Perforator Selection with Computed Tomography Angiography for Unilateral Breast Reconstruction: A Clinical Multicentre Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozen, W.M.; Bhullar, H.K.; Hunter-Smith, D. How to assess a CTA of the abdomen to plan an autologous breast reconstruction. Gland. Surg. 2019, 8 (Suppl. S4), S291–S296. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.; Ströbel, A.; Ludolph, I.; Hauck, T.; May, M.S.; Beier, J.P.; Horch, R.E.; Arkudas, A. Improving the Safety of DIEP Flap Transplantation: Detailed Perforator Anatomy Study Using Preoperative CTA. J. Pres. Med. 2022, 12, 701. [Google Scholar] [CrossRef]
- Cheng, A.; Losken, A. Essential elements of the preoperative breast reconstruction evaluation. Gland Surg. 2015, 4, 93–96. [Google Scholar] [CrossRef]
- Lim, B.; Cevik, J.; Seth, I.; Sofiadellis, F.; Ross, R.J.; Rozen, W.M.; Cuomo, R.; Lim, B.; Cevik, J.; Seth, I.; et al. Evaluating Artificial Intelligence’s Role in Teaching the Reporting and Interpretation of Computed Tomographic Angiography for Preoperative Planning of the Deep Inferior Epigastric Artery Perforator Flap. JPRAS Open 2024, 40, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Seth, I.; Xie, Y.; Ross, R.J.; Hunter-Smith, D.J.; Rozen, W.M.; Cuomo, R. Artificial Intelligence Language Model Performance for Rapid Intraoperative Queries in Plastic Surgery: ChatGPT and the Deep Inferior Epigastric Perforator Flap. J. Clin. Med. 2024, 13, 900. [Google Scholar] [CrossRef]
- Garg, R.K.; Urs, V.L.; Agarwal, A.A.; Chaudhary, S.K.; Paliwal, V.; Kar, S.K. Exploring the role of ChatGPT in patient care (diagnosis and treatment) and medical research: A systematic review. Health Promot. Perspect. 2023, 13, 183–191. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Feng, B.; Zhao, A.; Wu, Y. Innovating Healthcare: The Role of ChatGPT in Streamlining Hospital Workflow in the Future. Ann. Biomed. Eng. 2024, 52, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; He, D. The Potential Applications and Challenges of ChatGPT in the Medical Field. Int. J. Gen. Med. 2024, 17, 817–826. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Khayatkhoei, M.; Ilievski, F.; Sun, M. Exploring Perceptual Limitation of Large Language Models. arXiv 2024. [Google Scholar] [CrossRef]
- Sahlgren, M.; Carlsson, F. The Singleton Fallacy: Why Current Critiques of Language Models Miss the Point. Front. Artif. Intell. 2021, 4, 682578. [Google Scholar] [CrossRef]
- Ullah, E.; Parwani, A.; Baig, M.M.; Singh, R. Challenges and barriers of using large language models (LLM) such as ChatGPT for diagnostic medicine with a focus on digital pathology—A recent scoping review. Diagn. Pathol. 2024, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, M.; Hamam, S. AI-Driven Clinical Decision Support Systems: An Ongoing Pursuit of Potential. Cureus 2024, 16, e57728. [Google Scholar] [CrossRef] [PubMed]
- Karalis, V.D. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- Safranek, C.W.; Sidamon-Eristoff, A.E.; Gilson, A.; Chartash, D. The Role of Large Language Models in Medical Education: Applications and Implications. JMIR Med. Educ. 2023, 9, e50945. [Google Scholar] [CrossRef]
- Choudhury, A.; Chaudhry, Z. Large Language Models and User Trust: Consequence of Self-Referential Learning Loop and the Deskilling of Health Care Professionals. J. Med. Internet Res. 2024, 26, e56764. [Google Scholar] [CrossRef]
- Spotnitz, M.; Idnay, B.; Gordon, E.R.; Shyu, R.; Zhang, G.; Liu, C.; Cimino, J.J.; Weng, C. A Survey of Clinicians’ Views of the Utility of Large Language Models. Appl. Clin. Inform. 2024, 15, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Petersson, L.; Larsson, I.; Nygren, J.M.; Nilsen, P.; Neher, M.; Reed, J.E.; Tyskbo, D.; Svedberg, P. Challenges to implementing artificial intelligence in healthcare: A qualitative interview study with healthcare leaders in Sweden. BMC Health Serv. Res. 2022, 22, 850. [Google Scholar] [CrossRef]
- Asan, O.; Bayrak, A.E.; Choudhury, A. Artificial Intelligence and Human Trust in Healthcare: Focus on Clinicians. J. Med. Internet Res. 2020, 22, e15154. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, J.; Jiang, X.; Almeida, D.; Wainwright, C.; Mishkin, P.; Zhang, C.; Agarwal, S.; Slama, K.; Ray, A.; et al. Training language models to follow instructions with human feedback. arXiv 2022. [Google Scholar] [CrossRef]
- Nassiri, K.; Akhloufi, M.A. Recent Advances in Large Language Models for Healthcare. BioMedInformatics 2024, 4, 1097–1143. [Google Scholar] [CrossRef]
- Fang, X.; Che, S.; Mao, M.; Zhang, H.; Zhao, M.; Zhao, X. Bias of AI-generated content: An examination of news produced by large language models. Sci. Rep. 2024, 14, 5224. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, A.; Stubbersfield, J.M. Large language models show human-like content biases in transmission chain experiments. Proc. Natl. Acad. Sci. USA 2023, 120, e2313790120. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Chhaya, N.; Nsiah-Sarbeng, P.; Mosahebi, A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—Better for the patient, the surgeon, and the hospital. Clin. Radiol. 2013, 68, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.Y.; Kim, D.J.; Lee, N.; Kim, E.K. A Comprehensive evaluation of ChatGPT consultation quality for augmentation Mammoplasty: A comparative analysis between plastic surgeons and laypersons. Int. J. Med. Inform. 2023, 179, 105219. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Seth, I.; Hunter-Smith, D.J.; Rozen, W.M.; Ross, R.; Lee, M. Aesthetic Surgery Advice and Counseling from Artificial Intelligence: A Rhinoplasty Consultation with ChatGPT. Aesthetic Plast. Surg. 2023, 47, 1985–1993. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionising healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef]
| Scenario | Consultant 1 | Consultant 2 | Consultant 3 | Consultant 4 | Consultant 5 | Consultant 6 |
|---|---|---|---|---|---|---|
| 1 | Right DIEP | Right DIEP | Right DIEP | Right DIEP | Right DIEP | Right DIEP |
| 2 | Left DIEP | Right DIEP | Right MS TRAM | Right DIEP | Left MS TRAM | Left DIEP |
| 3 | Right DIEP | Right DIEP | Right DIEP | Right DIEP | Right DIEP | Right DIEP |
| 4 | Right DIEP | Right DIEP | Right DIEP | Right MS TRAM | Right MS TRAM | Right DIEP |
| 5 | Left DIEP | Right DIEP | Right MS TRAM | Left DIEP | Left DIEP | Left DIEP |
| 6 | Left DIEP | Right DIEP | Left DIEP | Left DIEP | Right DIEP | Left DIEP |
| 7 | Right DIEP | Left DIEP | Left DIEP | Right DIEP | Right DIEP | Right DIEP |
| 8 | Left MS TRAM | Right DIEP | Left TRAM | Left SIEA | Left TRAM | Left MS TRAM |
| 9 | Left DIEP | Left DIEP | Left DIEP | Left DIEP | Left DIEP | Left DIEP |
| 10 | Left DIEP | Left DIEP | Left DIEP | Left DIEP | Left DIEP | Left DIEP |
| Scenario | ChatGPT4o | ChatGPT4 | Bing CoPilot | Claude |
|---|---|---|---|---|
| 1 | Left DIEP | Right DIEP | Right DIEP | Right DIEP |
| 2 | Left DIEP | Right DIEP | Right DIEP | Left DIEP |
| 3 | Right DIEP | Right DIEP | Left DIEP | Right DIEP |
| 4 | Left DIEP | Right DIEP | Left DIEP | Left DIEP |
| 5 | Right DIEP | Left DIEP | Left DIEP | Right DIEP |
| 6 | Right DIEP | Left DIEP | Right DIEP | Right DIEP |
| 7 | Left DIEP | Left DIEP | Right DIEP | Left DIEP |
| 8 | Left DIEP | Right DIEP | Right DIEP | Right MS TRAM |
| 9 | Right DIEP | Right DIEP | Right DIEP | Left DIEP |
| 10 | Right DIEP | Right DIEP | Right DIEP | Right DIEP |
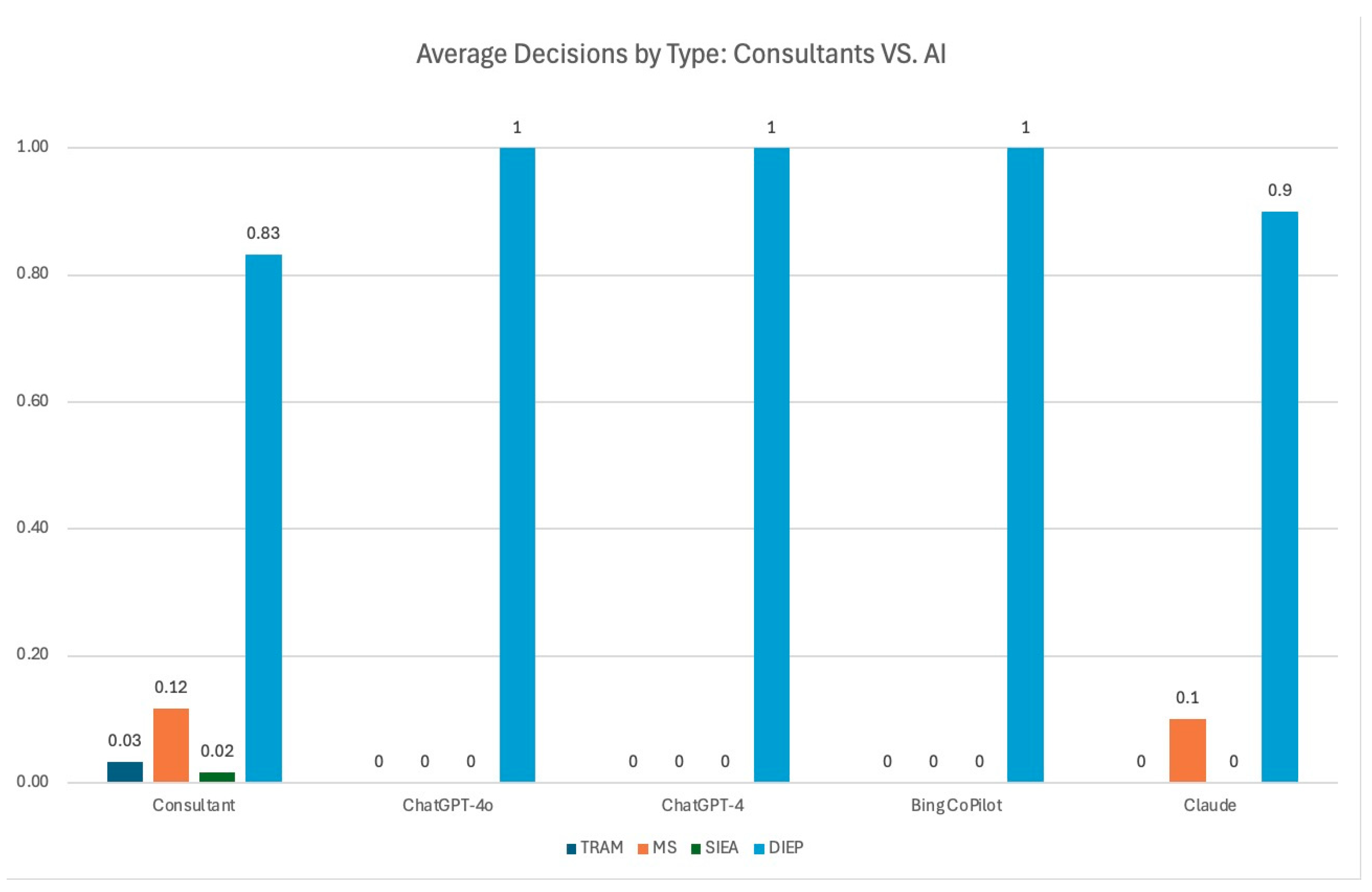
| TRAM | MS | SIEA | DIEP | |
|---|---|---|---|---|
| Consultant | 0.03 | 0.11 | 0.02 | 0.83 |
| ChatGPT-4o | 0 | 0 | 0 | 1 |
| ChatGPT-4 | 0 | 0 | 0 | 1 |
| Bing CoPilot | 0 | 0 | 0 | 1 |
| Claude | 0.1 | 0 | 0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seth, I.; Lim, B.; Phan, R.; Xie, Y.; Kenney, P.S.; Bukret, W.E.; Thomsen, J.B.; Cuomo, R.; Ross, R.J.; Ng, S.K.-H.; et al. Perforator Selection with Computed Tomography Angiography for Unilateral Breast Reconstruction: A Clinical Multicentre Analysis. Medicina 2024, 60, 1500. https://doi.org/10.3390/medicina60091500
Seth I, Lim B, Phan R, Xie Y, Kenney PS, Bukret WE, Thomsen JB, Cuomo R, Ross RJ, Ng SK-H, et al. Perforator Selection with Computed Tomography Angiography for Unilateral Breast Reconstruction: A Clinical Multicentre Analysis. Medicina. 2024; 60(9):1500. https://doi.org/10.3390/medicina60091500
Chicago/Turabian StyleSeth, Ishith, Bryan Lim, Robert Phan, Yi Xie, Peter Sinkjær Kenney, William E. Bukret, Jørn Bo Thomsen, Roberto Cuomo, Richard J. Ross, Sally Kiu-Huen Ng, and et al. 2024. "Perforator Selection with Computed Tomography Angiography for Unilateral Breast Reconstruction: A Clinical Multicentre Analysis" Medicina 60, no. 9: 1500. https://doi.org/10.3390/medicina60091500








