Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Participants
2.5. Data Analysis Methods
3. Results
3.1. Socio-Demographic Data
3.2. Univariate Descriptive Analysis
3.3. Bivariate Correlation Analysis
3.4. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. The Global Health Observatory. Global Health Estimates: Leading Causes of Death. Available online: https://Www.Who.Int/Data/Gho/Data/Themes/Mortality-and-Global-Health-Estimates/Ghe-Leading-Causes-of-Death (accessed on 20 May 2023).
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global Epidemiology of Cirrhosis—Aetiology, Trends and Predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Jalilova, A.S. The spread of cirrhosis of the liver by etiological factors. Orient. Renaiss. Innov. Educ. Nat. Soc. Sci. 2022, 2, 253–257. [Google Scholar]
- Patel, R.; Mueller, M. Alcoholic Liver Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Llamosas-Falcón, L.; Probst, C.; Buckley, C.; Jiang, H.; Lasserre, A.M.; Puka, K.; Tran, A.; Zhu, Y.; Rehm, J. How Does Alcohol Use Impact Morbidity and Mortality of Liver Cirrhosis? A Systematic Review and Dose–Response Meta-Analysis. Hepatol. Int. 2024, 18, 216–224. [Google Scholar] [CrossRef]
- GinËs, P.; Krag, A.; Abraldes, J.G. Liver Cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Cheemerla, S.; Balakrishnan, M. Epidemiologia Globală a Bolii Hepatice Cronice. Boală Hepatică Clin. 2021, 17, 365–370. [Google Scholar]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Aguirre-Villarreal, D.; Servin-Rojas, M.; Sánchez-Cedillo, A.; Chávez-Villa, M.; Hernandez-Alejandro, R.; Arab, J.P.; Ruiz, I.; Avendaño-Castro, K.P.; Matamoros, M.A.; Adames-Almengor, E.; et al. Liver Transplantation in Latin America: Reality and Challenges. Lancet Reg. Health–Am. 2023, 28, 100633. [Google Scholar] [CrossRef]
- Punekar, P.; Sharma, A.K.; Jain, A. A Study of Thyroid Dysfunction in Cirrhosis of Liver and Correlation with Severity of Liver Disease. Indian J. Endocrinol. Metab. 2018, 22, 645–650. [Google Scholar] [CrossRef]
- Gangadharam, D.Y.; Veeranarayana, D.G.; Kanha, D.M.M. A Study on Thyroid Function Tests as a Biomarker to Differentiate Acute from Chronic Liver Disease. J. Cardiovasc. Dis. Res. 2023, 14, 1–11. [Google Scholar]
- Nardin, G.D.; Colombo, B.d.S.; Ronsoni, M.F.; Silva, P.E.S.e; Fayad, L.; Wildner, L.M.; Bazzo, M.L.; Dantas-Correa, E.B.; Narciso-Schiavon, J.L.; Schiavon, L.d.L. Thyroid Hormone Profile Is Related to Prognosis in Acute Decompensation of Cirrhosis. Arch. Endocrinol. Metab. 2024, 68, e230249. [Google Scholar] [CrossRef]
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Thyroid Gland Disorders. In Harrison’s Manual of Medicine; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Kharb, S.; Garg, M.K.; Puri, P.; Brar, K.S.; Pandit, A.; Srivastava, S. Assessment of Thyroid and Gonadal Function in Liver Diseases. Indian J. Endocrinol. Metab. 2015, 19, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742. [Google Scholar] [CrossRef]
- Kim, H.J. Importance of Thyroid-Stimulating Hormone Levels in Liver Disease. J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Gionfra, F.; De Vito, P.; Pallottini, V.; Lin, H.-Y.; Davis, P.J.; Pedersen, J.Z.; Incerpi, S. The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer. Front. Endocrinol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct Effects of Thyroid Hormones on Hepatic Lipid Metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The Interplay between Thyroid and Liver: Implications for Clinical Practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Marino, L.; Kim, A.; Ni, B.; Celi, F.S. Thyroid Hormone Action and Liver Disease, a Complex Interplay. Hepatology 2023, 10-1097. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, V.; Tiwari, P.; Joge, N.; Misra, R. Thyroid Profile in Patients of Cirrhosis of Liver: A Cross-Sectional Study. J. Clin. Diagn. Res. 2017, 11, 6. [Google Scholar] [CrossRef]
- Ruf, A.; Dirchwolf, M.; Freeman, R.B. From Child-Pugh to MELD Score and beyond: Taking a Walk down Memory Lane. Ann. Hepatol. 2022, 27, 100535. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]
- Nallagangula, K.S.; Nagaraj, S.K.; Venkataswamy, L.; Chandrappa, M. Liver Fibrosis: A Compilation on the Biomarkers Status and Their Significance During Disease Progression. Future Sci. OA 2018, 4, FSO250. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, V.; Kaur, I.; Pandov, V.; Singh, A.; Sibia, R.P. Prevalence of Thyroid Dysfunction in Patients of Cirrhosis of Liver and Its Correlation with Severity of Cirrhosis. Int. J. Adv. Res. 2020, 8, 91–95. [Google Scholar] [CrossRef]
- Zucchi, R. Thyroid Hormone Analogues: An Update. Thyroid 2020, 30, 1099–1105. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Royston, J.P. An Extension of Shapiro and Wilk’s W Test for Normality to Large Samples. J. R. Stat. Soc. Ser. C Appl. Stat. 1982, 31, 115–124. [Google Scholar] [CrossRef]
- Royston, P. Remark AS R94: A Remark on Algorithm AS 181: The W-Test for Normality. J. R. Stat. Soc. Ser. C Appl. Stat. 1995, 44, 547–551. [Google Scholar] [CrossRef]
- Templ, M.; Kowarik, A.; Alfons, A.; de Cillia, G.; Prantner, B.; Rannetbauer, W. VIM: Visualization and Imputation of Missing Values. R Package Version 2022, 2, 2. [Google Scholar]
- West Haven Criteria for Hepatic Encephalopathy—GPnotebook. Available online: https://gpnotebook.com/pages/surgery/west-haven-criteria-for-hepatic-encephalopathy (accessed on 26 August 2024).
- Mandiga, P.; Kommu, S.; Bollu, P.C. Hepatic Encephalopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vincken, S.; Reynaert, H.; Schiettecatte, J.; Kaufman, L.; Velkeniers, B. Liver Cirrhosis and Thyroid Function: Friend or Foe? Acta Clin. Belg. 2017, 72, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhai, M.; Long, J.; Gong, Y.; Ren, C.; Zhang, D.; Lin, X.; Liu, S. The Burden of Liver Cirrhosis in Mortality: Results from the Global Burden of Disease Study. Front. Public Health 2022, 10, 909455. [Google Scholar] [CrossRef]
- Trajkovic, M.; Visser, T.J.; Mittag, J.; Horn, S.; Lukas, J.; Darras, V.M.; Raivich, G.; Bauer, K.; Heuer, H. Abnormal Thyroid Hormone Metabolism in Mice Lacking the Monocarboxylate Transporter 8. J. Clin. Investig. 2007, 117, 627–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
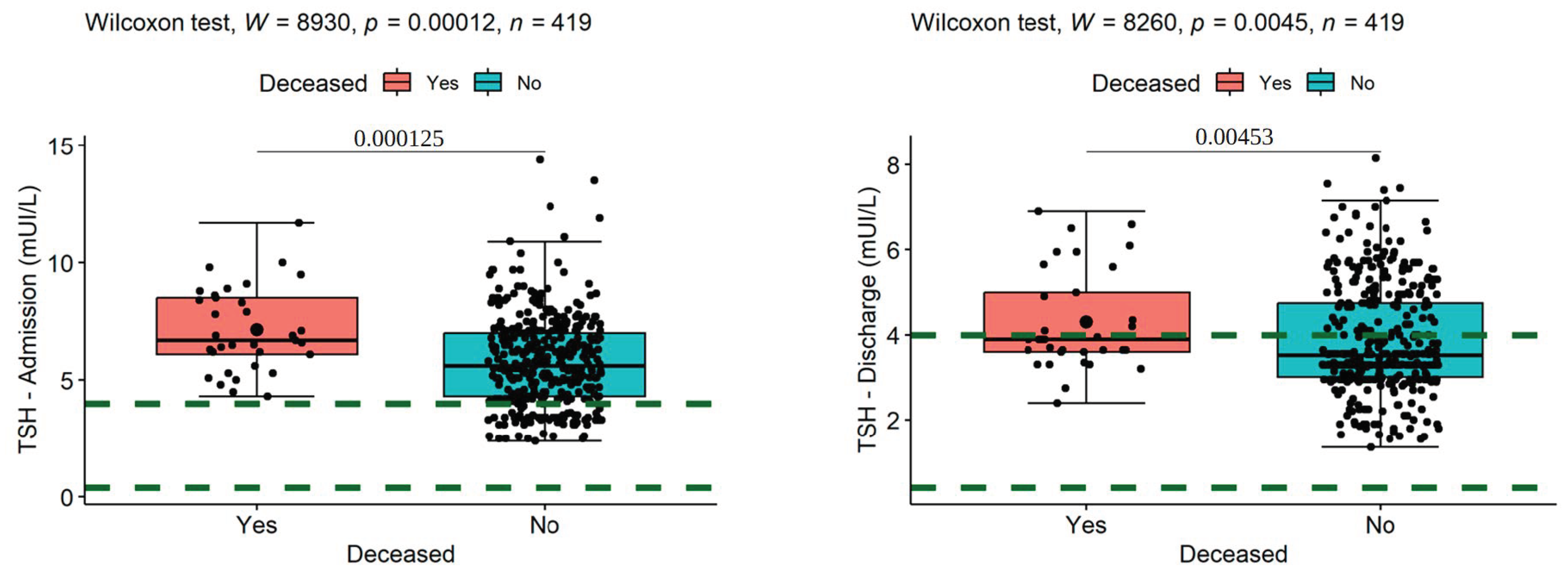
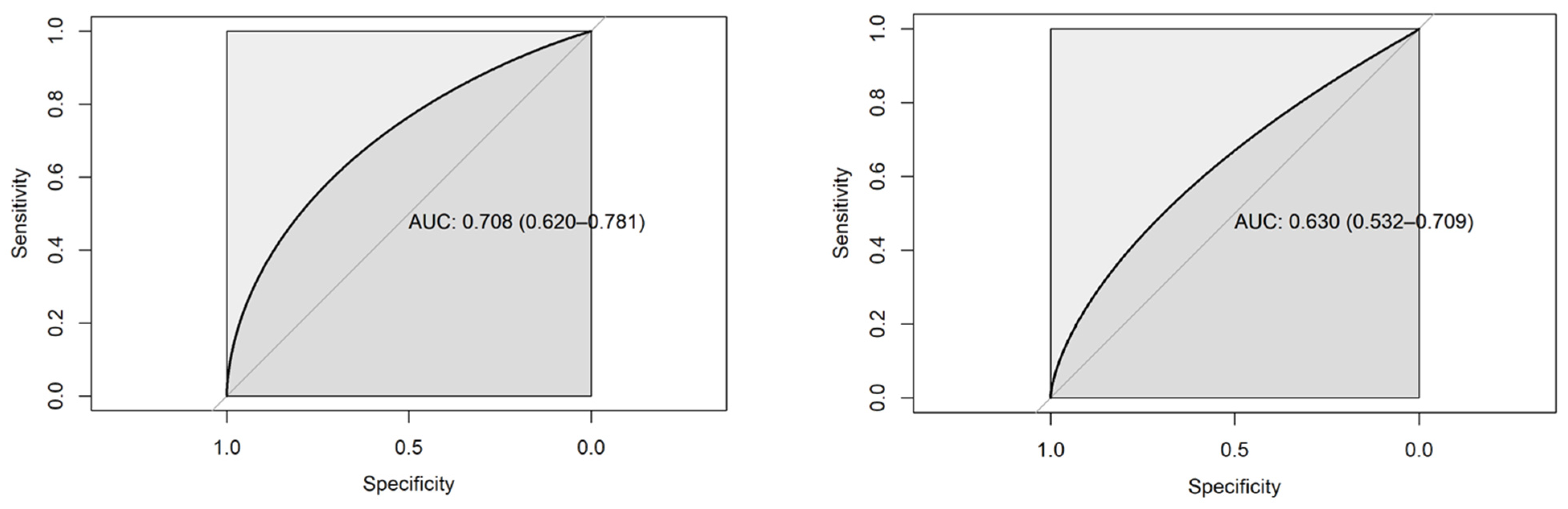
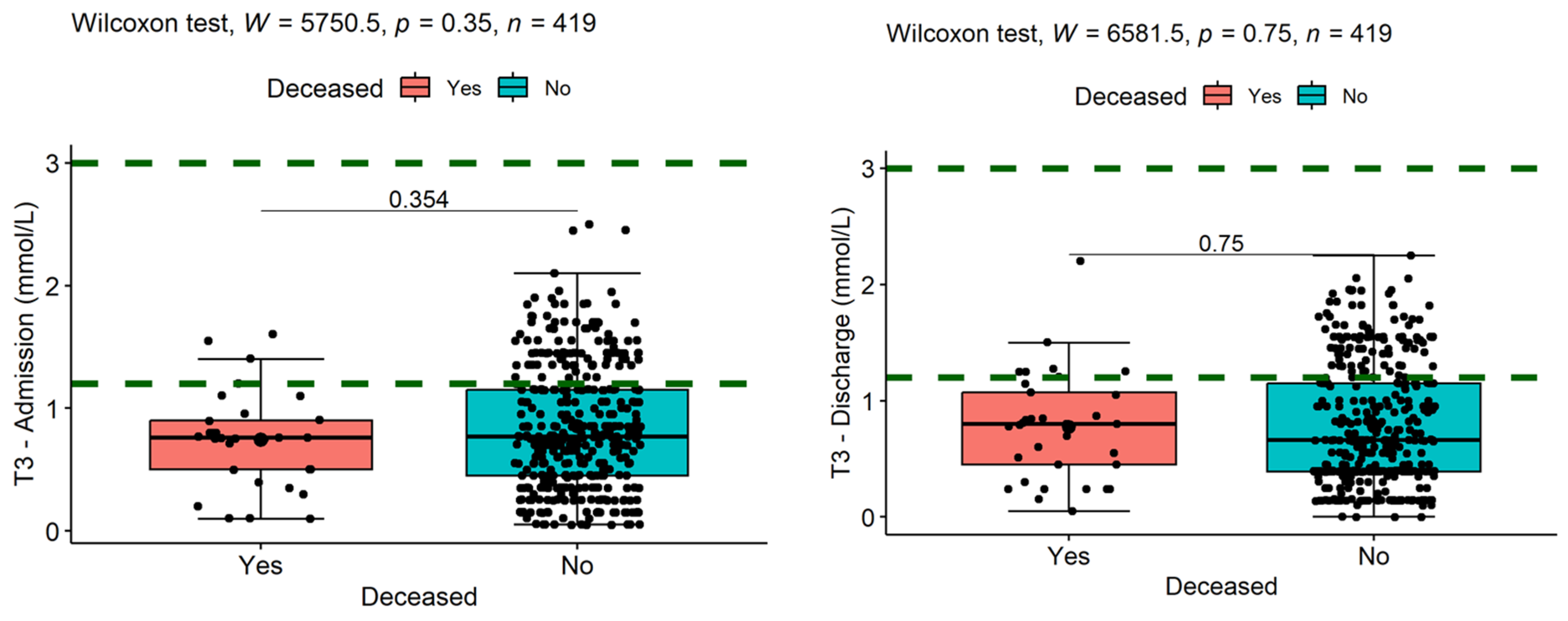
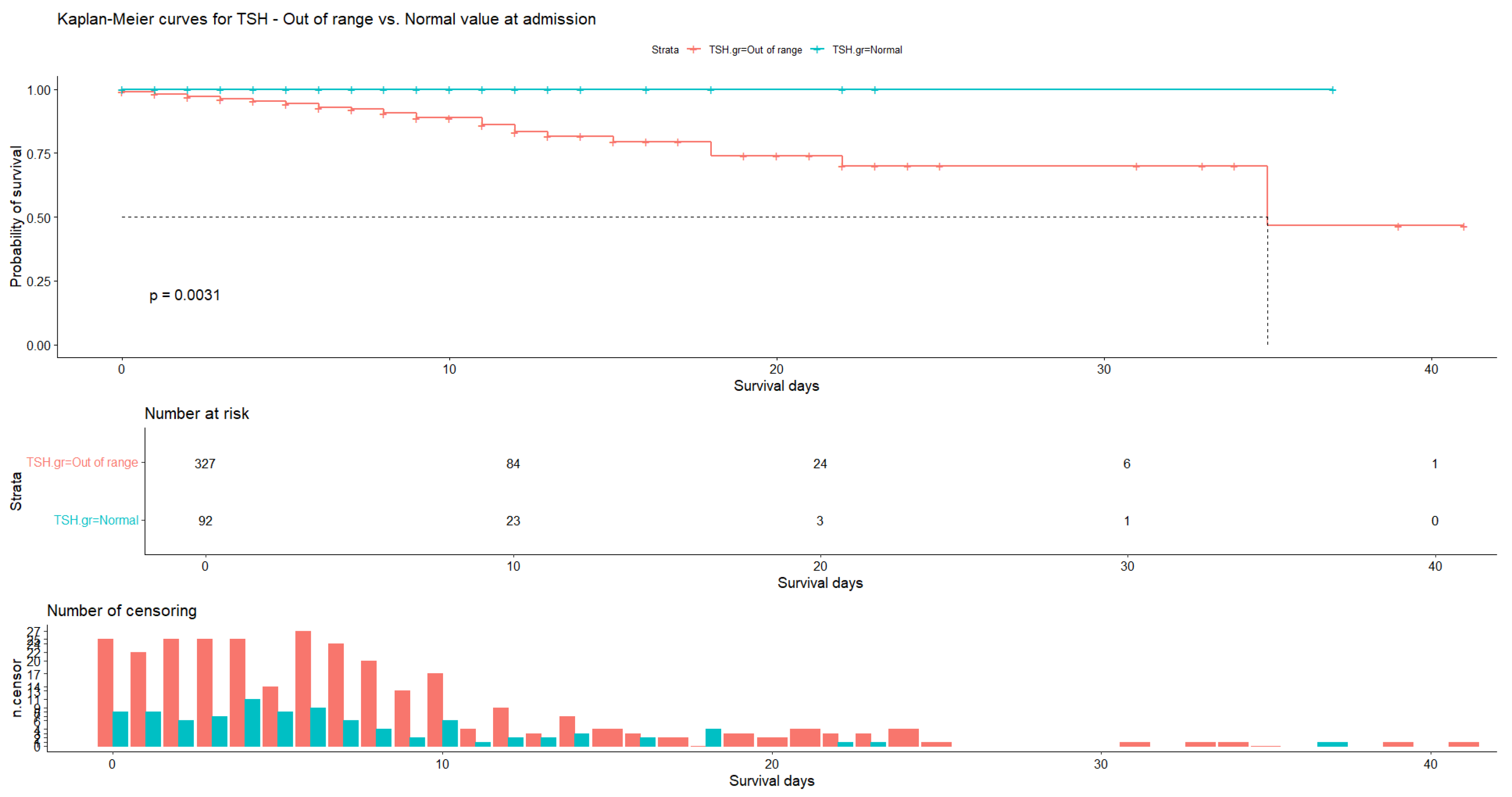
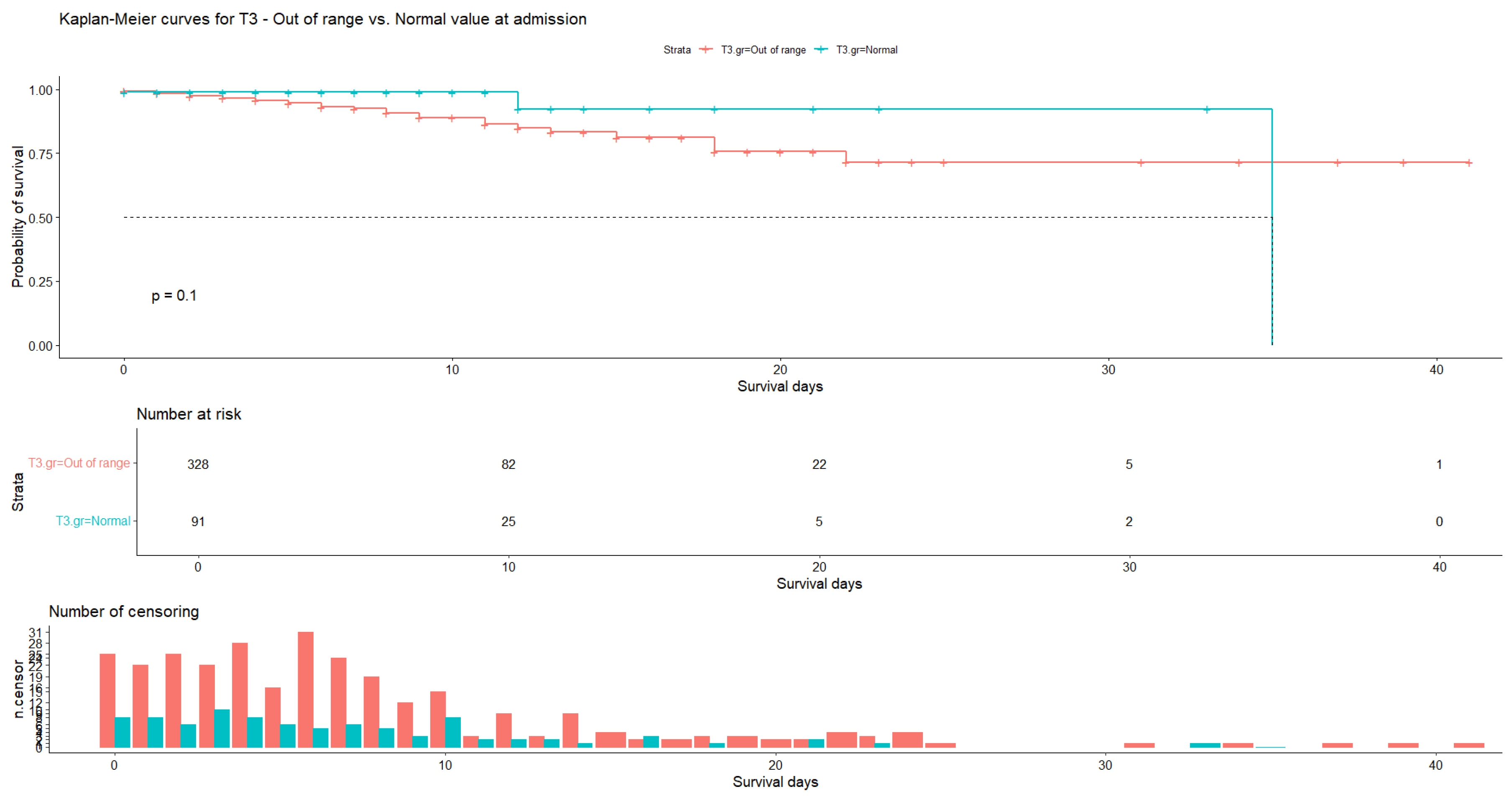
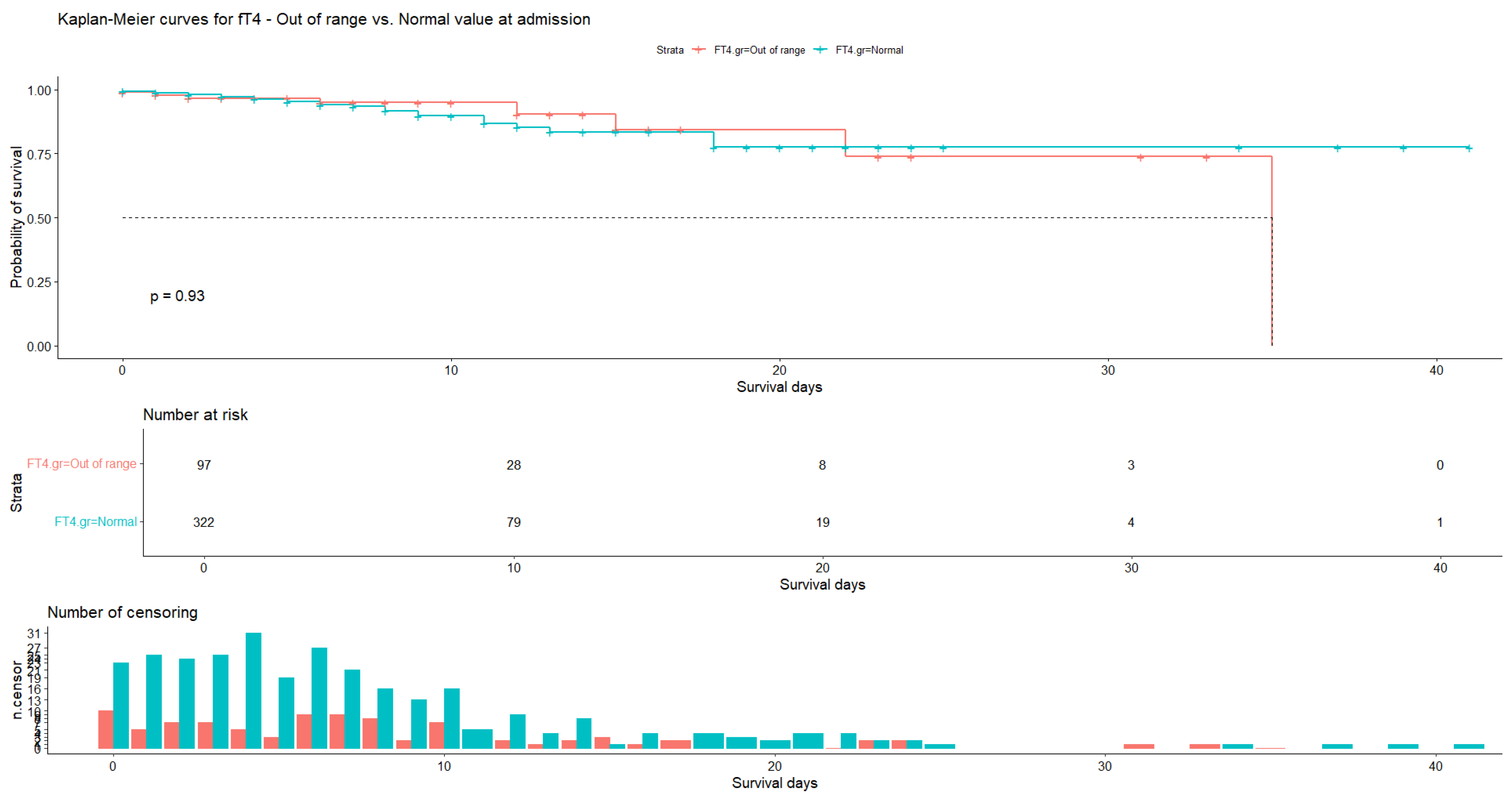
| Variable | N | Mean | Ab. Std | Median | Min | Max | Skew (ES) | Kurt (ES) | Normal |
|---|---|---|---|---|---|---|---|---|---|
| MELD Score | 419 | 14.08 | 4.32 | 14 | 5 | 31 | 0.72 (0.12) | 0.92 (0.24) | |
| TSH (mUI/L) | 419 | 5.89 | 1.92 | 5.7 | 2.4 | 14.4 | 0.70 (0.12) | 1.03 (0.24) | 0.4–4.0 |
| T3 (pmol/L) | 419 | 0.84 | 0.50 | 0.77 | 0.05 | 2.5 | 0.51 (0.12) | −0.24 (0.24) | 1.2–3.0 |
| fT4 (pmol/L) | 419 | 14.13 | 2.62 | 13.5 | 8.8 | 23 | 0.83 (0.12) | 0.43 (0.24) | 12.0–22.0 |
| Variable | N | Mean | Ab. Std | Median | Min | Max | Skew (ES) | Kurt (ES) | Normal |
|---|---|---|---|---|---|---|---|---|---|
| TSH (mUI/L) | 419 | 3.89 | 1.26 | 3.55 | 1.35 | 8.15 | 0.68 (0.12) | 0.04 (0.24) | 0.4–4.0 |
| T3 (pmol/L) | 419 | 0.78 | 0.52 | 0.65 | 0.00 | 2.25 | 0.64 (0.12) | −0.64 (0.24) | 1.2–3.0 |
| fT4 (pmol/L) | 419 | 12.76 | 3.04 | 12.4 | 8.8 | 23.4 | 0.86 (0.12) | 0.26 (0.24) | 12.0–22.0 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) MELD | - | ||||||||
| (2) Non-surviving patients | −0.09 | - | |||||||
| (3) Hospitalization (days) | −0.03 | −0.02 | - | ||||||
| (4) TSH—Admission | 0.27 *** | −0.19 *** | 0.03 | - | |||||
| (5) TSH—Discharge | 0.21 *** | −0.14 ** | 0.00 | 0.75 *** | - | ||||
| (6) T3—Admission | −0.10 | 0.04 | 0.01 | −0.35 *** | −0.28 *** | - | |||
| (7) T3—Discharge | −0.17 *** | −0.02 | −0.01 | −0.23 *** | −0.14 ** | 0.46 *** | - | ||
| (8) fT4—Admission | −0.02 | 0.01 | 0.06 | −0.18 *** | −0.07 | 0.23 *** | 0.13 ** | - | |
| (9) fT4—Discharge | 0.08 | 0.02 | 0.07 | 0.05 | 0.20 *** | 0.09 | 0.51 *** | 0.42 *** | - |
| Media | 14.08 | 1.92 | 7.21 | 5.89 | 3.89 | 0.84 | 0.78 | 14.13 | 12.76 |
| Standard deviations | 4.32 | 0.27 | 6.73 | 1.92 | 1.26 | 0.50 | 0.52 | 2.62 | 3.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belu, A.M.; Nicoara, A.D.; Belu, D.M.; Circo, E. Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis. Medicina 2024, 60, 1474. https://doi.org/10.3390/medicina60091474
Belu AM, Nicoara AD, Belu DM, Circo E. Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis. Medicina. 2024; 60(9):1474. https://doi.org/10.3390/medicina60091474
Chicago/Turabian StyleBelu, Anca M., Alina D. Nicoara, Daniela M. Belu, and Eduard Circo. 2024. "Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis" Medicina 60, no. 9: 1474. https://doi.org/10.3390/medicina60091474
APA StyleBelu, A. M., Nicoara, A. D., Belu, D. M., & Circo, E. (2024). Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis. Medicina, 60(9), 1474. https://doi.org/10.3390/medicina60091474









