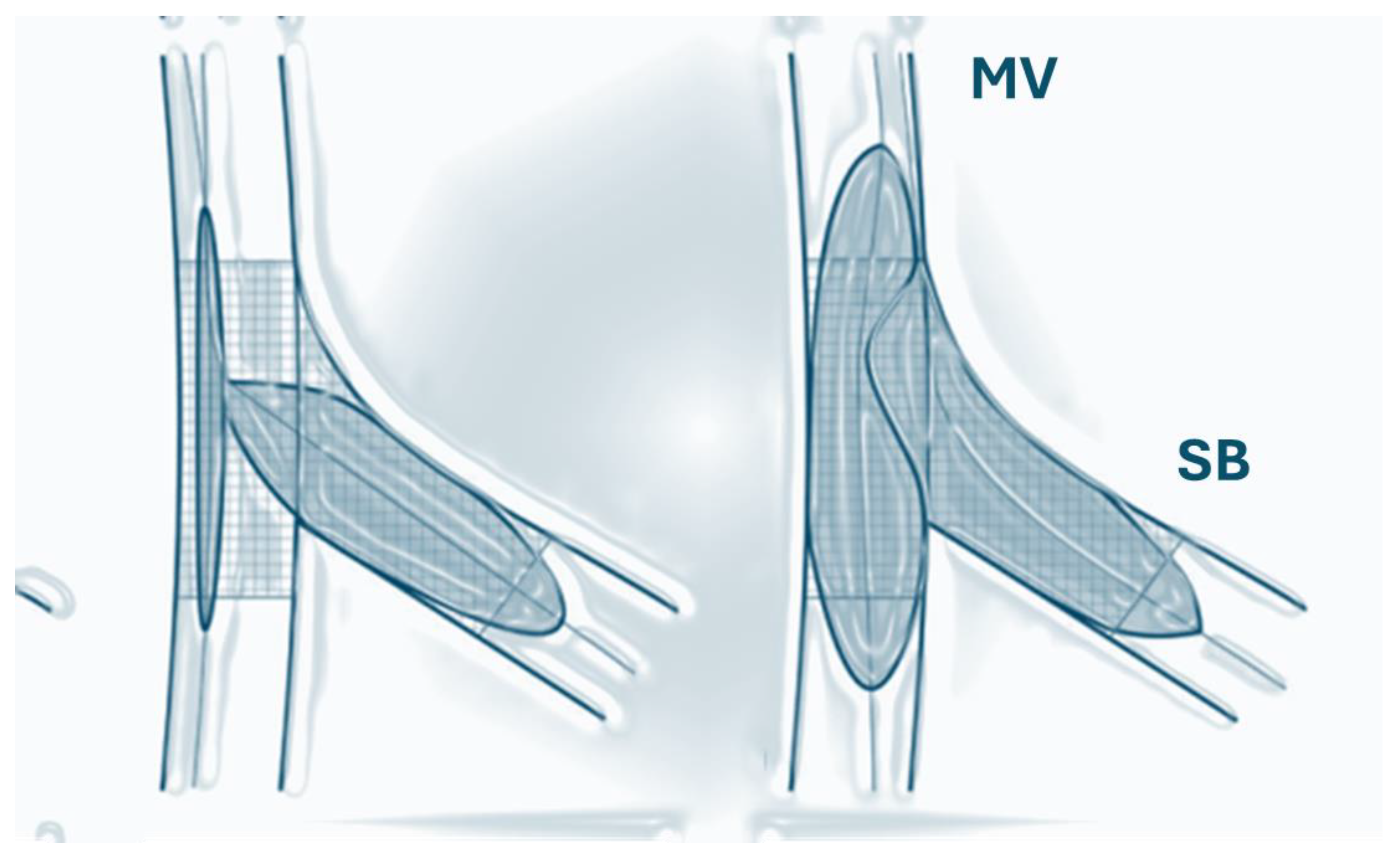
2. The Coronary Bifurcation Structure
3. Epidemiology
4. Side Branch Compromise
4.1. The Balloon Angioplasty Era
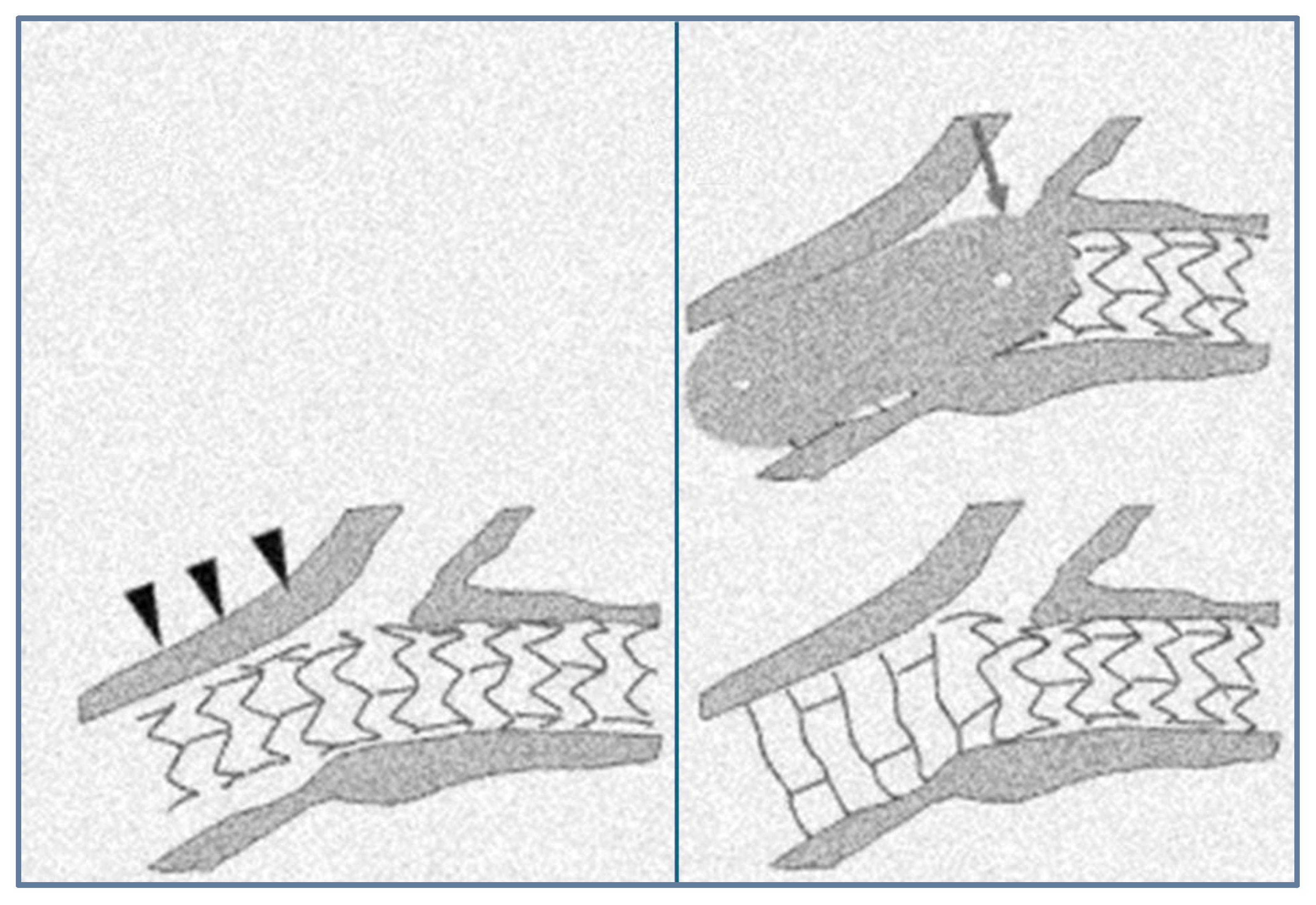
4.2. The Stent Era
- -
- -
- -
- -
- -
- -
- -
5. Restenosis in Bifurcation Lesions
6. Bifurcation Optimization Techniques
6.1. The Kissing Balloon Inflation (KBI) Technique
6.2. The Proximal Optimization Technique (POT)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burzotta, F.; Louvard, Y.; Lassen, J.F.; Lefèvre, T.; Finet, G.; Collet, C.; Legutko, J.; Lesiak, M.; Hikichi, Y.; Albiero, R.; et al. Percutaneous coronary intervention for bifurcation coronary lesions using optimised angiographic guidance: The 18th consensus document from the European Bifurcation Club. EuroIntervention 2024, 20, e915–e926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassilev, D.; Mileva, N.; Collet, C.; Nikolov, P.; Karamfiloff, K.; Naunov, V.; Sonck, J.; Hristova, I.; Georgieva, D.; Rigatelli, G.; et al. Determinants of functional significance of coronary bifurcation lesions and clinical outcomes after physiology-guided treatment. Int. J. Cardiol. Heart Vasc. 2021, 38, 100929. [Google Scholar] [CrossRef]
- Vassilev, D.; Mileva, N.; Panayotov, P.; Nikolov, P.; Dosev, L.; Karamfiloff, K.; Rigatelli, G.; Gil, R.J.; Stankovic, G.; Louvard, Y. Side branch predilatation during percutaneous coronary bifurcation intervention: Long-term mortality analysis. Kardiol. Pol. 2024, 82, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.D. The physiological principle of minimum work applied to the angle of branching of arteries. J. Gen. Physiol. 1926, 9, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Togawa, T. Optimal branching structure of the vascular tree. Bull. Math. Biophys. 1972, 34, 431–438. [Google Scholar] [CrossRef]
- Zamir, M. Cost analysis of arterial branching in the cardiovascular systems of man and animals. J. Theor. Biol. 1986, 120, 111–123. [Google Scholar] [CrossRef]
- Zamir, M.; Bigelow, D.C. Cost of departure from optimality in arterial branching. J. Theor. Biol. 1984, 109, 401–409. [Google Scholar] [CrossRef]
- Caro, C.G.; Fitz-Gerald, J.M.; Schroter, R.C. Arterial wall shear and distribution of early atheroma in man. Nature 1969, 223, 1159–1160. [Google Scholar] [CrossRef]
- Caro, C.G.; Fitz-Gerald, J.M.; Schroter, R.C. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfe mechanism for atherogenesis. Proc. R. Soc. Lond. B Biol. Sci. 1971, 177, 109–159. [Google Scholar]
- Kamiya, A.; Togawa, T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 1980, 239, H14–H21. [Google Scholar] [CrossRef]
- Vassilev, D.I.; Kassab, G.S.; Collet, C.; Gutiérrez-Chico, J.L.; Rigatelli, G.; Gil, R.J.; Serruys, P.W. Elliptical stretch as a cause of side branch ostial compromise after main vessel stenting in coronary bifurcations: New insights from numerical analysis. Cardiol. J. 2020, 27, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, D.; Mileva, N.; Panayotov, P.; Georgieva, D.; Koleva, G.; Collet, C.; Rigatelli, G.; Gil, R.J. A novel technique of proximal optimization with kissing balloon inflation in bifurcation lesions. Cardiol. J. 2022, 29, 899–905. [Google Scholar] [CrossRef]
- Weissman, N.J.; Sheris, S.J.; Chari, R.; Mendelsohn, F.O.; Anderson, W.D.; Breall, J.A.; Tanguay, J.F.; Diver, D.J. Intravascular ultrasonic analysis of plaque characteristics associated with coronary artery remodeling. Am. J. Cardiol. 1999, 84, 37–40. [Google Scholar] [CrossRef]
- Fujii, K.; Kobayashi, Y.; Mintz, G.S.; Hirose, M.; Moussa, I.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kreps, E.; Collins, M.; et al. Dominant contribution of negative remodeling to development of significant coronary bifurcation narrowing. Am. J. Cardiol. 2003, 92, 59–61. [Google Scholar] [CrossRef]
- Reidy, M.A.; Bowyer, D.E. Scanning electron microscopy of arteries: The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis 1977, 26, 181–194. [Google Scholar] [CrossRef]
- Silkworth, J.B.; McLean, B.; Stehbens, W.E. The effect of hypercholesterolemia on aortic endothelium studied en face. Atherosclerosis 1975, 22, 335–348. [Google Scholar] [CrossRef]
- Payling, H. Wright Mitosis patterns in aortic endothelium. Atherosclerosis 1972, 15, 93–100. [Google Scholar]
- Ellis, S.G.; Vandormael, M.G.; Cowley, M.J.; DiSciascio, G.; Deligonul, U.; Topol, E.J.; Bulle, T.M. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Progn. Study Group Circ. 1990, 82, 1193–1202. [Google Scholar]
- Al Suwaidi, J.; Yeh, W.; Cohen, H.A.; Detre, K.M.; Williams, D.O.; Holmes, D.R., Jr. Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am. J. Cardiol. 2001, 87, 1139–1144. [Google Scholar] [CrossRef]
- Garot, P.; Lefèvre, T.; Savage, M.; Louvard, Y.; Bamlet, W.R.; Willerson, J.T.; Morice, M.C.; Holmes, D.R., Jr. Nine-month outcome of patients treated by percutaneous coronary interventions for bifurcation lesions in the recent era: A report from the Prevention of Restenosis with Tranilast and its Outcomes (PRESTO) trial. J. Am. Coll. Cardiol. 2005, 46, 606–612. [Google Scholar] [CrossRef]
- Kelbaek, H.; Thuesen, L.; Helqvist, S.; Kløvgaard, L.; Jørgensen, E.; Aljabbari, S.; Saunamäki, K.; Krusell, L.R.; Jensen, G.V.; Bøtker, H.E.; et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial. J. Am. Coll. Cardiol. 2006, 47, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, L.; Kelbaek, H.; Kløvgaard, L.; Helqvist, S.; Jørgensen, E.; Aljabbari, S.; Krusell, L.R.; Jensen, G.V.; Bøtker, H.E.; Saunamäki, K.; et al. Comparison of sirolimus-eluting and bare metal stents in coronary bifurcation lesions: Subgroup analysis of the Stenting Coronary Arteries in Non-Stress/Benestent Disease Trial (SCANDSTENT). Am. Heart J. 2006, 152, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Colombo, A.; Lefèvre, T.; Oldroyd, K.G.; Guetta, V.; Guagliumi, G.; von Scheidt, W.; Ruzyllo, W.; Hamm, C.W.; Bressers, M.; et al. The clinical outcome of percutaneous treatment of bifurcation lesions in multivessel coronary artery disease with the sirolimus-eluting stent: Insights from the Arterial Revascularization Therapies Study part II (ARTS II). Eur. Heart J. 2007, 28, 433–442. [Google Scholar] [CrossRef]
- Meier, B.; Gruentzig, A.R.; King, S.B., 3rd; Douglas, J.S., Jr.; Hollman, J.; Ischinger, T.; Aueron, F.; Galan, K. Risk of side branch occlusion during coronary angioplasty. Am. J. Cardiol. 1984, 53, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.W.; Cowley, M.J.; Wolfgang, T.C.; Ducey, K.C. Effects of percutaneous transluminal coronary angioplasty on lesion-associated branches. Am. Heart J. 1985, 109, 921–925. [Google Scholar] [CrossRef]
- Boxt, L.M.; Meyerovitz, M.F.; Taus, R.H.; Ganz, P.; Friedman, P.L.; Levin, D.C. Side branch occlusion complicating percutaneous transluminal coronary angioplasty. Radiology 1986, 161, 681–683. [Google Scholar] [CrossRef]
- Weinstein, J.S.; Baim, D.S.; Sipperly, M.E.; McCabe, C.H.; Lorell, B.H. Salvage of branch vessels during bifurcation lesion angioplasty: Acute and long-term follow-up. Cathet. Cardiovasc. Diagn. 1991, 22, 1–6. [Google Scholar] [CrossRef]
- Pinkerton, C.A.; Slack, J.D.; Van Tassel, J.W.; Orr, C.M. Angioplasty for dilatation of complex coronary artery bifurcation stenosis. Am. J. Cardiol. 1985, 55, 1626–1628. [Google Scholar] [CrossRef]
- George, B.S.; Myler, R.K.; Stertzer, S.H.; Clark, D.A.; Cote, G.; Shaw, R.E.; Fishman-Rosen, J.; Murphy, M. Balloon angioplasty of coronary bifurcation lesions: The kissing balloon technique. Cathet. Cardiovasc. Diagn. 1986, 12, 124–138. [Google Scholar] [CrossRef]
- Piscione, F.; Beatt, K.; de Feyter, P.J.; Serruys, P.W. Sequential dilatation of septal and left anterior descending artery: Single guiding catheter and double guide wire technique. Cathet. Cardiovasc. Diagn. 1987, 13, 33–38. [Google Scholar] [CrossRef]
- Thomas, E.S.; Williams, D.O. Simultaneous double balloon coronary angioplasty through a single guiding catheter for bifurcation lesions. Cathet. Cardiovasc. Diagn. 1988, 15, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Bowser, M.A.; Lozner, E.C.; Johnson, L.W. Simplified two-wire technique for bifurcation lesions during coronary angioplasty. Cathet. Cardiovasc. Diagn. 1989, 16, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Renkin, J.; Wijns, W.; Hanet, C.; Michel, X.; Cosyns, J.; Col, J. Angioplasty of coronary bifurcation stenoses: Immediate and long-term results of the protecting branch technique. Cathet. Cardiovasc. Diagn. 1991, 22, 167–173. [Google Scholar] [CrossRef]
- Zack, P.M.; Ischinger, T. Experience with a technique for coronary angioplasty of bifurcational lesions. Cathet. Cardiovasc. Diagn. 1984, 10, 433–443. [Google Scholar] [CrossRef]
- Meier, B. Kissing balloon coronary angioplasty. Am. J. Cardiol. 1984, 54, 918–920. [Google Scholar] [CrossRef]
- Ciampricotti, R.; el Gamal, M.; van Gelder, B.; Bonnier, J.; Taverne, R. Coronary angioplasty of bifurcational lesions without protection of large side branches. Cathet. Cardiovasc. Diagn. 1992, 27, 191–196. [Google Scholar] [CrossRef]
- Morimoto, S.; Hiramitsu, S.; Yamada, K.; Uemura, A.; Kubo, N.; Mizuno, Y. Lesions in side branches of arteries having undergone percutaneous transluminal coronary angioplasty: A histopathologic study. Am. Heart J. 1990, 120, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Serruys, P.W.; de Jaegere, P.; Kiemeneij, F.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Emanuelsson, H.; Marco, J.; Legrand, V.; Materne, P.; et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994, 331, 489–495. [Google Scholar] [CrossRef]
- Abdelmeguid, A.E.; Whitlow, P.L.; Sapp, S.K.; Ellis, S.G.; Topol, E.J. Long-term outcome of transient, uncomplicated in-laboratory coronary artery closure. Circulation 1995, 91, 2733–2741. [Google Scholar] [CrossRef]
- Kini, A.; Kini, S.; Marmur, J.D.; Bertea, T.; Dangas, G.; Cocke, T.P.; Sharma, S.K. Incidence and mechanism of creatine kinase-MB enzyme elevation after coronary intervention with different devices. Cathet. Cardiovasc. Interv. 1999, 48, 123–129. [Google Scholar] [CrossRef]
- Kini, A.; Marmur, J.D.; Kini, S.; Dangas, G.; Cocke, T.P.; Wallenstein, S.; Brown, E.; Ambrose, J.A.; Sharma, S.K. Creatine kinase-MB elevation after coronary intervention correlates with diffuse atherosclerosis, and low-to-medium level elevation has a benign clinical course: Implications for early discharge after coronary intervention. J. Am. Coll. Cardiol. 1999, 34, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kornowski, R.; Leon, M.B. Differential Impact on Survival of Electrocardiographic Q-Wave versus Enzymatic Myocardial Infarction after Percutaneous Intervention: A Device-Specific Analysis of 7147 Patients. Circulation 2001, 104, 642–647. [Google Scholar] [CrossRef]
- Herrmann, J. Peri-procedural myocardial injury: 2005 update. Eur. Heart J. 2005, 26, 2493–2519. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.C.; Haldis, T.; Feit, F.; Hu, T.; Kleiman, N.S.; Topol, E.J.; Lincoff, A.M.; REPLACE-2 Investigators. Angiographic adverse events, creatine kinase-MB elevation, and ischemic end points complicating percutaneous coronary intervention (a REPLACE-2 substudy). Am. J. Cardiol. 2006, 97, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Topol, E.J. Periprocedural Cardiac Enzyme Elevation Predicts Adverse Outcomes. Circulation 2005, 112, 906–922. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Kuntz, R.E. Cardiac Enzyme Elevation after Successful Percutaneous Coronary Intervention Is Not an Independent Predictor of Adverse Outcomes. Circulation 2005, 112, 916–923. [Google Scholar] [CrossRef]
- Ellis, S.G.; Chew, D.; Chan, A.; Whitlow, P.L.; Schneider, J.P.; Topol, E.J. Death Following Creatine Kinase-MB Elevation after Coronary Intervention: Identification of an Early Risk Period: Importance of Creatine Kinase-MB Level, Completeness of Revascularization, Ventricular Function, and Probable Benefit of Statin Therapy. Circulation 2002, 106, 1205–1210. [Google Scholar] [CrossRef]
- Natarajan, M.K.; Kreatsoulas, C.; Velianou, J.L.; Mehta, S.R.; Pericak, D.; Goodhart, D.M. Incidence, Predictors, and Clinical Significance of Troponin-I Elevation without Creatine Kinase Elevation following Percutaneous Coronary Interventions. Am. J. Cardiol. 2004, 93, 750–753. [Google Scholar] [CrossRef]
- Cai, Q.; Skelding, K.A.; Armstrong, A.T., Jr.; Desai, D.; Wood, G.C.; Blankenship, J.C. Predictors of periprocedural creatine kinase-myocardial band elevation complicating elective percutaneous coronary intervention. Am. J. Cardiol. 2007, 99, 616–620. [Google Scholar] [CrossRef]
- Klein, L.W.; Kramer, B.L.; Howard, E.; Lesch, M. Incidence and clinical significance of transient creatine kinase elevations and the diagnosis of non-Q wavemyocardial infarction associated with coronary angioplasty. J. Am. Coll. Cardiol. 1991, 17, 621–626. [Google Scholar] [CrossRef]
- Karim, M.A.; Shinn, M.; Oskarsson, H.; Windle, J.; Deligonul, U. Significance of cardiac troponin T release after percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1995, 76, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.J.; Wu, E.; Davidson, C.J.; Choi, K.M.; Klocke, F.J.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation 2001, 103, 2780–2783. [Google Scholar] [CrossRef] [PubMed]
- Selvanayagam, J.B.; Porto, I.; Channon, K.; Petersen, S.E.; Francis, J.M.; Neubauer, S.; Banning, A.P. Troponin Elevation after Percutaneous Coronary Intervention Directly Represents the Extent of Irreversible Myocardial Injury Insights from Cardiovascular Magnetic Resonance Imaging. Circulation 2005, 111, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.; Selvanayagam, J.B.; Van Gaal, W.J.; Prati, F.; Cheng, A.; Channon, K.; Neubauer, S.; Banning, A.P. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: Insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation 2006, 114, 662–669. [Google Scholar]
- Selvanayagam, J.B.; Cheng, A.S.; Jerosch-Herold, M.; Rahimi, K.; Porto, I.; van Gaal, W.; Channon, K.M.; Neubauer, S.; Banning, A.P. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: A quantitative magnetic resonance perfusion study. Circulation 2007, 116, 1458–1464. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Chan, A.K.; Brown, K.A.; Chan, C.W.; Reynolds, H.G.; Tsang, S.; Davis, R.B. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006, 113, 2733–2743. [Google Scholar] [CrossRef]
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Schofer, J.; Schluter, M.; Gershlick, A.H.; Wijns, W.; Garcia, E.; Schampaert, E.; Breithardt, G. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: Double-blind, randomized controlled trial (E-SIRIUS). Lancet 2003, 362, 1093–1099. [Google Scholar] [CrossRef]
- Fischman, D.L.; Savage, M.P.; Leon, M.B.; Schatz, R.A.; Ellis, S.; Cleman, M.W.; Hirshfeld, J.W.; Teirstein, P.; Bailey, S.; Walker, C.M.; et al. Fate of lesion-related side branches after coronary artery stenting. J. Am. Coll. Cardiol. 1993, 22, 1641–1646. [Google Scholar] [CrossRef]
- Iñiguez, A.; Macaya, C.; Alfonso, F.; Goicolea, J.; Hernandez, R.; Zarco, P. Early angiographic changes of side branches arising from a Palmaz-Schatz stented coronary segment: Results and clinical implications. J. Am. Coll. Cardiol. 1994, 23, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.; Grinstead, W.C.; Hakim, A.H.; Dabaghi, S.F.; Abukhalil, J.M.; Ali, N.M.; Joseph, J.; French, B.A.; Raizner, A.E. Fate of side branches after intracoronary implantation of the Gianturco-Roubin flex-stent for acute or threatened closure after percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1994, 74, 1207–1210. [Google Scholar] [CrossRef]
- Pan, M.; Medina, A.; Suárez de Lezo, J.; Romero, M.; Melián, F.; Pavlovic, D.; Hernández, E.; Segura, J.; Marrero, J.; Torres, F.; et al. Follow-up patency of side branches covered by intracoronary Palmaz-Schatz stent. Am. Heart J. 1995, 129, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, R.; Hara, K.; Ikari, Y.; Yamasaki, M.; Yamaguchi, T.; Tamura, T. Patency of intermediate size side branches after Palmaz-Schatz stent implantation. JPN Heart J. 1997, 38, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, R.M.; Ling, F.S. Distortion of Palmaz-Schatz stent geometry following side-branch balloon dilation through the stent in a rabbit model. Cathet. Cardiovasc. Diagn. 1997, 40, 422–426. [Google Scholar] [CrossRef]
- Hayashi, S.; Tohyama, S.; Shindo, T.; Fukui, K.; Shiba, K.; Nakao, M.; Nakagawa, M.; Harada, K.; Ishii, M. Risk of side branch occlusion after coronary Palmaz-Schatz stenting. J. Cardiol. 1997, 29, 261–266. [Google Scholar]
- Kinoshita, T.; Kobayashi, Y.; De Gregorio, J.; Nameki, M.; Kuroda, N.; Yamamoto, Y.; Miyazaki, A.; Masuda, Y. Difference in security of stent jail between Palmaz-Schatz, NIR, and Multi-Link stents: The effect of balloon inflation through stent struts. Cathet. Cardiovasc. Interv. 1999, 48, 230–234. [Google Scholar] [CrossRef]
- Bhargava, B.; Waksman, R.; Lansky, A.J.; Kornowski, R.; Mehran, R.; Leon, M.B. Clinical outcomes of compromised side branch (stent jail) after coronary stenting with the NIR stent. Cathet. Cardiovasc. Interv. 2001, 54, 295–300. [Google Scholar] [CrossRef]
- Aliabadi, D.; Tilli, F.V.; Bowers, T.R.; Benzuly, K.H.; Safian, R.D.; Goldstein, J.A.; Grines, C.L.; O’Neill, W.W. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am. J. Cardiol. 1997, 80, 994–997. [Google Scholar] [CrossRef]
- Yilmaz, H.; Demir, I.; Belgi, A.; Kabukçu, M.; Yalçinkaya, S.; Sancaktar, O. Side branch occlusion in direct intracoronary stenting: Predictors and results. J. Invasive Cardiol. 2001, 13, 578–581. [Google Scholar]
- Timurkaynak, T.; Ciftci, H.; Ozdemir, M.; Cengel, A.; Tavil, Y.; Kaya, M.; Erdem, G.; Cemri, M.; Dortlemez, O.; Dortlemez, H. Side branch occlusion after coronary stenting with or without balloon predilation: Direct versus conventional stenting. J. Invasive Cardiol. 2002, 14, 497–501. [Google Scholar] [PubMed]
- Tanabe, K.; Serruys, P.W.; Degertekin, M.; Regar, E.; van Domburg, R.T.; Sousa, J.E.; Wülfert, E.; Morice, M.C. Fate of side branches after coronary arterial sirolimuseluting stent implantation. Am. J. Cardiol. 2002, 90, 937–941. [Google Scholar] [CrossRef]
- Cho, G.Y.; Lee, C.W.; Hong, M.K.; Kim, J.J.; Park, S.W.; Park, S.J. Side-branch occlusion after rotational atherectomy of in-stent restenosis: Incidence, predictors, and clinical significance. Cathet. Cardiovasc. Interv. 2000, 50, 406–410. [Google Scholar] [CrossRef]
- Cho, G.Y.; Lee, C.W.; Hong, M.K.; Kim, J.J.; Park, S.W.; Park, S.J. Effects of stent design on side branch occlusion after coronary stent placement. Cathet. Cardiovasc. Interv. 2001, 52, 18–23. [Google Scholar]
- Grundeken, M.J.; Lesiak, M.; Asgedom, S.; Garcia, E.; Bethencourt, A.; Norell, M.S.; Damman, P.; Woudstra, P.; Koch, K.T.; Vis, M.M.; et al. Clinical outcomes after final kissing balloon inflation compared with no final kissing balloon inflation in bifurcation lesions treated with a dedicated coronary bifurcation stent. Heart 2014, 100, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Airoldi, F.; Iakovou, I.; Cosgrave, J.; Michev, I.; Sangiorgi, G.M.; Montorfano, M.; Chieffo, A.; Carlino, M.; Corvaja, N.; et al. Clinical and angiographic outcome after implantation of drug-eluting stents in bifurcation lesions with the crush stent technique: Importance of final kissing balloon post-dilation. J. Am. Coll. Cardiol. 2005, 46, 613–620. [Google Scholar] [CrossRef]
- Almeda, F.Q.; Nathan, S.; Calvin, J.E.; Parrillo, J.E.; Klein, L.W. Frequency of abrupt vessel closure and side branch occlusion after percutaneous coronary intervention in a 6.5-year period (1994 to 2000) at a single medical center. Am. J. Cardiol. 2002, 89, 1151–1155. [Google Scholar] [CrossRef]
- Yamawaki, M.; Muramatsu, T.; Kozuma, K.; Ito, Y.; Kawaguchi, R.; Kotani, J.; Yokoi, H.; Nakamura, M.; Saito, S. Long-term clinical outcome of a single stent approach with and without a final kissing balloon technique for coronary bifurcation. Circ. J. 2014, 78, 110–121. [Google Scholar] [CrossRef]
- Kralev, S.; Poerner, T.C.; Basorth, D.; Lang, S.; Wolpert, C.; Haghi, D.; Borggrefe, M.; Haase, K.K.; Süselbeck, T. Side branch occlusion after coronary stent implantation in patients presenting with ST-elevation myocardial infarction: Clinical impact and angiographic predictors. Am. Heart J. 2006, 151, 153–157. [Google Scholar] [CrossRef]
- Prasad, N.; Seidelin, P.H. Side branch compromise during percutaneous coronary interventions. J. Invasive Cardiol. 2002, 14, 138–145. [Google Scholar]
- Mintz, G.S.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Popma, J.J.; Leon, M.B. Axial plaque redistribution as a mechanism of percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1996, 77, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Louvard, Y.; Lefevre, T. Bifurcation lesion stenting. In Problem Oriented Approaches in Interventional Cardiology; Colombo, A., Stankovic, G., Eds.; Informa Healthcare: Dubai, United Arab Emirates, 2007; pp. 37–55. [Google Scholar]
- Iakovou, I.; Ge, L.; Colombo, A. Contemporary stent treatment of coronary bifurcations. J. Am. Coll. Cardiol. 2005, 46, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Louvard, Y.; Lefèvre, T.; Morice, M.C. Percutaneous coronary intervention for bifurcation coronary disease. Heart 2004, 90, 713–722. [Google Scholar] [CrossRef]
- Anzuini, A.; Briguori, C.; Rosanio, S.; Tocchi, M.; Pagnotta, P.; Bonnier, H.; Gimelli, G.; Airoldi, F.; Margonato, A.; Legrand, V.; et al. Immediate and long-term clinical and angiographic results from Wiktor stent treatment for true bifurcation narrowings. Am. J. Cardiol. 2001, 88, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Maeng, M.; Holm, N.R.; Erglis, A.; Kumsars, I.; Niemelä, M.; Kervinen, K.; Jensen, J.S.; Galløe, A.; Steigen, T.K.; Wiseth, R.; et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J. Am. Coll. Cardiol. 2013, 62, 30–34. [Google Scholar] [CrossRef]
- Koo, B.K.; Kang, H.J.; Youn, T.J.; Chae, I.H.; Choi, D.J.; Kim, H.S. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J. Am. Coll. Cardiol. 2005, 46, 633–637. [Google Scholar] [CrossRef]
- Lefèvre, T.; Louvard, Y.; Morice, M.C.; Loubeyre, C.; Piéchaud, J.F.; Dumas, P. Stenting of bifurcation lesions: A rational approach. J. Interv. Cardiol. 2001, 14, 573–585. [Google Scholar] [CrossRef]
- Pan, M.; Suárez de Lezo, J.; Medina, A.; Romero, M.; Segura, J.; Ramírez, A.; Pavlovic, D.; Hernández, E.; Ojeda, S.; Adamuz, C. A stepwise strategy for the stent treatment of bifurcated coronary lesions. Cathet. Cardiovasc. Interv. 2002, 55, 50–57. [Google Scholar] [CrossRef]
- Watanabe, Y.; Murasato, Y.; Yamawaki, M.; Kinoshita, Y.; Okubo, M.; Yumoto, K.; Masuda, N.; Otake, H.; Aoki, J.; Nakazawa, G.; et al. Proximal optimisation technique versus final kissing balloon inflation in coronary bifurcation lesions: The randomised, multicentre PROPOT trial. EuroIntervention 2021, 17, 747–756. [Google Scholar] [CrossRef]
| Author | Randomization | Technique | Number of Patients | Follow-Up (Months) | Restenosis Rate, % | TLR (%) |
|---|---|---|---|---|---|---|
| Suwaidi | No | S + S | 77 | 12 | - | 19.4 |
| S + B | 54 | - | 20.5 | |||
| Chevalier | No | S + S | 50 | 6 | 28 | 24 |
| Yamashita | No | S + S | 53 | 6 | 62 | 38 |
| S + B | 39 | 48 | 36 | |||
| Pan | No | S + S | 23 | 18 | 43 | 39 |
| S + B | 47 | 19 | 17 | |||
| Anzuini | No | S + S | 45 | 12 | 25 | 35.5 |
| S + B | 45 | 12.5 | 15.5 | |||
| Brunel | No | S + S | 50 | 6 | 57 | 43 |
| S + B | 56 | 21 | 8 | |||
| Brunel | No | PTS | 186 | 7 | MB—9.1 | 15.9 |
| SB—25.3 | ||||||
| Rux | No | S + B | 370 | 6 | 13 | 15.7 |
| S + S | 45 | 16.2 | 33.3 | |||
| Beijk | No | S + B | 465 | 12 | 11.3 | 8.6 |
| R stent | ||||||
| Lefevre | No | S + B | 724 | 7 | MB/SB - | 13.6 |
| 7.3/7.3 | ||||||
| S + S | 425 | MB/SB - | 24.1 | |||
| 3.2/11.5 |
| Author | Randomization | Stent | Technique | Number of Patients | Follow-Up (Months) | Restenosis Rate, % | TLR (%) |
|---|---|---|---|---|---|---|---|
| Colombo | да | SES | S + S | 63 | 6 | 28 | 9.5 |
| S + B | 22 | 18.7 | 4.5 | ||||
| Pan | да | SES | S + S | 47 | 11 | 20 | 5 |
| S + B | 44 | 7 | 2 | ||||
| Ge | не | SES | S + S | 117 | 9 | 24 | 8.9 |
| S + B | 57 | 10 | 5.4 | ||||
| Ge | не | SES + PES | S+ S | 181 | 9 | MB—11.5 | 14.9 |
| SB—21.6 | |||||||
| Sharma | не | SES | S+ S | 200 | 8 | 4 | |
| Hoye | не | SES + PES | S+ S | 23 | 9 | MB—18.8 | 5.3 |
| SB—12.5 | |||||||
| Hoye | не | SES + PES | S+ S | 231 | 9 | MB—9.1 | 9.7 |
| SB—25.3 | |||||||
| Ge | не | SES + PES | S + S − T | 61 | 12 | 13 | 11.1 |
| S + S − CR | 121 | 16.2 | 14 | ||||
| Moussa | не | SES | S + S − CR | 120 | 6 | 11.3 | 11.3 |
| Steigen | да | SES | S + S | 206 | 8 | MB/SB – | 1.0 |
| 5.1/11.5 | |||||||
| S + B | 207 | MB/SB – | 1.9 | ||||
| 4.6/19.2 | |||||||
| Ferenc | да | SES | S + B + S | 101 | 12 | MB/SB – | 10.9 |
| 7.3/7.3 | |||||||
| S + S − T | 101 | MB/SB – | 8.9 | ||||
| 3.2/11.5 | |||||||
| De Mario | не | PES | S + S | 118 | 12 | 16.5 | 18.3 |
| S + B | 32 | 13.1 | 21 |
| Study | Design | Patients, n KBI Non-KBI | Follow-Up (Months) | CVD KBI vs. Non-KBI | MI | TVR | Stent Thrombosis | MACE | SB Stenosis, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Provisional stenting | ||||||||||
| THUEBIS | Random-ized | 56 | 54 | 6 | 0% vs. 3.7% | 3.6% vs. 1.9% | 18% vs. 15% | 3.6% vs. 1.9% | 23.2% vs. 24.1% | 37% vs. 32% |
| Nordic III | Random-ized | 238 | 239 | 6 | 0.8% vs. 0% | 0.4–1.3% | 1.3% vs. 1.7% | 0.4% vs. 0.4% | 2.1% vs. 2.5% | 25% vs. 30% |
| COBIS | Registry | 736 | 329 | 22 | 0.9–0.7% | 0.6–1.3% | 9.1–3.4% | NA | 10–4.9% | NA |
| Yamawaki et al. [79] | Registry | 132 | 124 | 36 | 0–0.1% | 0–0% | 12–5% | 0–0% | 15–7% | NA |
| Two stents | ||||||||||
| Ge et al. [77] | Observa-tional | 116 | 65 | 9 | 1.7–0% | 10–14% | 9.5–24.6% | 2.6–3.1% | 20–39% | 24–38% |
| Grundeken et al. [76] | Registry | 624 | 121 | 12 | 1.7–4.6% | 5.0–4.6% | 4.7–2.9% | 0.3–0.9% | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panayotov, P.; Mileva, N.; Vassilev, D. Current Challenges in Coronary Bifurcation Interventions. Medicina 2024, 60, 1439. https://doi.org/10.3390/medicina60091439
Panayotov P, Mileva N, Vassilev D. Current Challenges in Coronary Bifurcation Interventions. Medicina. 2024; 60(9):1439. https://doi.org/10.3390/medicina60091439
Chicago/Turabian StylePanayotov, Panayot, Niya Mileva, and Dobrin Vassilev. 2024. "Current Challenges in Coronary Bifurcation Interventions" Medicina 60, no. 9: 1439. https://doi.org/10.3390/medicina60091439






