Copper Serum Levels in the Hemodialysis Patient Population
Abstract
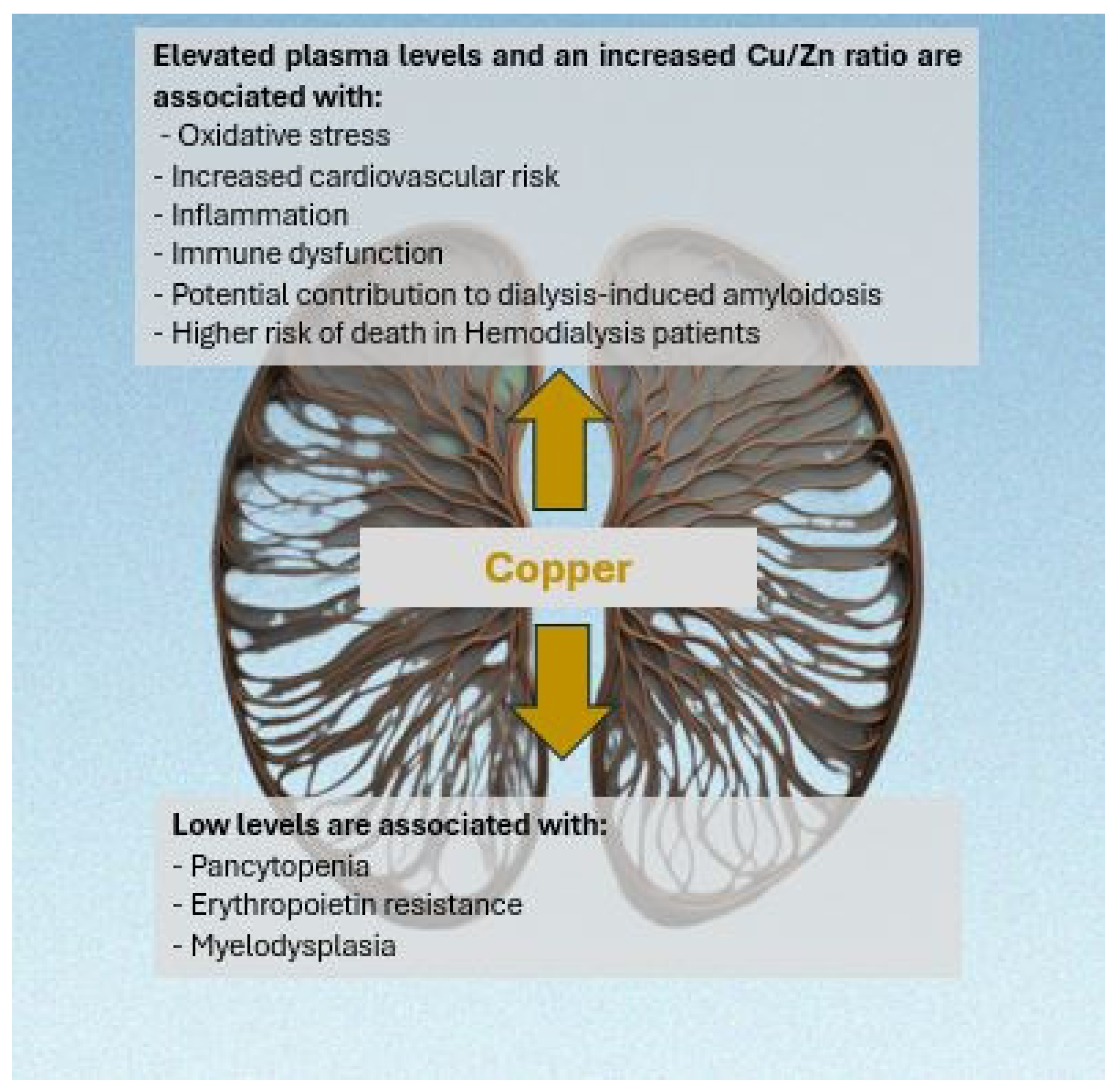
1. Introduction
2. Copper in Hemodialysis
2.1. Copper and Anemia in Hemodialysis
2.2. Balance of Copper and Zinc in Hemodialysis
2.3. Copper/Zinc Ratio in Hemodialysis
2.4. Copper and Hemodialysis-Related Amyloidosis
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| CKD | Chronic Kidney Disease |
| CRRT | Continuous Renal Replacement Therapy |
| Cu | Copper |
| CuD | Copper-Deficient Diet |
| CuS | Copper-Supplemented Diet |
| DM | Diabetes Mellitus |
| EPO | Erythropoietin |
| ESRD | End-Stage Renal Disease |
| HD | Hemodialysis |
| hs-CRP | High-Sensitivity C Reactive Protein |
| HVHF | High-Volume Hemofiltration |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| IL-1 beta | Interleukin-1 Beta |
| LDL | Low-Density Lipoprotein |
| pCU | Plasma Copper |
| PD | Peritoneal Dialysis |
| RBC | Red Blood Cells |
| RRT | Renal Replacement Therapy |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| TSF | Transferrin |
| Zn | Zinc |
References
- Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2022, 13, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, J.-J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.-T.; Antholine, W.E.; Wamer, W. Dual role of selected antioxidants found in dietary supplements: Crossover between anti-and pro-oxidant activities in the presence of copper. J. Agric. Food Chem. 2012, 60, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Patil, M.; Sheth, K.A.; Krishnamurthy, A.C.; Devarbhavi, H. A review and current perspective on Wilson disease. J. Clin. Exp. Hepatol. 2013, 3, 321–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, A. Role of Zn and copper in erythropoiesis in patients on hemodialysis. J. Ren. Nutr. 2022, 32, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef]
- Tahir, N.; Ashraf, A.; Waqar, S.H.; Rafae, A.; Kantamneni, L.; Sheikh, T.; Khan, R. Copper deficiency, a rare but correctable cause of pancytopenia: A review of literature. Expert Rev. Hematol. 2022, 15, 999–1008. [Google Scholar] [CrossRef]
- US Department of Health and Human Services; Public Health Service; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Copper; US Department of Health and Human Services: Atlanta, GA, USA, 2004. [Google Scholar]
- Ikee, R.; Tsunoda, M.; Sasaki, N.; Sato, N.; Hashimoto, N. Clinical factors associated with serum copper levels and potential effect of sevelamer in hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 839–845. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Copper and iron in Alzheimer’s disease: A systematic review and its dietary implications. Br. J. Nutr. 2012, 107, 7–19. [Google Scholar] [CrossRef]
- Ay, A.; Alkanli, N.; Ustundag, S. Investigation of the Relationship Between IL-18 (-607 C/A), IL-18 (-137 G/C), and MMP-2 (-1306 C/T) Gene Variations and Serum Copper and Zinc Levels in Patients Diagnosed with Chronic Renal Failure. Biol. Trace Elem. Res. 2021, 200, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Bello, A.; Field, C.J.; Gill, J.S.; Hemmelgarn, B.R.; Holmes, D.T.; Jindal, K.; Klarenbach, S.W.; Manns, B.J.; et al. Concentrations of Trace Elements and Clinical Outcomes in Hemodialysis Patients: A Prospective Cohort Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 907–915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zima, T.; Mestek, O.; Němeček, K.; Bártová, V.; Fialová, J.; Tesař, V.; Suchánek, M. Trace elements in hemodialysis and continuous ambulatory peritoneal dialysis patients. Blood Purif. 1998, 16, 253–260. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Shen, W.S.; Lee, L.Y.; Wu, T.L.; Ning, H.C.; Sun, C.F. Long-term changes in trace elements in patients undergoing chronic hemodialysis. Biol. Trace Elem. Res. 2006, 109, 115–121. [Google Scholar] [CrossRef]
- Koca, T.; Berber, A.; Koca, H.B.; Demir, T.A.; Koken, T. Effects of hemodialysis period on levels of blood trace elements and oxidative stress. Clin. Exp. Nephrol. 2010, 14, 463–468. [Google Scholar] [CrossRef]
- Emenaker, N.J.; DiSilvestro, R.A.; Nahman, N.S.; Percival, S. Copper-related blood indexes in kidney dialysis patients. Am. J. Clin. Nutr. 1996, 64, 757–760. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Filippidis, G.; Golfinopoulos, S.; Spanoulis, A.; Liakopoulos, V.; Stefanidis, I. Serum copper and ferroportin in monocytes of hemodialysis patients are both decreased but unassociated. Int. Urol. Nephrol. 2014, 46, 1825–1831. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Reyes-Pérez, A.; Lopez-Garcia, H.; Palomares-Bayo, M.; Olalla-Herrera, M.; Lopez-Martinez, M.C. Longitudinal study of serum zinc and copper levels in hemodialysis patients and their relation to biochemical markers. Biol. Trace Elem. Res. 2006, 113, 209–222. [Google Scholar] [CrossRef]
- Batista, M.N.; Cuppari, L.; Pedrosa LD, F.C.; Almeida MD, G.; Bruno de Almeida, J.; Queiroz de Medeiros, A.C.; Canziani ME, F. Effect of end-stage renal disease and diabetes on zinc and copper status. Biol. Trace Elem. Res. 2006, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reina de la Torre, M.L.; Navarro-Alarcón, M.; Del Moral, L.M.; de la Serrana, H.L.G.; Palomares-Bayo, M.; Oliveras López, M.J.; Herrera, R.M.B.; Agil, A. Serum Zn levels and Cu/Zn ratios worsen in hemodialysis patients, implying increased cardiovascular risk: A 2-year longitudinal study. Biol. Trace Elem. Res. 2014, 158, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A. Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis. Nutrients 2023, 15, 4887. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Ishimura, E.; Tsujimoto, Y.; Kakiya, R.; Tabata, T.; Mori, K.; Shoji, T.; Yasuda, H.; Nishizawa, Y.; Inaba, M. Trace elements in the hair of hemodialysis patients. Biol. Trace Elem. Res. 2011, 143, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Rakhshanizadeh, F. Serum Trace Elements in Children with End-Stage Renal Disease. J. Ren. Nutr. 2019, 29, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, S.T.; Hamidian, M.R.; Madani, A.; Ataei, N.; Mohseni, P.; Roudbari, M.; Haddadi, M. Serum zinc and copper levels in children with chronic renal failure. Pediatr Nephrol. 2006, 21, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.; Court Brown, F.; Wallace, D.; Reid, C.J.D.; Sinha, M.D. Trace element and vitamin concentrations in paediatric dialysis patients. Pediatr Nephrol. 2018, 33, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, Q.; He, T.; Zhou, Z.; Chen, D.; Yi, X.; Wang, Z.; Wang, R.; Tan, G.; Yu, P.; et al. Polydopamine-Assisted Immobilization of Copper Ions onto Hemodialysis Membranes for Antimicrobial. ACS Appl. Bio Mater. 2018, 1, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Munie, S.; Pintavorn, P. Erythropoietin-Resistant Anemia Secondary to Zinc-Induced Hypocupremia in a Hemodialysis Patient. Case Rep. Nephrol. Dial. 2021, 11, 167–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higuchi, T.; Matsukawa, Y.; Okada, K.; Oikawa, O.; Yamazaki, T.; Ohnishi, Y.; Fujita, T.; Fukuda, N.; Soma, M.; Matsumoto, K. Correction of copper deficiency improves erythropoietin unresponsiveness in hemodialysis patients with anemia. Intern. Med. 2006, 45, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.N.; Fregonesi, B.M.; Machado, C.S.; Zagui, G.S.; Kusumota, L.; Suzuki, A.K.; da Costa, J.A.C.; Llopart, J.S.; Nadal, M.; Domingo, J.L.; et al. Hemodialysis Water Parameters as Predisposing Factors for Anemia in Patients in Dialytic Treatment: Application of Mixed Regression Models. Biol. Trace Elem. Res. 2019, 190, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kurihara, S.; Anayama, M.; Makino, Y.; Nagasaw, M. Four cases of serum copper excess in patients with renal anemia receiving a hypoxia-inducible factor-prolyl hydroxylase inhibitor: A possible safety concern. Case Rep. Nephrol. Dial. 2022, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Nishime, K.; Kondo, M.; Saito, K.; Miyawaki, H.; Nakagawa, T. Zinc Burden Evokes Copper Deficiency in the Hypoalbuminemic Hemodialysis Patients. Nutrients 2020, 12, 577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López Melero, E.; Ruíz-Roso, G.; Botella, I.; Ortego Pérez, S.; Delgado, M.; Fernández Lucas, M. Pancitopenia secundaria a déficit de cobre en un paciente en hemodiálisis. Nefrología 2019, 39, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Marriott, B.P.; Birt, D.F.; Stallings, V.A.; Yates, A.A. (Eds.) Present Knowledge in Nutrition, 11th ed.; Wiley-Blackwell: Cambridge, MA, USA, 2020; pp. 393–408. [Google Scholar]
- Yaldizli, Ö.; Johansson, U.; Gizewski, E.R.; Maschke, M. Copper deficiency myelopathy induced by repetitive parenteral zinc supplementation during chronic hemohemodialysis. J. Neurol. 2006, 253, 1507–1509. [Google Scholar] [CrossRef]
- Szpanowska-Wohn, A.; Kolarzyk, E.; Chowaniec, E. Estimation of intake of zinc, copper and iron in the diet of patients with chronic renal failure treated by haemodialysis. Biol. Trace Elem. Res. 2008, 124, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Visconti, L.; Giuffrida, A.E.; Labbozzetta, V.; Peritore, L.; Lipari, A.; Calabrese, V.; Piccoli, G.B.; Torreggiani, M.; Siligato, R.; et al. Role of Zinc in Diabetic Kidney Disease. Nutrients 2022, 14, 1353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, C.H.; Wang, C.L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calabrese, M.F.; Miranker, A.D. Formation of a stable oligomer of beta-2 microglobulin requires only transient encounter with Cu(II). J. Mol. Biol. 2007, 367, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.F.; Miranker, A.D. Metal binding sheds light on mechanisms of amyloid assembly. Prion 2009, 3, 1–4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Lorenzi, E.; Colombo, R.; Sabella, S.; Corlin, D.B.; Heegaard, N.H. The influence of Cu(2+) on the unfolding and refolding of intact and proteolytically processed beta(2)-microglobulin. Electrophoresis 2008, 29, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, R.; Mendoza, V.L.; Bridgewater, J.D.; Zhang, G.; Vachet, R.W. Copper binding to beta-2-microglobulin and its pre-amyloid oligomers. Biochemistry 2009, 48, 9871–9881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, C.J.; Gelfand, M.; Atreya, C.; Miranker, A.D. Kidney dialysis-associated amyloidosis: A molecular role for copper in fiber formation. J. Mol. Biol. 2001, 309, 339–345. [Google Scholar] [CrossRef] [PubMed]
| Authors | Years | Patients | Serum Copper Levels | Note |
|---|---|---|---|---|
| Emenaker et al. [19] | 1996 | 23 HD patients (gender not specified) Mean age 50 (28–77) Dialysis vintage not specified Membrane of cellulose acetate, saponified cellulose esters, or polysulfone-based membranes | 134.6 ± 27.1 μg/dL | Significantly lower levels than control |
| Zima et al. [16] | 1998 | 36 HD patients (19 males, 17 females) Mean age 57 (24–77) Dialysis vintage not specified Low-flux dialyzers | 69 ± 3 μg/dL | No significant difference in serum copper levels between HD patients, PD patients, and control group |
| Batista et al. [22] | 2005 | 63 HD patients (31 males/32 females) 33 with DM2, 30 without DM2 Dialysis vintage 29.0 ± 27.4 months in DM group Dialysis vintage 43.3 ± 33.5 months in non-DM group Acetate cellulose membrane | Diabetic: 130.02 ± 36.7 μg/dL (pre-HD); Non-diabetic: 109.7 ± 26.2 μg/dL (pre-HD) | Increased plasma copper in diabetic group (p < 0.05) |
| Alarcon et al. [21] | 2005 | 48 HD patients (32 males, 16 females) Mean age: 50.1 ± 11.2 Dialysis vintage not specified Membrane not specified | Three samples were taken before each dialysis session at three different times, 3 months apart each First sampling: 113.6 ± 4.5 μg/dL Second sampling: 128.8 ± 4.7 μg/dL Third sampling: 118.8 ± 5 μg/dL | No statistically significant differences among the three blood samples. p > 0.05 In first and second samples, copper levels were higher in HD patients than in healthy controls; p < 0.05 Copper levels were not influenced by cause of ESRD |
| Hsieh et al. [17] | 2005 | 77 HD patients Mean age not specified Dialysis vintage not specified Membrane not specified | 94,190 ± 16,970 μg/dL | Cu concentration was statistically the same between the hemodialysis and control groups |
| Koca et al. [18] | 2010 | 110 HD patients (46 males, 54 females) Mean age 54 ± 14 Dialysis vintage: 0–2 years n = 31; 3–5 years n = 40; 6–8 years n = 27; 9–11 years (n = 13) Polysulfone-based membranes | 0–2 years of HD = 111.1 ± 28.6 μg/dL (pre-HD) 3–5 years of HD = 107.6 ± 21.4 μg/dL (pre-HD) 6–8 years of HD = 106.1 ± 33.3 μg/dL (pre-HD) 9–11 years of HD = 129.8 ± 34.7 μg/dL (pre-HD) | No statistically significant differences between control and HD groups and no significant difference between different dialysis age groups |
| Ikee et al. [10] | 2012 | 48 HD patients (28 males/20 females) Age 71 ± 10 Dialysis vintage 84 ± 72 months Polysulfone membranes in all patients | 94 μg/dL (pre-HD) | Positive correlations between serum copper and total cholesterol, LDL-cholesterol, and C-reactive protein and negative correlations with sevelamer dose |
| Eleftheriadis et al. [20] | 2014 | 34 HD patients (23 males, 11 females) Age 59.5 ± 13.6 Dialysis vintage 58.3 ± 40.2 (months) Polysulfone low-flux dialyzers | 64.77 μg/dL | Serum copper in HD patients was reduced compared to controls; p < 0.001 |
| Reina de la Torre et al. [23] | 2014 | 116 HD patients (51 male, 65 females) Age 74.6 ± 11.44 years Dialysis vintage at least 6 months, not further specified Low-flux polysulfone membranes | 102.2 ± 26.6 μg/dL (pre-HD) | Serum Cu levels did not significantly differ between HD patients and controls (p = 0.059) Copper was higher in those with diabetic nephropathy compared to glomerulonephritis, gout, or analgesic or polycystic nephropathy |
| Tonelli et al. [13] | 2018 | 1278 HD patients (784 males/494 females) Age 62 ± 10 Dialysis vintage not specified High-flux polysulfone membranes | Not specified | Higher copper concentrations were associated with higher risk of death (odds ratio, 1.07 per decile; 99.2% coincidence interval, 1.00 to 1.15) |
| Takahashi [6] | 2023 | 40 HD patients (20 males/20 females) Age 75 ± 11 Dialysis vintage 84 ± 60 Membranes not specified | 173 ± 27.9 (μg/dL) (no zinc no Roxadustat) 147.2 ± 35.1 (μg/dL) (zinc, no Roxadustat) 291.4 ± 46.4 (μg/dL) (no zinc, post Roxadustat) 182.1 ± 59.9 (μg/dL) (zinc, post Roxadustat) | Roxadustat increases the serum copper concentrations of patients, and zinc supplementation can reduce serum copper concentrations |
| Age | Cu RDAs for Male (mcg) | Cu RDAs for Female (mcg) | Zn RDAs for Male (mg) | Zn RDAs for Female (mg) |
|---|---|---|---|---|
| Birth to 6 months | 200 | 200 | 2 | 2 |
| 7–12 months | 220 | 220 | 3 | 3 |
| 1–3 yrs | 340 | 340 | 3 | 3 |
| 4–8 yrs | 440 | 440 | 5 | 5 |
| 9–13 yrs | 700 | 700 | 8 | 8 |
| 14–18 yrs | 890 | 890 | 11 | 9 |
| 19+ yrs | 900 | 900 | 11 | 8 |
| Microelements | Serum Levels |
|---|---|
| Copper | 10–25 mcmol/L (63.5–158.9 mcg/dL) |
| Zinc | 12 to 18 mcmol/L (80 to 120 mcg/dL) |
| Ceruloplasmin | 180–400 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Peritore, L.; Labbozzetta, V.; Giuffrida, A.E.; Lipari, A.; Spallino, E.; Calabrese, V.; Visconti, L.; Santoro, D. Copper Serum Levels in the Hemodialysis Patient Population. Medicina 2024, 60, 1484. https://doi.org/10.3390/medicina60091484
Gembillo G, Peritore L, Labbozzetta V, Giuffrida AE, Lipari A, Spallino E, Calabrese V, Visconti L, Santoro D. Copper Serum Levels in the Hemodialysis Patient Population. Medicina. 2024; 60(9):1484. https://doi.org/10.3390/medicina60091484
Chicago/Turabian StyleGembillo, Guido, Luigi Peritore, Vincenzo Labbozzetta, Alfio Edoardo Giuffrida, Antonella Lipari, Eugenia Spallino, Vincenzo Calabrese, Luca Visconti, and Domenico Santoro. 2024. "Copper Serum Levels in the Hemodialysis Patient Population" Medicina 60, no. 9: 1484. https://doi.org/10.3390/medicina60091484
APA StyleGembillo, G., Peritore, L., Labbozzetta, V., Giuffrida, A. E., Lipari, A., Spallino, E., Calabrese, V., Visconti, L., & Santoro, D. (2024). Copper Serum Levels in the Hemodialysis Patient Population. Medicina, 60(9), 1484. https://doi.org/10.3390/medicina60091484









