Novel Models for Assessing and Pathophysiology of Hepatic Ischemia–Reperfusion Injury Mechanisms
Abstract
:1. Introduction
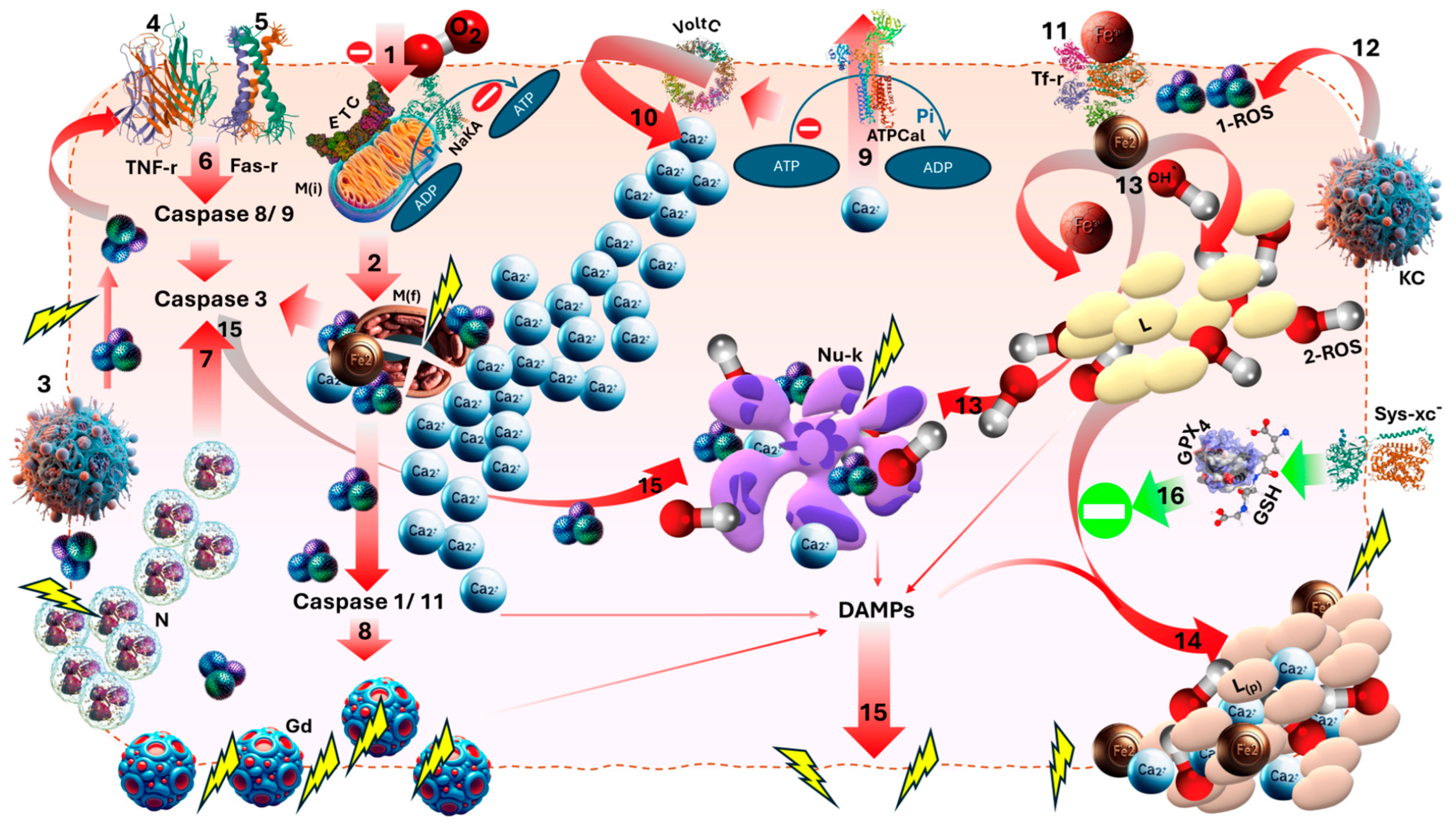
2. Pathophysiology
3. Murine Model
4. Porcine Model
5. Methods of Ischemia
6. Cell Lines
7. Normothermic Perfusion (NMP) Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Yanaga, K.; Kishikawa, K.; Kakizoe, S.; Shimada, M.; Sugimachi, K. Ischemic injury in liver transplantation: Difference in injury sites between warm and cold ischemia in rats. Hepatology 1992, 16, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Cai, J.-Z.; Dong, H. A Review of the Risk Factors and Approaches to Prevention of Post-Reperfusion Syndrome During Liver Transplantation. Organogenesis 2024, 20, 2386730. [Google Scholar] [CrossRef] [PubMed]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.-K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global Consequences of Liver Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef] [PubMed]
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid organ transplantation in the 21st century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.A.; Bilal, M.; Hmoud, B. Factors associated with waiting time on the liver transplant list: An analysis of the United Network for Organ Sharing (UNOS) database. Ann. Gastroenterol. 2018, 31, 84–89. [Google Scholar] [CrossRef]
- Israni, A.K.; Zaun, D.; Hadley, N.; Rosendale, J.D.; Schaffhausen, C.; McKinney, W.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 Annual Data Report: Deceased Organ Donation. Am. J. Transplant. 2020, 20, 509–541. [Google Scholar] [CrossRef]
- Bedogni, G.; Nobili, V.; Tiribelli, C. Epidemiology of fatty liver: An update. World J. Gastroenterol. 2014, 20, 9050–9054. [Google Scholar]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia-reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef]
- Cannistra, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Kristián, T.; Siesjö, B.K. Calcium in Ischemic Cell Death. Stroke 1998, 29, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, T.; Wang, T.; Yu, B.; Xia, K.; Guo, J.; Liu, Y.; Ma, X.; Zhang, L.; Zou, J.; et al. Research progress on the role of mitochondria in the process of hepatic ischemia-reperfusion injury. Gastroenterol. Rep. 2024, 12, goae066. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Perrelli, M.-G.; Pagliaro, P. Mitochondrial Pathways, Permeability Transition Pore, and Redox Signaling in Cardioprotection: Therapeutic Implications. Antioxidants Redox Signal. 2013, 18, 556–599. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, F.; Khori, V.; Alizadeh, A.M.; Khalighfard, S.; Khodayari, S.; Khodayari, H. Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci. 2016, 165, 43–55. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Taufani, I.P.; Tasminatun, S.; Harimurti, S.; Yang, L.-Y.; Huang, C.-Y.; Situmorang, J.H. Tannic Acid Suppresses Ferroptosis Induced by Iron Salophene Complex in Kidney Cells and Prevents Iron Overload-Induced Liver and Kidney Dysfunction in Rats. Biol. Trace Element Res. 2024, 202, 1–13. [Google Scholar] [CrossRef]
- Xu, Z.; Alloush, J.; Beck, E.; Weisleder, N. A Murine Model of Myocardial Ischemia-reperfusion Injury through Ligation of the Left Anterior Descending Artery. J. Vis. Exp. 2014, e51329. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, J.; Xia, Q. A Novel Mouse Model of Liver Ischemic/Reperfusion Injury and its Differences to the Existing Model. J. Investig. Surg. 2015, 28, 283–291. [Google Scholar] [CrossRef]
- Datta, G.; Fuller, B.J.; Davidson, B.R. Molecular mechanisms of liver ischemia reperfusion injury: Insights from transgenic knockout models. World J. Gastroenterol. 2013, 19, 1683–1698. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am. J. Gastrointest. Physiol. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Soroka, C.; Boyer, J. The Bile Salt Export Pump: Clinical and Experimental Aspects of Genetic and Acquired Cholestatic Liver Disease. Semin. Liver Dis. 2010, 30, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Karasawa, T.; Wakiya, T.; Sadatomo, A.; Ito, H.; Kamata, R.; Watanabe, S.; Komada, T.; Kimura, H.; Sanada, Y.; et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: Potential role of ferroptosis. Am. J. Transplant. 2020, 20, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hines, I.N.; Zibari, G.; Pavlick, K.; Gray, L.; Kitagawa, Y.; Grisham, M.B. Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free. Radic. Biol. Med. 2009, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ntonas, A.; Katsourakis, A.; Galanis, N.; Filo, E.; Noussios, G. Comparative Anatomical Study Between the Human and Swine Liver and Its Importance in Xenotransplantation. Cureus 2020, 12, e9411. [Google Scholar] [CrossRef]
- Maione, F.; Gilbo, N.; Lazzaro, S.; Friend, P.; Camussi, G.; Romagnoli, R.; Pirenne, J.; Jochmans, I.; Monbaliu, D. Porcine Isolated Liver Perfusion for the Study of Ischemia Reperfusion Injury. Transplantation 2018, 102, 1039–1049. [Google Scholar] [CrossRef]
- Ikeda, H.; Suzuki, Y.; Suzuki, M.; Koike, M.; Tamura, J.; Tong, J.; Nomura, M.; Itoh, G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 1998, 42, 530–537. [Google Scholar] [CrossRef]
- Palomino, J.; Echavarria, R.; Franco-Acevedo, A.; Moreno-Carranza, B.; Melo, Z. Opioids Preconditioning Upon Renal Function and Ischemia-Reperfusion Injury: A Narrative Review. Medicina 2019, 55, 522. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Noun, R.; Belghiti, J. Hepatic vascular occlusion: Which technique? Surg. Clin. N. Am. 2004, 84, 563–585. [Google Scholar] [CrossRef]
- Huang, J.; Xie, P.; Dong, Y.; An, W. Inhibition of Drp1 SUMOylation by ALR protects the liver from ischemia-reperfusion injury. Cell Death Differ. 2021, 28, 1174–1192. [Google Scholar] [CrossRef]
- Chouillard, E.K.; Gumbs, A.A.; Cherqui, D. Vascular clamping in liver surgery: Physiology, indications and techniques. Ann. Surg. Innov. Res. 2010, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Piriou, V.; Chiari, P.; Lhuillier, F.; Bastien, O.; Loufoua, J.; Raisky, O.; David, J.S.; Ovize, M.; Lehot, J.J. Pharmacological pre-conditioning: Comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br. J. Anaesth. 2002, 89, 486–491. [Google Scholar]
- Kunst, G.; Klein, A.A. Peri-operative anaesthetic myocardial preconditioning and protection–cellular mechanisms and clinical relevance in cardiac anaesthesia. Anaesthesia 2015, 70, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.F.; DeBoer, L.W.V.; Rude, R.E.; Lowenstein, E.; Maroko, P.R. The Effect of Halothane Anesthesia on Myocardial Necrosis, Hemodynamic Performance, and Regional Myocardial Blood Flow in Dogs Following Coronary Artery Occlusion. Anesthesiology 1983, 59, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Toller, W.G.; Kersten, J.R.; Pagel, P.S.; Hettrick, D.A.; Warltier, D.C. Sevoflurane Reduces Myocardial Infarct Size and Decreases the Time Threshold for Ischemic Preconditioning in Dogs. Anesthesiology 1999, 91, 1437. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Emadali, A.; Muscatelli-Groux, B.; Delom, F.; Jenna, S.; Boismenu, D.; Sacks, D.B.; Metrakos, P.P.; Chevet, E. Proteomic Analysis of Ischemia-Reperfusion Injury upon Human Liver Transplantation Reveals the Protective Role of IQGAP1. Mol. Cell. Proteom. 2006, 5, 1300–1313. [Google Scholar] [CrossRef]
- Huang, X.; Dai, J.; Fournier, J.; Ali, A.M.; Zhang, Q.; Frenkel, K. Ferrous ion autoxidation and its chelation in iron-loaded human liver HepG2 cells. Free. Radic. Biol. Med. 2002, 32, 84–92. [Google Scholar] [CrossRef]
- Honegger, P. Overview of Cell and Tissue Culture Techniques. Curr. Protoc. Pharmacol. 2001, 12, 11. [Google Scholar] [CrossRef]
- Fasciano, A.C.; Mecsas, J.; Isberg, R.R. New Age Strategies to Reconstruct Mucosal Tissue Colonization and Growth in Cell Culture Systems. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Yanaga, K.; Makowka, L.; Lebeau, G.; Hwang, R.R.; Shimada, M.; Kakizoe, S.; Demetris, A.J.; Starzl, T.E. A New Liver Perfusion and Preservation System for Transplantation Research in Large Animals. J. Investig. Surg. 1990, 3, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nwaduru, C.; Baker, E.; Buff, M.; Selim, M.; Ovalle, L.A.; Baker, T.B.; Zimmerman, M.A. Assessing Liver Viability: Insights from Mitochondrial Bioenergetics in Ischemia-Reperfusion Injury. Transplant. Proc. 2024, 56, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Akateh, C.; Beal, E.W.; Whitson, B.A.; Black, S.M. Normothermic Ex-vivo Liver Perfusion and the Clinical Implications for Liver Transplantation. J. Clin. Transl. Hepatol. 2018, 6, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Jayant, K.; Reccia, I.; Virdis, F.; Shapiro, A.M.J. The Role of Normothermic Perfusion in Liver Transplantation (TRaNsIT Study): A Systematic Review of Preliminary Studies. HPB Surg. 2018, 2018, 6360423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, Y.; Luo, L.; Chen, Y.; Luo, Q.; Chen, J. Impact of Prolonged Mechanical Ventilation on Ferroptosis in Renal Ischemia/Reperfusion Injury in Rats. BioMed Res. Int. 2020, 2020, 6097516. [Google Scholar] [CrossRef]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free. Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Blum, M.F.; Liu, Q.; Soliman, B.; Dreher, P.; Okamoto, T.; Poggio, E.D.; Goldfarb, D.A.; Baldwin, W.M., 3rd; Quintini, C. Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death. J. Surg. Res. 2017, 216, 35–45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whalen, C.; Verma, A.; Kurashima, K.; Carter, J.; Nazzal, H.; Jain, A. Novel Models for Assessing and Pathophysiology of Hepatic Ischemia–Reperfusion Injury Mechanisms. Medicina 2024, 60, 1507. https://doi.org/10.3390/medicina60091507
Whalen C, Verma A, Kurashima K, Carter J, Nazzal H, Jain A. Novel Models for Assessing and Pathophysiology of Hepatic Ischemia–Reperfusion Injury Mechanisms. Medicina. 2024; 60(9):1507. https://doi.org/10.3390/medicina60091507
Chicago/Turabian StyleWhalen, Connor, Arun Verma, Kento Kurashima, Jasmine Carter, Hala Nazzal, and Ajay Jain. 2024. "Novel Models for Assessing and Pathophysiology of Hepatic Ischemia–Reperfusion Injury Mechanisms" Medicina 60, no. 9: 1507. https://doi.org/10.3390/medicina60091507
APA StyleWhalen, C., Verma, A., Kurashima, K., Carter, J., Nazzal, H., & Jain, A. (2024). Novel Models for Assessing and Pathophysiology of Hepatic Ischemia–Reperfusion Injury Mechanisms. Medicina, 60(9), 1507. https://doi.org/10.3390/medicina60091507




