Abstract
The management of hidradenitis suppurativa (HS) in elderly patients presents unique challenges due to its chronic inflammatory nature, heterogeneous clinical presentation and comorbidities. While HS typically affects the anogenital and intertriginous regions, elderly patients may exhibit atypical features such as the involvement of the neck, mammary area and gluteal region. The prevalence of HS in the elderly population is lower and the average age of disease onset is higher than in patients under 65. In contrast, it is unclear whether HS in the elderly has different clinical features. The elderly frequently present multiple comorbidities, including obesity, diabetes, and heart disease, which further complicate management decisions. Therapeutic interventions must consider the frailty and increased risk of multimorbidity and adverse events in elderly patients. While systemic antibiotics remain a mainstay of HS treatment, biologic agents such as TNFα inhibitors and secukinumab offer promising options for refractory cases. However, their safety and efficacy in elderly patients, particularly those with multiple comorbidities, require careful consideration. A comprehensive approach to managing HS in elderly patients involves not only pharmacological interventions but also lifestyle modifications and surgical options where appropriate. Multidisciplinary collaboration between dermatologists, geriatricians and other specialists is essential for tailoring treatment strategies and optimizing long-term outcomes and quality of life in special population.
1. Introduction
Hidradenitis suppurativa (HS) is a chronic, non-infective inflammatory disease that usually involves the anogenital and intertriginous regions of the body. The major clinical feature is the presence of papules and/or deep-seated painful nodules that show a slow tendency to spontaneous healing and progress to abscess-like lesions that drain foul-smelling purulent material. Furthermore, since the disease is typically relapse-remitting, new lesions may develop in adjacent sites, leading to developing sinus tracts, fistulas and hypertrophic scars that deserve a surgical resolution.
HS typically occurs after puberty, with the average age of onset in the second and third decades of life [1]. HS onset in elderly is very uncommon, and studies on this fragile class of patients are lacking.
Van Den Weijden et al. suggest defining HS in the elderly as HS tarda and to subdivide it as late onset if it develops after 60 years of age and persistent HS tarda if it has an earlier onset and persists after 60 years [2]. It is interesting to note that the HS female to male ratio is 3.3:1.0 [3], while in the older population a lower female to male ratio is observed (1.7:1.0); this might be due to the role of menopause on HS development and course [2]. Additionally, the clinical presentation may be different in elderly patients. For example, in the cohort of patients studied by Cazzaniga et al., the neck and mammary regions were more likely involved in older people [4], whereas the armpits and buttocks were less commonly involved compared to younger patients [2]. Since HS is a multifactorial disease, both genetics and lifestyle factors, in particular body mass index (BMI) and cigarette smoking, play a key role for HS development and flare-ups [5]. Family history for HS is typically associated with an earlier onset of clinical presentation [4]; on the other hand, older patients with HS are more likely to be current or ex-smokers [6]. Furthermore, there is evidence that current smokers, especially older patients, have a more severe form of the disease and may be less likely to respond to medical therapies [7]. Blum et al. found that Hurley 3 stage disease is more prevalent in older adults, with more sinus tracts and larger abscesses [6]. Population aging is notably increasing worldwide: more than 2 billion people are estimated to be older than 65 years old by 2050 [8], so third millennium medicine will be focused on the management of patients with several comorbidities and polypharmacotherapy. In this regard, old patients with HS have an increased risk of developing chronic obstructive pulmonary disease, intestinal bowel diseases, cardiopathy, diabetes and kidney dysfunction [2]. Hence, the aim of this review is to focus on HS in the elderly at both clinical and therapeutic levels.
2. Materials and Methods
This study is a narrative review; we conducted a narrative analysis and covered a broad range of topics by using studies of various complexity and design.
Pubmed, Cochrane Skin and Embase databases were reviewed up to March 2024. Search terms included “hidradenitis suppurativa”, “diagnosis”, “elderly”, “smoking”, “old age”, “biological therapies”, “geriatric”, “translational studies”, in the abstract and title. Manuscripts were found, screened, analyzed for relevant data and compared further. Only English language manuscripts were included in this study. This article is based on previous studies and does not include any studies of human participants or animals performed by any of the authors. The criteria for inclusion were English-language articles, empirical studies, and review papers to provide context for our findings. We reviewed the articles and omitted those with restrictive medical content.
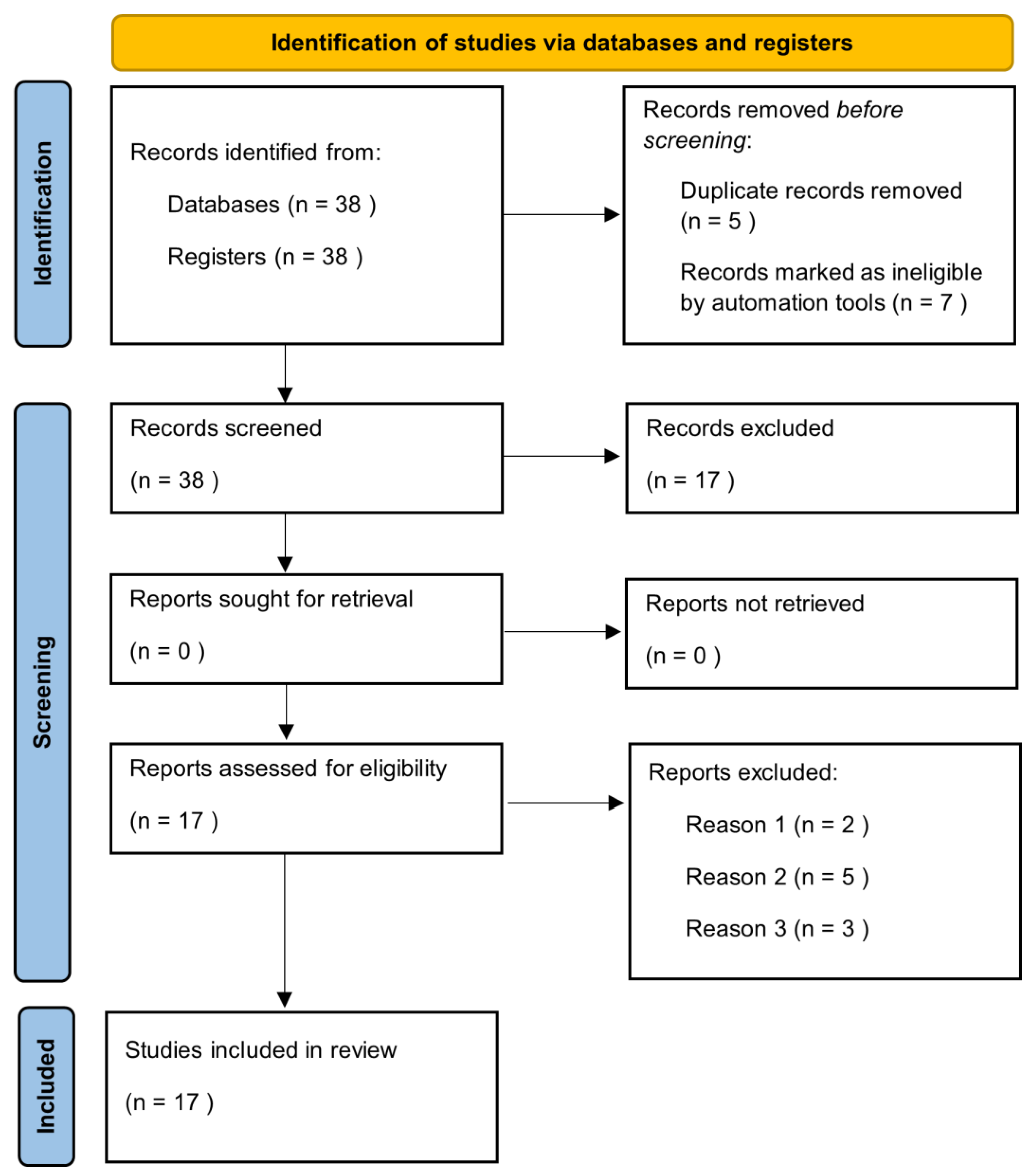
Manuscripts were identified, screened and extracted for relevant data following the PRISMA (Preferred Reporting Items for Systematic Reviews and meta-analyses) guideline (Figure 1).
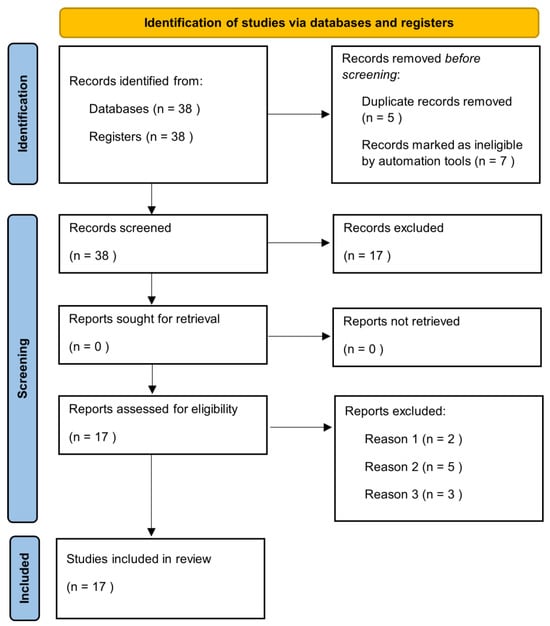
Figure 1.
Prisma check list.
3. Results
The literature search highlighted 38 articles, of which 17 satisfied the selection criteria for this narrative review. The results section has been divided into sub-sections in which the epidemiological and clinical characteristics of the elderly with HS and the impact of treatment on this category of patients are described.
3.1. Epidemiology
HS is an under-diagnosed disease, which affects approximately 1% of the world population but the exact prevalence in the general population is reported to be very variable. A retrospective study of 48 million people in the U.S. calculated a prevalence of 0.1%, but other works proposed values as high as 4.1% [9]. HS is four times more common in women and affects black and biracial people more frequently [9,10]. The mean age of onset is between the second and third decade, but it can appear at any age [11]. Two peaks of onset are estimated, in the late teens and around 40 years of age [12]. In total, 7.7% of patients with HS are under 13 years of age and knowledge on pediatric pathology is steadily increasing [13]. In contrast, data on HS in elderly population are scant. Among these people, the prevalence of HS is estimated at 0.8% [14]. According to Blum et al., the average age of disease onset is 41.8 in patients over 65 years, which is significantly higher than patients under 65 years, where the average is 20.1 [6]. Furthermore, in elderly HS, a reduction in the female–male ratio to 1.6 is observed [7] probably due to the menopause, which is associated with a decrease in the frequency of the condition [15]. For this reason, van der Weijden et al. defined the HS of the elderly, or tarda, from the age of 60, considering the menopause to be between 40 and 58 years. According to their estimates, the average age onset of HS was significantly higher in the elderly group than in the adult group (40 vs. 23), confirming the data of Blum et al. [2].
3.2. Clinical Features
Van der Weijden et al. also proposed a classification of HS tarda into late-onset HS, if it occurred after the age of 60, and persistent HS, if it began before the age of 60 and lasted beyond this age [2]. They found no clinical differences between these two categories, while there were some significant ones between adult and elderly patients. Indeed, among the elderly, the most affected sites were not those typical of adult HS, such as armpits, groin and buttocks (OR 0.595, 0.643 and 0.563 respectively), but of others (OR 1.887) [2]. They also investigated familiarity, finding that 33.8% of elderly patients had an affected relative, compared to 21.3% of adult patients [2]. In this study, the Hurley grading scale was used to compare the HS severity of the two groups, but no significant differences were observed [2]. In contrast, a cross-sectional study by Jiang et al. established a positive relationship between disease severity and age of onset. The main outcome was that the International Hidradenitis Suppurativa Severity Score System (IHS4) and the geometric mean ratio (GMR) were significantly higher in the group of late-onset patients than in the common-onset group [16]. The authors also compared other classifications, such as the Hurley stage and the Physician’s Global Assessment (PGA). Hurley stage III was present in 58% of patients with late-onset HS, compared to 28% of those with common-onset HS; in contrast, a ‘severe’ or ‘very severe’ PGA among the former was found in 37% of patients and among the latter in only 8% of cases [16]. BMI is reported to not particularly differ between elderly and adult HS patients, whereas it was significantly higher in elderly HS patients than in the age-matched controls [2]. Furthermore, elderly HS patients were more frequently ex-smokers than the adult HS group, with OR 2.035. In contrast, the latter were more commonly active smokers, with 38.4% of patients compared to 20.9% of the affected elderly [2]. Another aspect to be considered with regard to patients with HS tarda is that of comorbidities, which impact on the quality of life and treatment of these patients. According to van der Weijden et al., there was a significantly higher prevalence of psoriasis (20.1% vs. 7.9%), history of acne (16.7% vs. 4.8%), diabetes (9.2% vs. 4.8%), irritable bowel syndrome (71.4% vs. 56%), kidney disease (8.8% vs. 4.5%), chronic obstructive pulmonary disease (COPD) (14.1% vs. 8.1%) and fibromyalgia (45.1% vs. 27.2%) in the elderly patient group than in the adult HS group [2]. Another study of 1100 patients in the Italian population observed a higher prevalence of dermatitis (including atopic, seborrheic and perioral) among patients with late-onset HS than among early-onset HS (1.9% vs. 0.7%) [4]. This was also estimated for psoriasis (3.5% vs. 1.3%) and diabetes (1.2% vs. 0.2%) [4]. It must be underlined that the same study contradicts what is reported above; in fact, no significant differences were found in terms of disease location, with armpits, groin and buttocks comparably affected in both the early-onset and late-onset HS group [4]. In addition, a lower severity of disease was estimated in the late-onset group rather than its counterpart, as calculated with the Sartorius score [4]. The discrepancies are probably due to the different sample sizes and, above all, to the different division into groups compared with the work of van der Weijden et al. In fact, whereas in van der Weijden et al.’s study the cut-off was set at 60 years of age, in the work of Cazzaniga et al., the late teen years and age over 40 years are considered. In this regard, Nielsen et al. conducted a study of 700 patients with HS classifying them as van der Weijden et al. in over and under 60 years of age. Again, differences were found, such as a lower familiarity and higher frequency of buttock involvement in patients aged 60. Furthermore, the study estimated a higher severity of disease in the elderly patients using the IHS4 score, and they also found a positive correlation between age and IHS4 score in males [17].
3.3. Treatment
The treatment of HS in the elderly must necessarily take into consideration their frailty and the increased risk of multimorbidity that afflicts this category [2]. The European guidelines, however, do not report specific indications for this class of patients, but instead describe drugs contraindications. Their choice for elderly patients must therefore necessarily be based on their comorbidities. For example, TNFα inhibitors are contraindicated in patients with heart failure NYHA class III-IV, multiple sclerosis and severe infections. Polypharmacotherapy and possible drugs interactions must be considered in the elderly when selecting systemic antibiotics indicated for HS (tetracycline, clindamycin and/or rifampicin) [18]. For example, rifampin is a potent inducer of certain drug-metabolizing enzymes, such as cytochrome P450 (CYP) 3A4, and interacts with numerous drugs. In fact, it reduces the blood concentrations of beta-blockers and calcium antagonists, as well as various benzodiazepines [19]. For HS patients, however, secukinumab has received Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for HS. It is an anti-IL-17 not contraindicated in patients with heart failure; moreover, differently from anti-TNF, the risk of reactivation of latent tuberculosis seems to be a concern [20]. In addition, secukinumab has shown efficacy on HS in several studies. For instance, an Italian 52-Week Real-Life Study observed the achievement of Hidradenitis Suppurativa Clinical Response (HiSCR) score at 16 weeks in 57% of patients. Furthermore, from week 16 to 52, the HiSCR was achieved by 71% of the patients, and in all severity scales (IHS4, DLQI and VAS pain scale), the values showed a decrease [21]. However, according to guidelines and expert opinions, there is currently no reason not to consider systemic antibiotics as a first-line therapy in the elderly with HS [20]. The retrospective study by Denny et al. reinforces this concept by observing that in old age there are better responses to first-line therapies, such as topical and systemic antibiotics and intralesional corticosteroids [7]. On the other hand, a study by Prens et al. calculated drug survival, i.e., the measure of length of time until the discontinuation of a drug, in a population of adult patients with HS treated with biological agents; they found that older age, severity and duration of disease correlated positively with the survival of adalimumab and infliximab [22]. The authors explained this by hypothesizing that patients with a higher disease burden and who cared more about their health status, as older people do, had a greater compliance to accept long-term therapy such as anti-TNFα [22].
4. Discussion
Managing hidradenitis suppurativa (HS) in elderly patients presents a multifaceted challenge within dermatological practice, necessitating a comprehensive understanding of the disease’s nuances, treatment modalities and the unique physiological and clinical characteristics of older individuals. With HS being a chronic inflammatory condition primarily affecting the anogenital and intertriginous regions, its management in the elderly requires a tailored approach that considers not only the dermatological manifestations but also the impact of age-related changes on treatment efficacy and tolerability [23,24,25,26,27,28,29,30,31,32,33,34,35].
One of the key considerations in managing HS in older patients is the recognition of the disease’s atypical presentation and its potential overlap with other dermatological conditions commonly seen in this population. Clinical features such as the involvement of different anatomical sites, including the neck, mammary region and lower extremities, as well as variations in lesion morphology and distribution, may pose diagnostic challenges and necessitate a high index of suspicion for HS in elderly individuals [27,28,29,30].
Furthermore, the epidemiological profile of HS in the eldRingerly differs from that of younger cohorts, with studies suggesting a lower prevalence of the disease but a higher average age of onset. The categorization of HS tarda, defined as disease onset after the age of 60, underscores the importance of recognizing age-related variations in disease presentation and progression. Additionally, the shift in the female-to-male ratio observed in older patients may reflect the influence of hormonal changes, such as menopause, on disease pathogenesis and clinical course.
Understanding the interplay between age-related comorbidities and HS is paramount to optimizing treatment outcomes in elderly patients. Conditions such as obesity, diabetes mellitus and COPD are prevalent in this population and may impact both the severity of HS and the choice of therapeutic interventions. These comorbidities very often are associated with HS, such as obesity, but at the same time are independent conditions that affect elderly patients.
Moreover, the potential for drug–drug interactions and the need to consider the overall burden of polypharmacy highlight the importance of a holistic approach to treatment decision-making in elderly patients with HS [6].
In terms of therapeutic interventions, while systemic antibiotics remain a cornerstone of treatment for HS, particularly in the setting of acute flares and mild-to-moderate disease, the use of biologic agents such as TNFα inhibitors and secukinumab has emerged as a promising option for refractory cases. However, the safety and efficacy of these agents in elderly patients, particularly those with underlying cardiac comorbidities, warrant careful consideration and may necessitate individualized treatment strategies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
Although the current literature on HS does not allow us to establish with certainty the efficacy and safety of biologics, it is important to point out that, in contrast, for conditions such as psoriasis, the efficacy and safety of these drugs have been well-documented and demonstrated for many years. This extensive positive experience with biologics in the treatment of psoriasis provides us with a valuable foundation and allows us to approach their use in HS with a degree of optimism. Indeed, the successful approval and widespread adoption of biologics for psoriasis serve as a significant reference point [40,41,42]. This suggests that the benefits observed in psoriasis patients could potentially be mirrored in HS patients as well, pending further research and clinical trials. The hope is that, as research progresses and more clinical trials are conducted, similarly positive outcomes will be achieved for HS. This would significantly enhance the therapeutic options and improve the quality of life for those suffering from this condition.
A comprehensive approach to managing HS in elderly patients should encompass not only pharmacological interventions but also lifestyle modifications, wound care and surgical options where appropriate. Moreover, given the chronic and relapsing nature of HS, ongoing monitoring and regular follow-up are essential to assess treatment response, manage disease flares and address any emerging concerns or complications.
5. Conclusions
In conclusion, managing HS in elderly patients requires a nuanced understanding of age-related changes in disease presentation, comorbidity profiles and treatment responses. A multidisciplinary approach involving dermatologists, geriatricians and other specialists is crucial for tailoring treatment strategies to the unique needs of this patient population and optimizing long-term outcomes and quality of life.
Author Contributions
F.M.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. N.T.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. C.B.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. T.B.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. L.P.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. T.B.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. M.M.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This study was approved by the Italy Data Protection Agency. According to Italy legislation; neither approval from the ethics committee nor informed consent from the study populations are required for registry linkage studies.
Data Availability Statement
Data are reported in the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bukvić Mokos, Z.; Markota Čagalj, A.; Marinović, B. Epidemiology of hidradenitis suppurativa. Clin. Dermatol. 2023, 41, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, D.A.Y.; Koerts, N.D.K.; van Munster, B.C.; van der Zee, H.H.; Horváth, B. Hidradenitis suppurativa tarda: Defining an understudied elderly population. Br. J. Dermatol. 2023, 190, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.G.; Alikhan, A.; Weaver, A.L.; Wetter, D.A.; Davis, M.D. Incidence of hidradenitis suppurativa and associated factors: A population-based study of Olmsted County, Minnesota. J. Investig. Dermatol. 2013, 133, 97–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cazzaniga, S.; Pezzolo, E.; Garcovich, S.; Naldi, L.; IRHIS Study Group. Late-onset hidradenitis suppurativa: A cluster analysis of the National Italian Registry IRHIS. J. Am. Acad. Dermatol. 2021, 85, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.A.; Fragoulis, G.E.; Nikiphorou, E. Hidradenitis suppurativa: Infection, autoimmunity, or both? Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19895488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blum, F.R.; DeBarmore, B.M.; Sayed, C.J. Hidradenitis Suppurativa in Older Adults. JAMA Dermatol. 2023, 159, 216–219, Erratum in JAMA Dermatol. 2023, 159, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Denny, G.; Anadkat, M.J. The effect of smoking and age on the response to first-line therapy of hidradenitis suppurativa: An institutional retrospective cohort study. J. Am. Acad. Dermatol. 2017, 76, 54–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brivio, P.; Paladini, M.S.; Racagni, G.; Riva, M.A.; Calabrese, F.; Molteni, R. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr. Med. Chem. 2019, 26, 3685–3701. [Google Scholar] [CrossRef] [PubMed]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Alhusayen, R.; Lansang, P.; Shear, N.; Yeung, J. What is hidradenitis suppurativa? Can. Fam. Phys. 2017, 63, 114–120. [Google Scholar] [PubMed] [PubMed Central]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naik, H.B.; Paul, M.; Cohen, S.R.; Alavi, A.; Suàrez-Fariñas, M.; Lowes, M.A. Distribution of Self-reported Hidradenitis Suppurativa Age at Onset. JAMA Dermatol. 2019, 155, 971–973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deckers, I.E.; van der Zee, H.H.; Boer, J.; Prens, E.P. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J. Am. Acad. Dermatol. 2015, 72, 485–488. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Del Marmol, V. Hidradenitis suppurativa in the elderly: New insights. Br. J. Dermatol. 2023, 2023, ljad378. [Google Scholar] [CrossRef] [PubMed]
- Margesson, L.J.; Danby, F.W. Hidradenitis suppurativa. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.W.; Petty, A.J.; Jacobs, J.L.; Robinson, C.; Bhatia, S.M.; Kwock, J.T.; Liu, B.; Green, C.L.; Hall, R.P.; Cardones, A.R.; et al. Association between age at symptom onset and disease severity in older patients with hidradenitis suppurativa. Br. J. Dermatol. 2023, 188, 555–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, V.W.; Ring, H.C.; Holgersen, N.; Thomsen, S.F. Elderly male patients with hidradenitis suppurativa have more severe disease independent of disease duration. Br. J. Dermatol. 2024, 190, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Baddley, J.W.; Deodhar, A.A.; Magrey, M.; Rich, P.A.; Soriano, E.R.; Soung, J.; Bao, W.; Keininger, D.; Marfo, K.; et al. Association of Secukinumab Treatment With Tuberculosis Reactivation in Patients With Psoriasis, Psoriatic Arthritis, or Ankylosing Spondylitis. JAMA Dermatol. 2021, 157, 43–51, Erratum in JAMA Dermatol. 2021, 157, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martora, F.; Marasca, C.; Cacciapuoti, S.; Fariello, F.; Potestio, L.; Battista, T.; Scalvenzi, M.; Megna, M. Secukinumab in Hidradenitis Suppurativa Patients Who Failed Adalimumab: A 52-Week Real-Life Study. Clin. Cosmet. Investig. Dermatol. 2024, 17, 159–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prens, L.M.; Bouwman, K.; Aarts, P.; Arends, S.; van Straalen, K.R.; Dudink, K.; Horváth, B.; Prens, E.P. Adalimumab and infliximab survival in patients with hidradenitis suppurativa: A daily practice cohort study. Br. J. Dermatol. 2021, 185, 177–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martora, F.; Picone, V.; Fabbrocini, G.; Marasca, C. Hidradenitis suppurativa flares following COVID-19 vaccination: A case series. JAAD Case Rep. 2022, 23, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Martora, L.; Fabbrocini, G.; Marasca, C. A Case of Pemphigus Vulgaris and Hidradenitis Suppurativa: May Systemic Steroids Be Considered in the Standard Management of Hidradenitis Suppurativa? Skin. Appendage Disord. 2022, 8, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Marasca, C.; Battista, T.; Fabbrocini, G.; Ruggiero, A. Management of patients with hidradenitis suppurativa during COVID-19 vaccination: An experience from southern Italy. Comment on: ‘Evaluating the safety and efficacy of COVID-19 vaccination in patients with hidradenitis suppurativa’. Clin. Exp. Dermatol. 2022, 47, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Gioacchini, H.; Sapigni, C.; Diotallevi, F.; Brisigotti, V.; Rizzetto, G.; Offidani, A.; Simonetti, O. New Insight into the Molecular Pathomechanism and Immunomodulatory Treatments of Hidradenitis Suppurativa. Int. J. Mol. Sci. 2023, 24, 8428. [Google Scholar] [CrossRef] [PubMed]
- Preda-Naumescu, A.; Ahmed, H.N.; Mayo, T.T.; Yusuf, N. Hidradenitis suppurativa: Pathogenesis, clinical presentation, epidemiology, and comorbid associations. Int. J. Dermatol. 2021, 60, e449–e458. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Damiani, G.; Orenstein, L.A.V.; Hamzavi, I.; Jemec, G.B. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 50–61. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Diaz-Calvillo, P.; Rodriguez-Pozo, J.A.; Cuenca-Barrales, C.; Martinez-Lopez, A.; Arias-Santiago, S.; Molina-Leyva, A. The Burden of Hidradenitis Suppurativa Signs and Symptoms in Quality of Life: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6709. [Google Scholar] [CrossRef]
- Matusiak, Ł.; Szczęch, J.; Kaaz, K.; Lelonek, E.; Szepietowski, J.C. Clinical Characteristics of Pruritus and Pain in Patients with Hidradenitis Suppurativa. Acta Derm.-Venereol. 2018, 98, 191–194. [Google Scholar] [CrossRef]
- Ring, H.C.; Maul, J.T.; Yao, Y.; Wu, J.J.; Thyssen, J.P.; Thomsen, S.F.; Egeberg, A. Drug Survival of Biologics in Patients with Hidradenitis Suppurativa. JAMA Dermatol. 2022, 158, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Scalvenzi, M.; Battista, T.; Fornaro, L.; Potestio, L.; Ruggiero, A.; Megna, M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Hidradenitis Suppurativa: A Review of Existing Trials and Real-Life Data. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Hung, C.Y.; Tsai, T.F. Efficacy and Safety of Biologics and Small Molecules for Moderate-to-Severe Hidradenitis Suppurativa: A Systematic Review and Network Meta-Analysis. Pharmaceutics 2023, 15, 1351. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, I.H.; Tai, C.C.; Chi, C.C. Biologics and Small Molecule Inhibitors for Treating Hidradenitis Suppurativa: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1303. [Google Scholar] [CrossRef]
- Glatt, S.; Jemec, G.B.E.; Forman, S.; Sayed, C.; Schmieder, G.; Weisman, J.; Rolleri, R.; Seegobin, S.; Baeten, D.; Ionescu, L.; et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Scalvenzi, M.; Ruggiero, A.; Potestio, L.; Battista, T.; Megna, M. Hidradenitis Suppurativa and JAK Inhibitors: A Review of the Published Literature. Medicina 2023, 59, 801. [Google Scholar] [CrossRef]
- Martora, F.; Marasca, C.; Fabbrocini, G.; Ruggiero, A. Strategies adopted in a southern Italian referral centre to reduce adalimumab discontinuation: Comment on ‘Can we increase the drug survival time of biologic therapies in hidradenitis suppurativa?’. Clin. Exp. Dermatol. 2022, 47, 1864–1865. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Frew, J.W.; Giamarellos-Bourboulis, E.J.; Jemec, G.B.E.; Del Marmol, V.; Marzano, A.V.; Nikolakis, G.; Sayed, C.J.; Tzellos, T.; Wolk, K.; et al. Target molecules for future hidradenitis suppurativa treatment. Exp. Dermatol. 2021, 30 (Suppl. S1), 8–17. [Google Scholar] [CrossRef]
- Markota Čagalj, A.; Marinović, B.; Bukvić Mokos, Z. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. Int. J. Mol. Sci. 2022, 23, 3753. [Google Scholar] [CrossRef]
- Di Lernia, V.; Goldust, M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin. Biol. Ther. 2018, 18, 897–903. [Google Scholar] [CrossRef]
- Di Caprio, R.; Caiazzo, G.; Cacciapuoti, S.; Fabbrocini, G.; Scala, E.; Balato, A. Safety concerns with current treatments for psoriasis in the elderly. Expert Opin. Drug Saf. 2020, 19, 523–531. [Google Scholar] [CrossRef]
- Garber, C.; Plotnikova, N.; Au, S.C.; Sorensen, E.P.; Gottlieb, A. Biologic and Conventional Systemic Therapies Show Similar Safety and Efficacy in Elderly and Adult Patients With Moderate to Severe Psoriasis. J. Drugs Dermatol. 2015, 14, 846–852. [Google Scholar] [PubMed]
- Ricceri, F.; Bardazzi, F.; Chiricozzi, A.; Dapavo, P.; Ferrara, F.; Mugheddu, C.; Romanelli, M.; Rongioletti, F.; Prignano, F. Elderly psoriatic patients under biological therapies: An Italian experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 143–146. [Google Scholar] [CrossRef]
- Wride, A.M.; Chen, G.F.; Spaulding, S.L.; Tkachenko, E.; Cohen, J.M. Biologics for Psoriasis. Dermatol. Clin. 2024, 42, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, W.; Zouboulis, C.C.; Prens, E.; Jemec, G.B.; Tzellos, T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev. Endocr. Metab. Disord. 2016, 17, 343–351. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Fritz, K.; Goebeler, M.; Hetzer, F.H.; Just, E.; Kirsten, N.; Kokolakis, G.; Kurzen, H.; Nikolakis, G.; et al. S2k guideline for the treatment of hidradenitis suppurativa/acne inversa—Short version. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. 2024, 22, 868–889. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Dickinson-Blok, J.L.; Gulliver, W.; Horváth, B.; Hughes, R.; Kimball, A.B.; Kirby, B.; Martorell, A.; Podda, M.; et al. Hidradenitis suppurativa/acne inversa: A practical framework for treatment optimization—Systematic review and recommendations from the HS ALLIANCE working group. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 19–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).