Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases
Abstract
1. Introduction
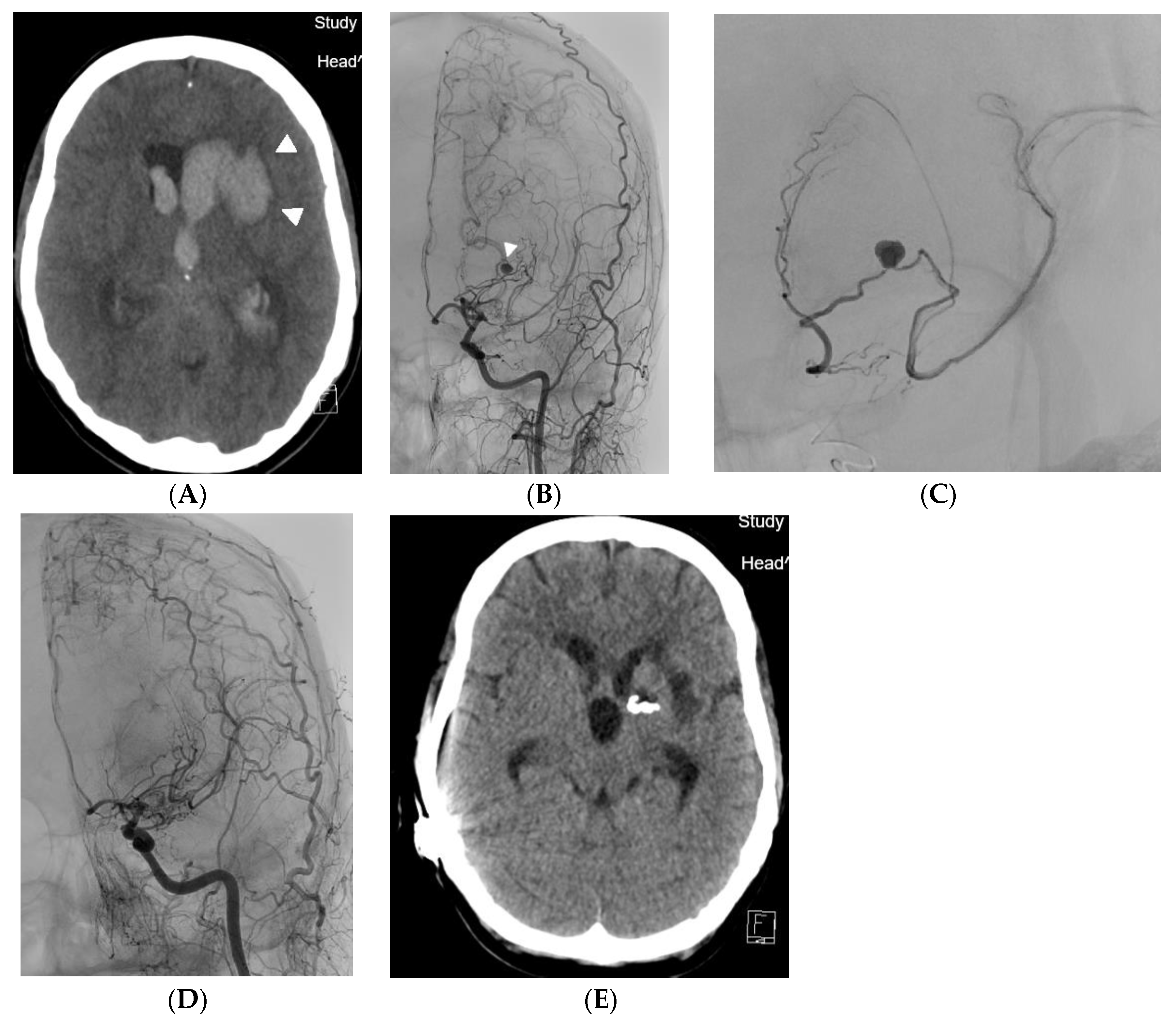
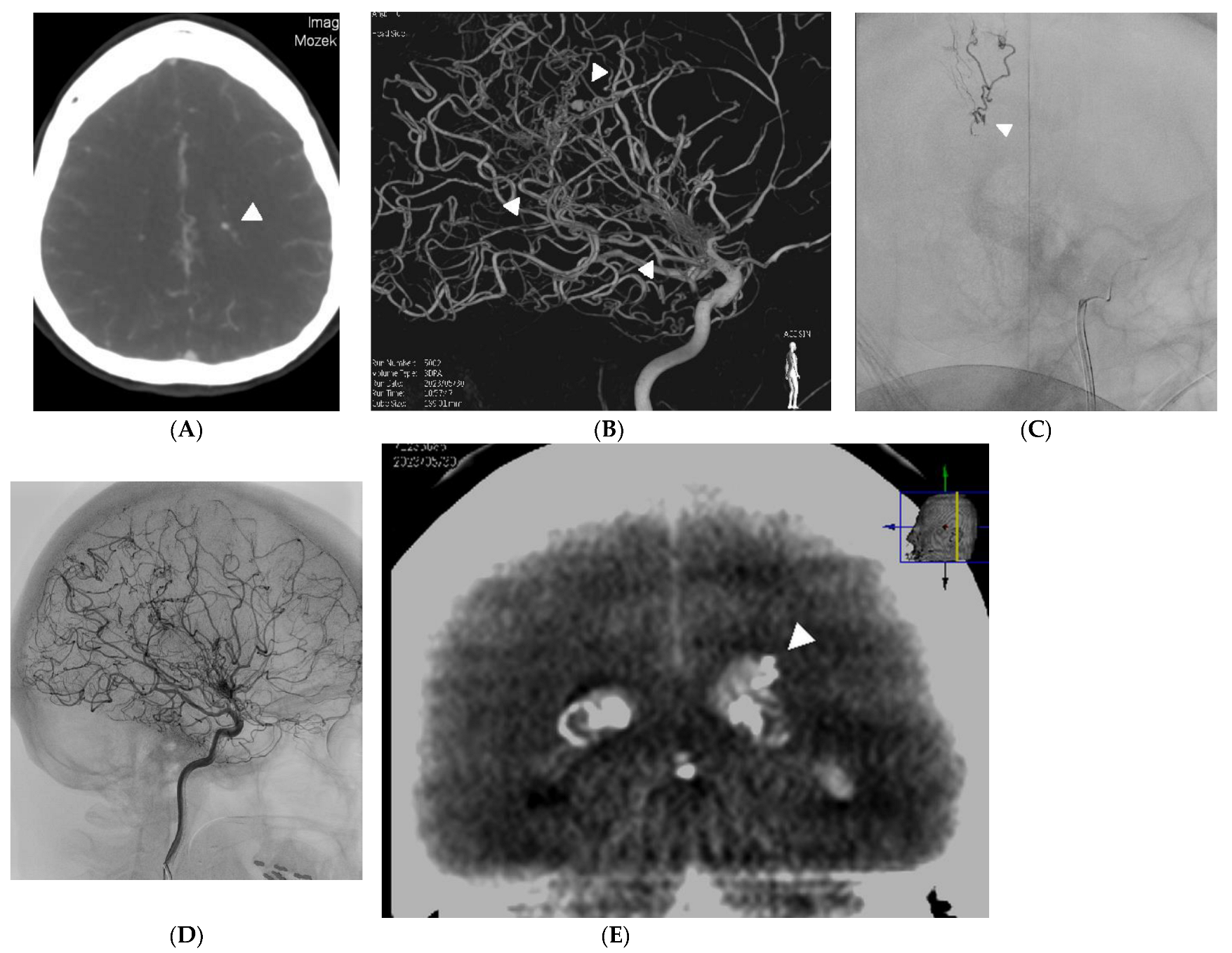
2. Case Reports
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, J.; Takaku, A. Cerebrovascular moyamoya disease: Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med.-Chir. 2012, 52, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Demartini, Z., Jr.; Teixeira, B.C.; Koppe, G.L.; Gatto, L.A.M.; Roman, A.; Munhoz, R.P. Moyamoya disease and syndrome: A review. Radiol. Bras. 2022, 55, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Besses, C. Cerebrovascular disease as the initial clinical presentation of haematological disorders. Eur. Neurol. 1997, 37, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Scott, R.M. Spontaneous occlusion of the circle of Willis in children: Pediatric moyamoya summary with proposed evidence-based practice guidelines: A review. J. Neurosurg. Pediatr. 2012, 9, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Czabanka, M.; Peña-Tapia, P.; Schubert, G.; Heppner, F.; Martus, P.; Horn, P.; Schmiedek, P.; Vajkoczy, P. Proposal for a new grading of moyamoya disease in adult patients. Cerebrovasc. Dis. 2011, 32, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.K.H.; Davidoff, C.; Lo Presti, A.; Ni, W.; Rhim, J.K.; Simons, M.; Stoodley, M.A. Treatment of ruptured aneurysms of the choroidal collateral system in moyamoya disease: A systematic review and data analysis. J. Neurosurg. 2021, 136, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Tominaga, T. Hemorrhagic moyamoya isease: A recent update. J. Korean Neurosurg. Soc. 2019, 62, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, C.K.; Park, E.K.; Shim, K.W.; Kim, D.S.; Chung, J.; Kim, Y.B.; Lee, J.W.; Park, K.Y. Clinical features and outcomes of intracranial aneurysm associated with moyamoya disease. J. Clin. Neurol. 2020, 16, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Wang, M.; Meng, Q.; Wang, C. Treatment strategies of ruptured intracranial aneurysms associated with moyamoya disease. Br. J. Neurosurg. 2021, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, Z.; Gao, X.; Su, J.; Jiang, H.; Yang, S.; Zhang, Q.; Ni, W.; Gu, Y. Safety and efficacy of remote ischemic conditioning in adult moyamoya disease patients undergoing revascularization surgery: A pilot study. Front. Neurol. 2023, 14, 1200534. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Li, G.; Luan, T.; Xu, K.; Yu, J. The prospects and pitfalls in the endovascular treatment of moyamoya disease–associated intracranial aneurysms. Neurosurg. Rev. 2021, 44, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Choulakian, A.; Drazin, D.; Alexander, M.J. NBCA embolization of a ruptured intraventricular distal anterior choroidal artery aneurysm in a patient with moyamoya disease. J. NeuroInterv. Surg. 2010, 2, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lv, X.; Li, Y.; Lv, M. Onyx embolization of a ruptured rotundum foramen artery aneurysm in a patient with moyamoya disease: A case report. World Neurosurg. 2015, 84, e1171–e1173. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhu, Q.; Liu, X.-Z.; Ling, H.-P.; Na, S.-J.; Liu, T.; Zhang, Y.-H.; Hang, C.-H.; Liu, K.-D.; Zhang, Q.-R. Efficacy of liquid embolic agent treatment in hemorrhagic peripheral intracranial aneurysms: A single-center experience. Brain Sci. 2022, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.S.; Rinaldo, L.; Brinjikji, W.; Lanzino, G. Location-based treatment of intracranial aneurysms in moyamoya disease: A systematic review and descriptive analysis. Neurosurg. Rev. 2021, 44, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Saga, I.; Kojima, A.; Horiguchi, T. Short-Term Spontaneous Resolution of Ruptured Peripheral Aneurysm in Moyamoya Disease. World Neurosurg. 2019, 126, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Jiang, H.; Xu, B.; Lei, Y.; Yang, H.; Su, J.; Gu, Y.; Mao, Y. Treatment of aneurysms in patients with moya moya disease: A 10 year single center experience. J. Neurosurg. 2018, 128, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.C.; Funaki, T.; Houkin, K.; Inoue, T.; Ogasawara, K.; Nakagawara, J.; Kuroda, S.; Yamada, K.; Miyamoto, S. Significance of the hemorrhagic site for recurrent bleeding: Prespecified analysis in the Japan adult moyamoya trial. Stroke 2016, 47, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.X.Y.; Carrazana, E.; Mitchell, C.; Viereck, J.; Liow, K.K.; Ghaffari-Rafi, A. Identification of associations and distinguishing moyamoya disease from ischemic strokes of other etiologies: A retrospective case-control study. Ann. Med. Surg. 2022, 78, 103771. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryška, P.; Lojík, M.; Habalová, J.; Kajzrová, C.; Česák, T.; Vítková, E.; Bartoš, M.; Bělobrádek, Z.; Krajina, A. Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases. Medicina 2024, 60, 1499. https://doi.org/10.3390/medicina60091499
Ryška P, Lojík M, Habalová J, Kajzrová C, Česák T, Vítková E, Bartoš M, Bělobrádek Z, Krajina A. Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases. Medicina. 2024; 60(9):1499. https://doi.org/10.3390/medicina60091499
Chicago/Turabian StyleRyška, Pavel, Miroslav Lojík, Jiřina Habalová, Carmen Kajzrová, Tomáš Česák, Eva Vítková, Michael Bartoš, Zdeněk Bělobrádek, and Antonín Krajina. 2024. "Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases" Medicina 60, no. 9: 1499. https://doi.org/10.3390/medicina60091499
APA StyleRyška, P., Lojík, M., Habalová, J., Kajzrová, C., Česák, T., Vítková, E., Bartoš, M., Bělobrádek, Z., & Krajina, A. (2024). Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases. Medicina, 60(9), 1499. https://doi.org/10.3390/medicina60091499




