Sanger Sequencing Reveals Novel Variants in GLO-1, ACE, and CBR1 Genes in Patients of Early and Severe Diabetic Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients/Participants
2.2. Ethics and Compliance
2.3. Biochemical Analysis
2.4. Genetic Analysis
2.5. Statistical Analysis and Software
2.6. In Silico Analysis
3. Results
3.1. Demographic Profile
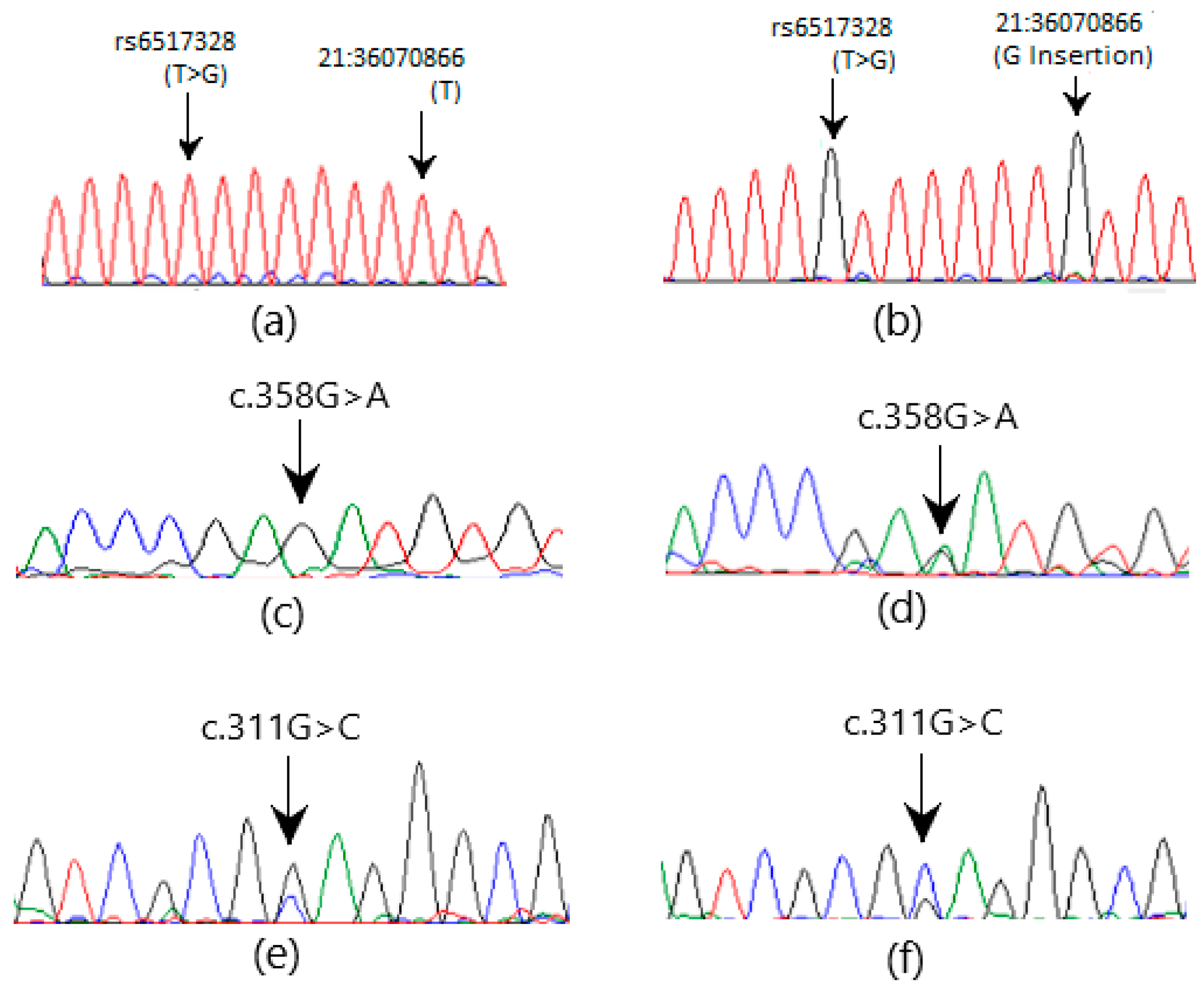
3.2. Sanger Sequencing
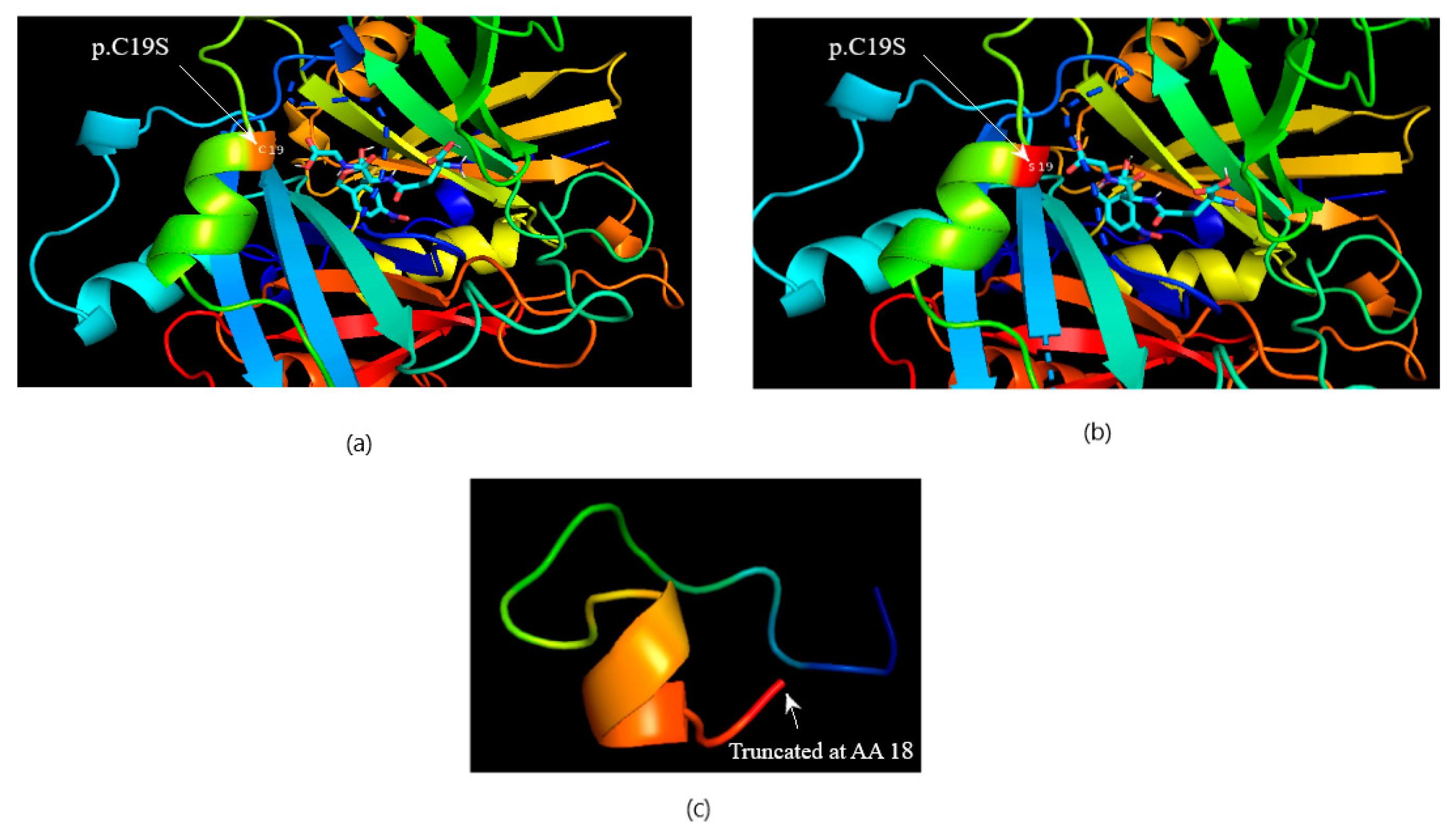
3.3. Computational Protein–Ligand Docking
4. Discussion
5. Conclusions
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Taal, M.W. Obesity, Metabolism, An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Shin, J.; Zhou, X.; Tan, J.T.; Hyppönen, E.; Benyamin, B.; Lee, S.H. Lifestyle modifies the diabetes-related metabolic risk, conditional on individual genetic differences. Front. Genet. 2022, 13, 759309. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, T.O.; Salisu, T.F. A review of type 2 diabetes mellitus predisposing genes. Curr. Diabetes Rev. 2020, 16, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Imtiaz, S.; Alam, A. Epidemiology and demography of Chronic Kidney Disease in Pakistan-A review of Pakistani literature. Pak. J. Kidney Dis. 2023, 7, 2–7. [Google Scholar] [CrossRef]
- Kato, S.; Matsumura, T.; Sugawa, H.; Nagai, R. Correlation between serum advanced glycation end-products and vascular complications in patient with type 2 diabetes. Sci. Rep. 2024, 14, 18722. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Zhang, D.-D.; Wang, Y.-N.; Tan, Y.-Q.; Yu, X.-Y.; Zhao, Y.-Y. AGE/RAGE in diabetic kidney disease and ageing kidney. Free. Radic. Biol. Med. 2021, 171, 260–271. [Google Scholar] [CrossRef]
- Saeed, M.; Kausar, M.A.; Singh, R.; Siddiqui, A.J.; Akhter, A. The role of glyoxalase in glycation and carbonyl stress induced metabolic disorders. Curr. Protein Pept. Sci. 2020, 21, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.L.; Li, H.Y.; Zhang, X.S.; Wang, T.; Zhou, S.P.; Xu, W.H. CBR1 decreases protein carbonyl levels via the ROS/Akt/CREB pathway to extend lifespan in the cotton bollworm. Helicoverpa Armigera 2023, 290, 2127–2145. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Guad, R.M.; Udupa, K.; Fuloria, N.K. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Stehouwer, C.D.; Schalkwijk, C.G. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertension 2019, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.H.; Rashid, A.; Majeed, A. Determination of Glyoxalase-1 levels and Identification of Genetic Variants in GLO1 Gene in Patients of Diabetic Nephropathy. Pak. J. Med. Sci. 2024, 40, 652–656. [Google Scholar] [CrossRef]
- Corredor, Z.; Filho, M.; Rodriguez-Ribera, L.; Velazquez, A.; Hernandez, A.; Catalano, C.; Hemminki, K.; Coll, E.; Silva, I.; Diaz, J.M.; et al. Genetic Variants Associated with Chronic Kidney Disease in a Spanish Population. Sci. Rep. 2020, 10, 144. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Stefanidis, I.; Zintzaras, E. The genetic map of diabetic nephropathy: Evidence from a systematic review and meta-analysis of genetic association studies. Clin. Kidney J. 2020, 13, 768–781. [Google Scholar] [CrossRef]
- Pai, D.; Adiga, S.; Suresh, G.; Adiga, U.; Chaitra, D.; Honalli, N.M. The association of ACE gene polymorphism and serum ACE levels with diabetic nephropathy-a cross-sectional study. J. Pharm. Sci. Appl. 2024, 14, 210–217. [Google Scholar] [CrossRef]
- Jankovic, M.; Novakovic, I.; Nikolic, D.; Mitrovic Maksic, J.; Brankovic, S.; Petronic, I.; Cirovic, D.; Ducic, S.; Grajic, M.; Bogicevic, D.; et al. Genetic and Epigenomic Modifiers of Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 4887. [Google Scholar] [CrossRef]
- Giani, J.F.; Veiras, L.C.; Shen, J.Z.; Bernstein, E.A.; Cao, D.; Okwan-Duodu, D.; Khan, Z.; Gonzalez-Villalobos, R.A.; Bernstein, K.E. Novel roles of the renal angiotensin-converting enzyme. Mol. Cell Endocrinol. 2021, 529, 111257. [Google Scholar] [CrossRef]
- Deng, X.; Li, D.; Tang, Q.; Chen, Y. ACEI and ARB lower the incidence of end-stage renal disease among patients with diabetic nephropathy: A meta-analysis. Comput. Math. Methods Med. 2022, 2022, 6962654. [Google Scholar] [CrossRef] [PubMed]
- Juin, S.K.; Ouseph, R.; Gondim, D.D.; Jala, V.R.; Sen, U. Diabetic Nephropathy and Gaseous Modulators. Antioxidants 2023, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Pharmacotherapy to delay the progression of diabetic kidney disease in people with type 2 diabetes: Past, present and future. Ther. Adv. Endocrinol. Metabolism. 2022, 13, 20420188221081601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tsukube, S.; Izawa, K.; Okochi, M.; Lim, T.-K.; Watanabe, S.; Harada, M.; Matsunaga, T. Electrochemical detection of HbA1c, a maker for diabetes, using a flow immunoassay system. Biosens. Bioelectron. 2007, 22, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pezzolesi, M.G. Advances in understanding the genetic basis of diabetic kidney disease. Acta Diabetol. 2018, 55, 1093–1104. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Mayr, A.; Zoppini, G.; Targher, G.; Bonadonna, R.C.; Willeit, J. High-Normal HbA1c Is a Strong Predictor of Type 2 Diabetes in the General Population. Diabetes Care 2011, 34, 1038–1040. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. (Eds.) Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, UK, 1990. [Google Scholar]
- Umeukeje, E.M.; Koonce, T.Y.; Kusnoor, S.V.; Ulasi, I.I.; Kostelanetz, S.; Williams, A.M.; Blasingame, M.N.; Epelbaum, M.I.; Giuse, D.A.; Apple, A.N.; et al. Systematic review of international studies evaluating MDRD and CKD-EPI estimated glomerular filtration rate (eGFR) equations in Black adults. PLoS ONE 2022, 17, e0276252. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35 (Suppl. S2), W71–W74. [Google Scholar] [CrossRef]
- Al-Shuhaib, M.B.S.; Hashim, H.O. Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. J. Genet. Eng. Biotechnol. 2023, 21, 115. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8; Schrodinger: New York, NY, USA, 2015. [Google Scholar]
- Mazani, M.; Mahdavifard, S.; Koohi, A. Crocetin ameliorative effect on diabetic nephropathy in rats through a decrease in transforming growth factor-β and an increase in glyoxalase-I activity. Clin. Nutr. ESPEN 2023, 58, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Kong, L.; Tang, Z.Z.; Zhang, Y.M.; Liu, Y.; Wang, T.Y.; Liu, Y.W. Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/ARE/glyoxalase 1 pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Ensemble. Ensemble Human Gene Browser 11. 2024. Available online: https://asia.ensembl.org/Homo_sapiens/Variation/Mappings?db=core;g=ENSG00000124767;r=6:38675925-38703145;t=ENST00000373365;v=rs1168871721;vdb=variation;vf=429570578 (accessed on 18 February 2024).
- Peculis, R.; Konrade, I.; Skapare, E.; Fridmanis, D.; Nikitina-Zake, L.; Lejnieks, A.; Pirags, V.; Dambrova, M.; Klovins, J. Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene 2013, 515, 140–143. [Google Scholar] [CrossRef]
- Tao, H.; Si, L.; Zhou, X.; Liu, Z.; Ma, Z.; Zhou, H.; Zhong, W.; Cui, L.; Zhang, S.; Li, Y.; et al. Role of glyoxalase I gene polymorphisms in late-onset epilepsy and drug-resistant epilepsy. J. Neurol. Sci. 2016, 363, 200–206. [Google Scholar] [CrossRef]
- Bora, S.; Adole, P.S.; Vinod, K.V.; Pillai, A.A.; Ahmed, S. The genetic polymorphisms and activity of glyoxalase 1 as a risk factor for acute coronary syndrome in South Indians with type 2 diabetes mellitus. Gene 2023, 885, 147701. [Google Scholar] [CrossRef]
- Maasen, K.; Hanssen, N.M.J.; van der Kallen, C.J.H.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J.; Schalkwijk, C.G. Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study. Antioxidants 2021, 10, 219. [Google Scholar] [CrossRef]
- Megias-Vericat, J.E.; Martinez-Cuadron, D.; Herrero, M.J.; Alino, S.F.; Poveda, J.L.; Sanz, M.A.; Montesinos, P. Pharmacogenetics of metabolic genes of anthracyclines in acute myeloid leukemia. Curr. Drug Metab. 2018, 19, 55–74. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Z.; Jiang, H. Flufenamic acid alleviates sepsis-induced lung injury by up-regulating CBR1. Drug Dev. Res. 2020, 81, 885–892. [Google Scholar] [CrossRef]
- Yanar, K.; Atayik, M.C.; Simsek, B.; Çakatay, U. Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 2020, 21, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, X.; Zheng, B.; Zheng, Z.; Zhang, H.; Zheng, J.; Sun, C.; Chen, H.; Yang, J.; Wang, Z.; et al. Maternal 25-hydroxyvitamin D deficiency promoted metabolic syndrome and downregulated Nrf2/CBR1 pathway in offspring. Front. Pharmacol. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Feng, F.; Liu, L.; Chen, L.; Lv, P.; Ma, S.; Chen, K.; Yao, Q. RNA-Seq analysis of the pathogenesis of STZ-induced male diabetic mouse liver. J. Diabetes Its Complicat. 2019, 34, 107444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, W.; Yan, R. Gbp3 is associated with the progression of lupus nephritis by regulating cell proliferation, inflammation and pyroptosis. Autoimmunity 2023, 56, 2250095. [Google Scholar] [CrossRef]
- Bell, R.M.; Villalobos, E.; Nixon, M.; Miguelez-Crespo, A.; Murphy, L.; Fawkes, A.; Coutts, A.; Sharp, M.G.; Koerner, M.V.; Allan, E.; et al. Carbonyl reductase 1 amplifies glucocorticoid action in adipose tissue and impairs glucose tolerance in lean mice. Mol. Metab. 2021, 48, 101225. [Google Scholar] [CrossRef]
- Ma, H.; Yu, C.; Wang, R. Association of ACE polymorphism and diabetic nephropathy susceptibility. Int. J. Clin. Exp. Med. 2015, 8, 2962–2965. [Google Scholar]
- Deepashree, G.A.; Ramprasad, E.; Jayakumar, M.; Paul, S.F.; Gnanasambandan, R. ACE ID gene polymorphism contributes to chronic kidney disease progression but not NOS3 gene among Type 2 diabetes with nephropathy patients. Endocrine Metab. Sci. 2021, 4, 100100. [Google Scholar]
- Taha, M.M.; Mahdy-Abdallah, H.; Shahy, E.M.; Helmy, M.A.; ElLaithy, L.S. Diagnostic efficacy of cystatin-c in association with different ACE genes predicting renal insufficiency in T2DM. Sci. Rep. 2023, 13, 5288. [Google Scholar] [CrossRef]
- Lubbe, L.; Cozier, G.E.; Oosthuizen, D.; Acharya, K.R.; Sturrock, E.D. ACE2 and ACE: Structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin. Sci. 2020, 134, 2851–2871. [Google Scholar] [CrossRef]




| Parameter | Target |
|---|---|
| Tool used | NCBI primer BLAST and Primer 3 plus |
| Genome | RefSeq representative genome (GRCh38) |
| Maximum hits in genome | 1 |
| 5′ self-complementarity | <6 |
| 5′ self-complementarity | <6 |
| Single base repetition (max) | 3 (no 4 Gs together) |
| Product size | 500 to 900 bp |
| Minimum length around the target sequence at both ends | 150 bp |
| Primer annealing temperature Tm | 55–63 °C |
| The maximum difference in Tm of the two primers | 4 °C |
| GC content | 45 to 60% |
| Target | Primer | Primer Sequence | Product Size | Primer Tm (°C) | Reaction Tm (°C) |
|---|---|---|---|---|---|
| GLO1 Exon 1 | Forward | 5′ TTCTACCAAATTGCAGCCCTGA 3′ | 725 bp | 61.0 | 61.8 |
| Reverse | 5′ CAGCCACCGTCGCAACATA 3′ | 62.3 | |||
| GLO1 Exon 2 | Forward | 5′ TTGCAAGTTGTAGGTGGTAGGTT 3′ | 280 bp | 60.3 | 61.2 |
| Reverse | 5′ AAGATGGGTCTGAAAACACTCTC 3′ | 59.4 | |||
| GLO1 Exon 2 (second set) | Forward | 5′ TCTGACACTTTGGACTTGCATCA 3′ | 761 bp | 60.7 | 61.2 |
| Reverse | 5′ TTTCAGGCTGGCTGGGATAGA 3′ | 63.6 | |||
| CBR1 Exon 1 | Forward | 5′ GTCCATAACGCCTCCCTAGG 3′ | 413 bps | 61.3 | 61.2 |
| Reverse | 5′ GTCCATAACGCCTCCCTAGG 3′ | 59.3 | |||
| CBR1 Exon 2 | Forward | 5′ AACTTTGTGTTTCCCTGGCTGGG 3′ | 812 bp | 63.6 | 64.8 |
| Reverse | 5′ GGATGGACTCCCACGCAGAG 3′ | 62.3 | |||
| ACE Exon 1 | Forward | 5′ AGAGGAGGCCCTTTCTCCAGCT 3′ | 716 bp | 68.6 | 65.0, |
| Reverse | 5′ ACCCTCATCCATCCAACTCG 3′ | 62.9 | 66.0 | ||
| ACE Exon 2 | Forward | 5′ TCCGCAAACTAAGGTCTCCC 3′ | 549 bp | 62.7 | 62.0 |
| Reverse | 5′ TTGGCTTCCTACTCCAGAATGC 3′ | 61.7 | |||
| ACE Exon 2 (second set) | Forward | 5′ AAGCCCTTGGCCTTCCTC 3′ | 325 bp | 64.2 | 67.0 |
| Reverse | 5′ CACGATGGGGCACTAGGAG 3′ | 63.9 |
| Parameters 1 | Control Mean (SD) (n = 100) | Diabetic Nephropathy Mean (SD) (n = 113) | p Value | |
|---|---|---|---|---|
| Age | (years) | 54.6 ± 10.3 | 57.2 ± 11.4 | 0.27 |
| Gender | Male n (%) | 59 (59) | 68 (60.2) | 0.13 |
| Female n (%) | 41 (41) | 45 (39.8) | 0.32 | |
| BMI | 25.5 ± 4.7 | 27.3 ± 3.2 | 0.19 | |
| Gene | DNA Sequence Change (GRCh38) | Amino Acid Residue Change | Status | Locus/Site | Type of Change | Control n = 100 n (%) | DN n = 113 n (%) |
|---|---|---|---|---|---|---|---|
| GLO1 | c.102G>T | p.P4Q | rs1168871721 | 6:38703044 | Missense | 0 (0) | 3 (2.65) |
| c.147C>G | p.C19S | rs17855424 | 6:38702999 | Missense | 0 (0) | 8 (7.08) | |
| c.148G>T | p.C19X | Novel | 6:38702998 | Nonsense | 0 (0) | 8 (7.08) | |
| CBR1 | c.358G>A | p.D120N | Novel | 21:36071018 | Missense | 0 (0) | 3 (2.65) |
| c.311G>C | p.L73L | rs25678 | 21:36070334 | Silent | 0 (0) | 5 (4.42) | |
| ACE | c.337A>C | p.T113M | Novel | 17:63478018 | Missense | 0 (0) | 3 (2.65) |
| Gene | Location (GRCh38) | Variation | Status | Control n = 100 n (%) | DN n = 113 n (%) |
|---|---|---|---|---|---|
| GLO1 | 6:38703061 | G>A | rs1049346 | 0 (0) | 13 (11.5) |
| 6:38703141 | G>T | rs1761734427 | 0 (0) | 1 (0.9) | |
| 6:38703186 | C>A | Novel | 0 (0) | 1 (0.9) | |
| 6:38686709 | G>C | Novel | 0 (0) | 1 (0.9) | |
| 6:38686760 | G>A | Novel | 0 (0) | 1 (0.9) | |
| 6:38686799 | G>A | Novel | 0 (0) | 1 (0.9) | |
| 6:38686823 | G>A | Novel | 0 (0) | 1 (0.9) | |
| 6:38686886 | T>C | Novel | 0 (0) | 1 (0.9) | |
| CBR1 | 21:36070068 | G>A | rs11542168 | 0 (0) | 1 (0.9) |
| 21:36070774 | G>A | Novel | 0 (0) | 1 (0.9) | |
| 21:36070859 | T>G | rs6517328 | 0 (0) | 7 (6.19) | |
| 21:36070866 | X>G | Novel | 0 (0) | 5 (4.42) | |
| 21:36070872 | X>G | Novel | 0 (0) | 1 (0.9) | |
| 21:36070881 | A>T | rs1469692824 | 0 (0) | 1 (0.9) | |
| 21:36070907 | A>C | Novel | 0 (0) | 1 (0.9) | |
| ACE | 17:63477094 | C>G | rs887305522 | 0 (0) | 1 (0.9) |
| 17:63477454 | C>X | Novel | 0 (0) | 1 (0.9) | |
| 17:63477926 | A>C | Novel | 0 (0) | 1 (0.9) | |
| 17:63477929 | X>C | Novel | 0 (0) | 2 (1.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.Z.H.; Rashid, A.; Majeed, A.; Ghafoor, T.; Azam, N. Sanger Sequencing Reveals Novel Variants in GLO-1, ACE, and CBR1 Genes in Patients of Early and Severe Diabetic Nephropathy. Medicina 2024, 60, 1540. https://doi.org/10.3390/medicina60091540
Shah SZH, Rashid A, Majeed A, Ghafoor T, Azam N. Sanger Sequencing Reveals Novel Variants in GLO-1, ACE, and CBR1 Genes in Patients of Early and Severe Diabetic Nephropathy. Medicina. 2024; 60(9):1540. https://doi.org/10.3390/medicina60091540
Chicago/Turabian StyleShah, Syed Zubair Hussain, Amir Rashid, Asifa Majeed, Tariq Ghafoor, and Nadeem Azam. 2024. "Sanger Sequencing Reveals Novel Variants in GLO-1, ACE, and CBR1 Genes in Patients of Early and Severe Diabetic Nephropathy" Medicina 60, no. 9: 1540. https://doi.org/10.3390/medicina60091540
APA StyleShah, S. Z. H., Rashid, A., Majeed, A., Ghafoor, T., & Azam, N. (2024). Sanger Sequencing Reveals Novel Variants in GLO-1, ACE, and CBR1 Genes in Patients of Early and Severe Diabetic Nephropathy. Medicina, 60(9), 1540. https://doi.org/10.3390/medicina60091540






