Association of Pregnancy Complications with Endometrial or Ovarian or Breast Cancer: A Case Control Study
Abstract
1. Introduction
2. Methods
Data Source
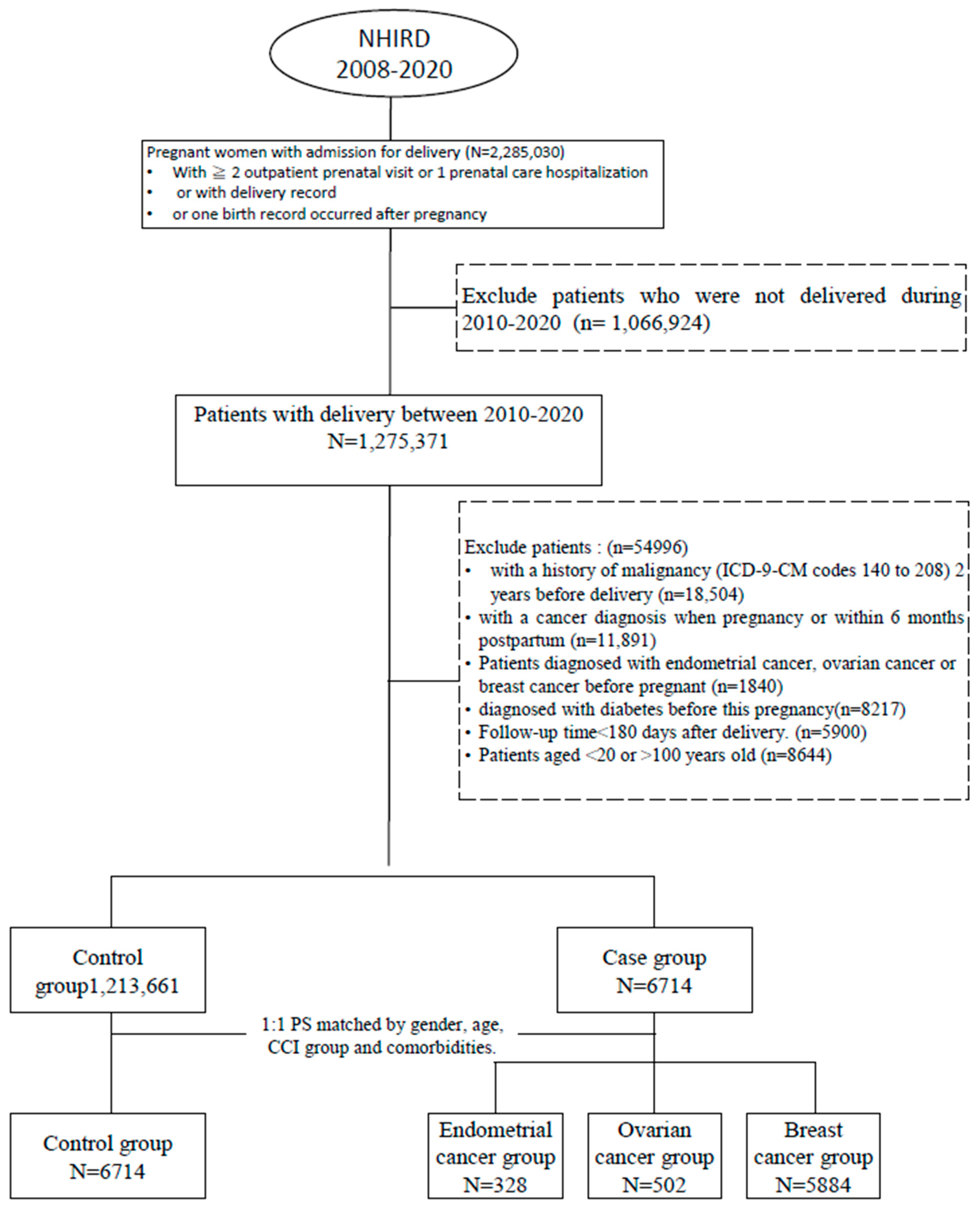
3. Study Cohort
4. Case Identification and Control Selection
5. Confounding Variables
6. Statistical Analysis
7. Results
Study Populations
8. Pregnancy Complications Associated with Gynecological Cancers and Breast Cancer
9. Discussion
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 20 May 2023).
- Health Promotion Administration, Ministry of Health and Welfare. Available online: http://www.hpa.gov.tw/bhpnet/English/Index.aspx (accessed on 20 May 2023).
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Setiawan, V.W.; Pike, M.C.; Karageorgi, S.; Deming, S.L.; Anderson, K.; Bernstein, L.; Brinton, L.A.; Cai, H.; Cerhan, J.R.; Cozen, W.; et al. Age at last birth in relation to risk of endometrial cancer: Pooled analysis in the epidemiology of endometrial cancer consortium. Am. J. Epidemiol. 2012, 176, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Main, C.; Chen, X.; Zhao, M.; Chamley, L.W.; Chen, Q. Understanding How Pregnancy Protects Against Ovarian and Endometrial Cancer Development: Fetal Antigens May Be Involved. Endocrinology 2022, 163, bqac141. [Google Scholar] [CrossRef] [PubMed]
- Högnäs, E.; Kauppila, A.; Hinkula, M.; Tapanainen, J.S.; Pukkala, E. Incidence of cancer among grand multiparous women in Finland with special focus on non-gynecological cancers: A population-based cohort study. Acta Oncol. 2016, 55, 370–376. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/reproductive-history-fact-sheet (accessed on 15 June 2023).
- Opdahl, S.; Romundstad, P.R.; Alsaker, M.D.; Vatten, L.J. Hypertensive diseases in pregnancy and breast cancer risk. Br. J. Cancer 2012, 107, 176–182. [Google Scholar] [CrossRef]
- Ruiz, R.; Herrero, C.; Strasser-Weippl, K.; Touya, D.; Louis, J.S.; Bukowski, A.; Goss, P.E. Epidemiology and pathophysiology of pregnancy-associated breast cancer: A review. Breast 2017, 35, 136–141. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.; Yang, H.; Liu, M.; Wang, S.; Guo, J.; Yu, L.; Zhou, F.; Wang, F.; Xiang, Y.; et al. The Impact of Reproductive Factors on the Risk of Breast Cancer by ER/PR and HER2: A Multicenter Case-Control Study in Northern and Eastern China. Oncologist 2022, 27, e1–e8. [Google Scholar] [CrossRef]
- De Oliveira Andrade, F.; Verma, V.; Hilakivi-Clarke, L. Maternal obesity and resistance to breast cancer treatments among offspring: Link to gut dysbiosis. Cancer Rep. 2022, 5, e1752. [Google Scholar] [CrossRef]
- Calderon-Margalit, R.; Friedlander, Y.; Yanetz, R.; Deutsch, L.; Perrin, M.C.; Kleinhaus, K.; Tiram, E.; Harlap, S.; Paltiel, O. Preeclampsia and subsequent risk of cancer: Update from the Jerusalem Perinatal Study. Am. J. Obs. Gynecol. 2009, 200, 63.e1–63.e5. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Cheng, W.; Huo, N.; Zhang, S. Preeclampsia and cancer risk in women in later life: A systematic review and meta-analysis of cohort studies. Menopause 2021, 28, 1070–1078. [Google Scholar] [CrossRef]
- Jordao, H.; Herink, K.; Ka, E.; McVicker, L.; Kearns, C.; McMenamin, Ú.C. Pre-eclampsia during pregnancy and risk of endometrial cancer: A systematic review and meta-analysis. BMC Womens Health 2023, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Sheng, J.; Sun, X.; Chen, G.Q.; Zhao, M.; Chen, Q. Complications of Pregnancy and the Risk of Developing Endometrial or Ovarian Cancer: A Case-Control Study. Front. Endocrinol. 2021, 12, 642928. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.; Giannakeas, V.; Read, S.; Lega, I.C.; Shah, B.R.; Lipscombe, L.L. Risk of Breast Cancer After Diabetes in Pregnancy: A Population-based Cohort Study. Can. J. Diabetes 2024, 48, 171–178.e1. [Google Scholar] [CrossRef]
- Fuchs, O.; Sheiner, E.; Meirovitz, M.; Davidson, E.; Sergienko, R.; Kessous, R. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch. Gynecol. Obs. 2017, 295, 731–736. [Google Scholar] [CrossRef]
- Pace, R.; Rahme, E.; Dasgupta, K. Gestational diabetes mellitus and risk of incident primary cancer: A population-based retrospective cohort study. J. Diabetes 2020, 12, 87–90. [Google Scholar] [CrossRef]
- Shim, S.H.; Noh, E.; Lee, A.J.; Jang, E.B.; Kim, M.; Hwang, H.S.; Cho, G.J. Risk of adverse obstetric outcomes in patients with a history of endometrial cancer: A nationwide population-based cohort study. BJOG 2023, 130, 1662–1668. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, F.; Jin, H.Y.; Lau, S.; Stone, P.; Chamley, L. Phagocytosis of apoptotic trophoblastic debris protects endothelial cells against activation. Placenta 2012, 33, 548–553. [Google Scholar] [CrossRef]
- Jordan, S.J.; Na, R.; Johnatty, S.E.; Wise, L.A.; Adami, H.O.; Brinton, L.A.; Chen, C.; Cook, L.S.; Dal Maso, L.; De Vivo, I.; et al. Breastfeeding and Endometrial Cancer Risk: An Analysis From the Epidemiology of Endometrial Cancer Consortium. Obs. Gynecol. 2017, 129, 1059–1067. [Google Scholar] [CrossRef]
- Roy, D.; Liehr, J.G. Estrogen, DNA damage and mutations. Mutat. Res. 1999, 424, 107–115. [Google Scholar] [CrossRef]
- Chen, G.G.; Zeng, Q.; Tse, G.M. Estrogen and its receptors in cancer. Med. Res. Rev. 2008, 28, 954–974. [Google Scholar] [CrossRef]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.J.; Spartz, A.; Austin, J.R.; Lange, C.A. Reevaluating the Role of Progesterone in Ovarian Cancer: Is Progesterone Always Protective? Endocr. Rev. 2023, 44, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Gao, Y.; Zeng, K.; Yin, Y.; Zhao, M.; Wei, J.; Chen, Q. The levels of the sex hormones are not different between type 1 and type 2 endometrial cancer. Sci. Rep. 2016, 6, 39744. [Google Scholar] [CrossRef]
- Placenta: Overview, Anatomy, Function & Complications. Available online: https://my.clevelandclinic.org (accessed on 27 October 2024).
- Russo, J.; Moral, R.; Balogh, G.A.; Mailo, D.; Russo, I.H. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005, 7, 131–142. [Google Scholar] [CrossRef]
- Nechuta, S.; Paneth, N.; Velie, E.M. Pregnancy characteristics and maternal breast cancer risk: A review of the epidemiologic literature. Cancer Causes Control 2010, 21, 967–989. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Lefevre, G.; Alfaidy, N.; Abi Nahed, R.; Vincent, J.; Oudinet, J.P.; Pianos, A.; Cambourg, A.; Rozenberg, P.; et al. Abnormal steroidogenesis and aromatase activity in preeclampsia. Placenta 2018, 69, 40–49. [Google Scholar] [CrossRef]
- Baud, O.; Berkane, N. Hormonal Changes Associated With Intra-Uterine Growth Restriction: Impact on the Developing Brain and Future Neurodevelopment. Front. Endocrinol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Trabert, B.; Troisi, R.; Grotmol, T.; Ekbom, A.; Engeland, A.; Gissler, M.; Glimelius, I.; Madanat-Harjuoja, L.; Sørensen, H.T.; Tretli, S.; et al. Associations of pregnancy-related factors and birth characteristics with risk of endometrial cancer: A Nordic population-based case–control study. Int. J. Cancer 2020, 146, 1523–1531. [Google Scholar] [CrossRef]
- Ebrahim Valojerdi, A.; Janani, L. A brief guide to propensity score analysis. Med. J. Islam. Repub. Iran. 2018, 32, 122. [Google Scholar]
- Parsons, L.S.; Ovation Research Group. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques; SAS Support: Cary, NC, USA, 2001. [Google Scholar]
- Taiwan’s Declining Birth Rate Difficult to Reverse: Official. Available online: https://focustaiwan.tw/society/202410240013 (accessed on 16 November 2024).
- Peng, Y.S.; Lin, J.R.; Cheng, B.H.; Ho, C.; Lin, Y.H.; Shen, C.H.; Tsai, M.H. Incidence and relative risk for developing cancers in women with gestational diabetes mellitus: A nationwide cohort study in Taiwan. BMJ Open 2019, 9, e024583. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Gates, K.M.; Al-Geizi, H.; Baah, E.; Clunes, L.A.; Kollias, T.F. The Relationship Between Gestational Diabetes and the Risk of Cancer: A Systematic Review. Cureus 2024, 16, e53328. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.M.H.; Lee, Y.S.; Venkataraman, K.; Khoo, E.Y.H.; Tai, E.S.; Chong, Y.S.; Gluckman, P.; Leow, M.K.S.; Khoo, C.M. Ethnic differences in insulin sensitivity and beta-cell function among Asian men. Nutr. Diabetes 2015, 5, e173. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Gao, H.; Salim, A.; Lee, J.; Tai, E.S.; Van Dam, R.M. Can body fat distribution, adiponectin levels and inflammation explain differences in insulin resistance between ethnic Chinese, Malays and Asian Indians? Int. J. Obes. 2012, 36, 1086–1093. [Google Scholar] [CrossRef]
- Seah, J.Y.H.; Sim, X.; Khoo, C.M.; Tai, E.S.; van Dam, R.M. Differences in type 2 diabetes risk between East, South, and Southeast Asians living in Singapore: The multi-ethnic cohort. BMJ Open Diabetes Res. Care 2023, 11, e003385. [Google Scholar] [CrossRef]
- Lorincz, A.M.; Sukumar, S. Molecular links between obesity and breast cancer. Endocr. Relat. Cancer 2006, 13, 279–292. [Google Scholar] [CrossRef]
- Katsanis, W.A.; Shields, L.B.; Spinnato, J.A.; Gerçel-Taylor, C.; Taylor, D.D. Immune recognition of endometrial tumor antigens induced by multiparity. Gynecol. Oncol. 1998, 70, 33–39. [Google Scholar] [CrossRef]
- Shields, L.B.; Gerçel-Taylor, Ç.; Yashar, C.M.; Wan, T.C.; Katsanis, W.A.; Spinnato, J.A.; Taylor, D.D. Induction of immune responses to ovarian tumor antigens by multiparity. J. Soc. Gynecol. Investig. 1997, 4, 298–304. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bustoros, M.; Lin, X.; De Mejia, C.M.; Gurzenda, E.; Chavez, M.; Hanna, I.; Aguiari, P.; Perin, L.; Hanna, N. Placental extracellular vesicles-associated microRNA-519c mediates endotoxin adaptation in pregnancy. Am. J. Obs. Gynecol. 2021, 225, 681.e1–681.e20. [Google Scholar] [CrossRef]
- Pillay, P.; Vatish, M.; Duarte, R.; Moodley, J.; Mackraj, I. Exosomal microRNA profiling in early and late onset preeclamptic pregnant women reflects pathophysiology. Int. J. Nanomed. 2019, 14, 5637–5657. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, M.E.; Derdaki, M.; Quyou, A. Advanced Maternal Age, Gestational Diabetes, and Parity: A Moderated Mediation Model for Preeclampsia. Tanzan. J. Health Res. 2024, 1, 524–541. [Google Scholar]
- Yang, Y.; Wu, N. Gestational Diabetes Mellitus and Preeclampsia: Correlation and Influencing Factors. Front. Cardiovasc. Med. 2022, 9, 831297. [Google Scholar] [CrossRef] [PubMed]
| Variables | Controls (n = 6714) | EC (n = 328) | OC (n = 502) | BC (n = 5884) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Complication | |||||||||
| No | 5331 | 79.40 | 253 | 77.13 | 406 | 80.88 | 4578 | 77.80 | 0.0827 |
| Yes | 1383 | 20.60 | 75 | 22.87 | 96 | 19.12 | 1306 | 22.20 | 0.0827 |
| Complication | |||||||||
| GDM, gestational diabetes | 1253 | 18.66 | 71 | 21.65 | 86 | 17.13 | 1214 | 20.63 | 0.0146 |
| Intrauterine growth restriction or slow intrauterine growth | 12 | 0.18 | 0 | 0 | 0 | 0 | 11 | 0.19 | 0.9649 |
| Large for gestational age newborn | 17 | 0.25 | 0 | 0 | 0 | 0 | 14 | 0.24 | 0.5562 |
| Preeclampsia | 162 | 2.41 | 9 | 2.74 | 17 | 3.39 | 130 | 2.21 | 0.3689 |
| Age | <0.001 | ||||||||
| 20–30 | 298 | 4.44 | 23 | 7.01 | 63 | 12.55 | 170 | 2.89 | |
| 31–40 | 3697 | 55.06 | 182 | 55.49 | 305 | 60.76 | 3090 | 52.52 | |
| 41–50 | 2653 | 39.51 | 118 | 35.98 | 130 | 25.90 | 2578 | 43.81 | |
| >50 | 66 | 0.98 | 5 | 1.52 | 4 | 0.80 | 46 | 0.78 | |
| mean, (SD) | 39.22 | 5.10 | 38.92 | 5.61 | 36.92 | 5.63 | 39.74 | 4.85 | <0.001 |
| Age at first birth (years, median/range) | 35 (32–38) | 34 (31–37) | 33 (30–36) | 34 (32–37) | <0.001 | ||||
| Age at diagnosis (years, median/range) | 40 (36–43) | 39 (36–42) | 37 (33–41) | 40 (36–43) | <0.001 | ||||
| Comorbidites | |||||||||
| Myocardial infarction | 0 | 0 | 0 | 0 | 0 | 0 | ≤3 | 0.8713 | |
| Congestive heart failure | 8 | 0.12 | 0 | 0 | 0 | 0 | 5 | 0.08 | 0.7444 |
| Peripheral vascular disease | 27 | 0.40 | 0 | 0 | ≤3 | 30 | 0.51 | 0.7594 | |
| Hypertension | 354 | 5.27 | 21 | 6.40 | 25 | 4.98 | 299 | 5.08 | 0.7414 |
| Cerebrovascular disease | 48 | 0.71 | ≤3 | 6 | 1.20 | 40 | 0.68 | 0.5960 | |
| Chronic pulmonary disease | 1028 | 15.31 | 47 | 14.33 | 66 | 13.15 | 899 | 15.28 | 0.5877 |
| Connective tissue disease | 37 | 0.55 | 6 | 1.83 | ≤3 | 32 | 0.54 | 0.0234 | |
| Ulcer disease | 1088 | 16.20 | 63 | 19.21 | 74 | 14.74 | 949 | 16.13 | 0.3924 |
| Mild liver disease | 99 | 1.47 | 9 | 2.74 | 5 | 1.00 | 86 | 1.46 | 0.2206 |
| Dyslipidemia | 405 | 6.03 | 28 | 8.54 | 22 | 4.38 | 354 | 6.02 | 0.1093 |
| Ovarian dysfunction | 89 | 1.33 | 14 | 4.27 | 14 | 2.79 | 57 | 0.97 | <0.001 |
| Infertility | 989 | 14.73 | 78 | 23.78 | 93 | 18.53 | 825 | 14.02 | <0.001 |
| Obesity | 82 | 1.22 | 9 | 2.74 | 9 | 1.79 | 66 | 1.12 | 0.0455 |
| Alcohol-related disease | 18 | 0.27 | 0 | 0 | 0 | 0 | 22 | 0.37 | 0.4927 |
| Charlson comorbidity index | 0.1071 | ||||||||
| 1 | 6602 | 98.33 | ≤3 | ≤3 | 5789 | 98.39 | |||
| 2–3 | 103 | 1.53 | 86 | 1.46 | |||||
| >3 | 9 | 0.13 | 9 | 0.15 | |||||
| Parity (number, %) | <0.001 | ||||||||
| 1 | 4766 | 70.99 | 273 | 83.23 | 363 | 72.31 | 4212 | 71.58 | |
| ≥2 | 1948 | 29.01 | 55 | 16.77 | 139 | 27.69 | 1672 | 28.42 | |
| Abortion (number, %) | 0.022 | ||||||||
| 0 | 5496 | 81.86 | 254 | 77.44 | 410 | 81.67 | 4737 | 80.51 | |
| 1 | 919 | 13.69 | 49 | 14.94 | 70 | 13.94 | 874 | 14.85 | |
| 2 | 217 | 3.23 | 13 | 3.96 | 16 | 3.19 | 198 | 3.37 | |
| ≥3 | 82 | 1.22 | 12 | 3.66 | 6 | 1.20 | 75 | 1.27 | |
| Variable | Non-EC (n = 328) | EC (n = 328) | cOR | (95% CI) | p-Value | aOR | (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||||
| GDM | 65 | 19.82 | 71 | 21.65 | 1.14 | (0.87, 1.49) | 0.3306 | 1.10 | (0.84, 1.44) | 0.4867 |
| Intrauterine growth restriction or slow intrauterine growth | 0 | 0 | 0.00 | |||||||
| Large for gestational age newborn | 0 | 0 | 0.00 | |||||||
| Preeclampsia | 10 | 3.05 | 9 | 2.74 | 0.90 | (0.36, 2.24) | 0.816 | 0.91 | (0.34, 2.45) | 0.849 |
| Variable | Non-OC (n = 502) | OC (n = 502) | cOR | (95% CI) | p-value | aOR | (95% CI) | p-value | ||
| n | % | n | % | |||||||
| GDM | 85 | 16.93 | 86 | 17.13 | 1.01 | (0.73, 1.41) | 0.933 | 1.02 | (0.73, 1.43) | 0.896 |
| Intrauterine growth restriction or slow intrauterine growth | 0 | 0 | 199,762 | (0.00, NA) | 0.978 | 501,883 | (0.00, NA) | 0.986 | ||
| Large for gestational age newborn | - | - | - | - | - | - | ||||
| Preeclampsia | 11 | 2.19 | 17 | 3.39 | 1.56 | (0.73, 3.37) | 0.254 | 1.87 | (0.82, 4.27) | 0.139 |
| Variable | Non-BC (n = 5884) | BC (n = 5884) | cOR | (95% CI) | p-value | aOR | (95% CI) | p-value | ||
| n | % | n | % | |||||||
| GDM | 1103 | 18.75 | 1214 | 20.63 | 1.13 | (1.03, 1.23) * | 0.010 * | 1.12 | (1.02,1.23) * | 0.013 |
| Intrauterine growth restriction or slow intrauterine growth | 8 | 0.14 | 11 | 0.19 | 1.37 | (0.55, 3.41) | 0.496 | 1.42 | (0.57, 3.55) | 0.454 |
| Large for gestational age newborn | 16 | 0.27 | 14 | 0.24 | 0.87 | (0.43, 1.79) | 0.715 | 0.88 | (0.43, 1.80) | 0.720 |
| Preeclampsia | 141 | 2.40 | 130 | 2.21 | 0.92 | (0.72, 1.17) | 0.499 | 0.92 | (0.72, 1.18) | 0.519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.C.; Leung, H.W.C.; Lin, H.-J.; Leung, J.H.; Chan, A.L.F. Association of Pregnancy Complications with Endometrial or Ovarian or Breast Cancer: A Case Control Study. Medicina 2025, 61, 1. https://doi.org/10.3390/medicina61010001
Han LC, Leung HWC, Lin H-J, Leung JH, Chan ALF. Association of Pregnancy Complications with Endometrial or Ovarian or Breast Cancer: A Case Control Study. Medicina. 2025; 61(1):1. https://doi.org/10.3390/medicina61010001
Chicago/Turabian StyleHan, Lin Cheng, Henry W. C. Leung, Heng-Jun Lin, John Hang Leung, and Agnes L. F. Chan. 2025. "Association of Pregnancy Complications with Endometrial or Ovarian or Breast Cancer: A Case Control Study" Medicina 61, no. 1: 1. https://doi.org/10.3390/medicina61010001
APA StyleHan, L. C., Leung, H. W. C., Lin, H.-J., Leung, J. H., & Chan, A. L. F. (2025). Association of Pregnancy Complications with Endometrial or Ovarian or Breast Cancer: A Case Control Study. Medicina, 61(1), 1. https://doi.org/10.3390/medicina61010001






