The Effect of Local Melatonin Application on Bone Fracture Healing in Rat Tibias
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Surgical Procedures
2.3. Histomorphometric Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meeson, R.L.; Perpétuo, I.P.; Parsons, K.; Orriss, I.R.; Shah, M.; Pitsillides, A.A.; Michael, D. The in vitro behaviour of canine osteoblasts derived from different bone types. BMC Vet. Res. 2019, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Mishina, Y. Roles of osteoclasts in alveolar bone remodeling. Genesis 2022, 60, e23490. [Google Scholar] [CrossRef]
- Vertenten, G.; Gasthuys, F.; Cornelissen, M.; Schacht, E.; Vlaminck, L. Enhancing bone healing and regeneration: Present and future perspectives in veterinary orthopaedics. Vet. Comp. Orthop. Traumatol. VCOT 2010, 23, 153–162. [Google Scholar] [CrossRef]
- Lu, C.; Miclau, T.; Hu, D.; Hansen, E.; Tsui, K.; Puttlitz, C.; Marcucio, R.S. Cellular basis for age-related changes in fracture repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2005, 23, 1300–1307. [Google Scholar] [CrossRef]
- López-Martínez, F.; Olivares Ponce, P.N.; Guerra Rodríguez, M.; Martínez Pedraza, R. Melatonin: Bone metabolism in oral cavity. Int. J. Dent. 2012, 2012, 628406. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.J.; Acuna-Castroviejo, D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin as a chronobiotic/cytoprotective agent in bone. Doses involved. J. Pineal Res. 2024, 76, e12931. [Google Scholar] [CrossRef]
- Sethi, S.; Radio, N.M.; Kotlarczyk, M.P.; Chen, C.T.; Wei, Y.H.; Jockers, R.; Witt-Enderby, P.A. Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J. Pineal Res. 2010, 49, 222–238. [Google Scholar] [CrossRef]
- Zhang, L.; Su, P.; Xu, C.; Chen, C.; Liang, A.; Du, K.; Peng, Y.; Huang, D. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J. Pineal Res. 2010, 49, 364–372. [Google Scholar] [CrossRef]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Munmun, F.; Witt-Enderby, P.A. Melatonin effects on bone: Implications for use as a therapy for managing bone loss. J. Pineal Res. 2021, 71, e12749. [Google Scholar] [CrossRef]
- Arabaci, T.; Kermen, E.; Ozkanlar, S.; Kose, O.; Kara, A.; Kizildag, A.; Duman, S.B.; Ibisoglu, E. Therapeutic Effects of Melatonin on Alveolar Bone Resorption After Experimental Periodontitis in Rats: A Biochemical and Immunohistochemical Study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef]
- Gao, W.; Lin, M.; Liang, A.; Zhang, L.; Chen, C.; Liang, G.; Xu, C.; Peng, Y.; Chen, C.; Huang, D.; et al. Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells. J. Pineal Res. 2014, 56, 62–70. [Google Scholar] [CrossRef]
- Zhu, G.; Ma, B.; Dong, P.; Shang, J.; Gu, X.; Zi, Y. Melatonin promotes osteoblastic differentiation and regulates PDGF/AKT signaling pathway. Cell Biol. Int. 2020, 44, 402–411. [Google Scholar] [CrossRef]
- Zhang, B.; Bailey, W.M.; McVicar, A.L.; Gensel, J.C. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol. Aging 2016, 47, 157–167. [Google Scholar] [CrossRef]
- Halici, M.; Öner, M.; Güney, A.; Canöz, Ö.; Narin, F.; Halıcı, C. Melatonin promotes fracture healing in the rat model. Jt. Dis. Relat. Surg. 2010, 21, 172–177. [Google Scholar]
- Ramírez-Fernández, M.P.; Calvo-Guirado, J.L.; de-Val, J.E.; Delgado-Ruiz, R.A.; Negri, B.; Pardo-Zamora, G.; Peñarrocha, D.; Barona, C.; Granero, J.M.; Alcaraz-Baños, M. Melatonin promotes angiogenesis during repair of bone defects: A radiological and histomorphometric study in rabbit tibiae. Clin. Oral Investig. 2013, 17, 147–158. [Google Scholar] [CrossRef]
- Yamada, Y.; Tamura, T.; Hariu, K.; Asano, Y.; Sato, S.; Ito, K. Angiogenesis in newly augmented bone observed in rabbit calvarium using a titanium cap. Clin. Oral Implant. Res. 2008, 19, 1003–1009. [Google Scholar] [CrossRef]
- Lu, X.; Yu, S.; Chen, G.; Zheng, W.; Peng, J.; Huang, X.; Chen, L. Insight into the roles of melatonin in bone tissue and bone-related diseases (Review). Int. J. Mol. Med. 2021, 47, 82. [Google Scholar] [CrossRef]
- Dundar, S.; Yaman, F.; Saybak, A.; Ozupek, M.F.; Toy, V.E.; Gul, M.; Ozercan, I.H. Evaluation of Effects of Topical Melatonin Application on Osseointegration of Dental Implant: An Experimental Study. J. Oral Implantol. 2016, 42, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Acikan, I.; Mehmet, G.; Artas, G.; Yaman, F.; Deniz, G.; Bulmus, O.; Kom, M.; Kirtay, M.; Dundar, S. Systemic melatonin application increases bone formation in mandibular distraction osteogenesis. Braz. Oral Res. 2018, 32, e85. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; López-Peña, M.; Miño, N.; Gómez-Moreno, G.; Guardia, J.; Cutando, A. Topical application of melatonin and growth hormone accelerates bone healing around dental implants in dogs. Clin. Implant. Dent. Relat. Res. 2012, 14, 226–235. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. The effects of melatonin in bone healing. Vet. Sci. Res. 2018, 3, 000155. [Google Scholar] [CrossRef]
- Tanrisever, M.; Istek, O.; Eroksuz, H.; Karabulut, B.; Ozcan, E.C.; Bingul, M.B.; Guler, R.; Dundar, S. Effects of local application of bovine amniotic fluid on fracture healing in rats (Rattus norvegicus). Rev. Cient. Fac. Vet. 2024, 34, 2–3. [Google Scholar] [CrossRef]
- Acikan, I.; Yaman, F.; Dundar, S.; Ozercan, I.H.; Atilgan, S.S. Protective effects of caffeic acid phenethyl ester (CAPE) and thymoquinone against cigarette smoke in experimental bone fracture healing. J. Oral Biol. Craniofacial Res. 2022, 12, 610–616. [Google Scholar] [CrossRef]
- Ascenzi, M.G.; Roe, A.K. The osteon: The micromechanical unit of compact bone. Front. Biosci. (Landmark Ed.) 2012, 17, 1551–1581. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Kos-Kudla, B.; Nowak, M.; Swietochowska, E.; Marek, B.; Gorski, J.; Kajdaniuk, D.; Wolkowska, K. The relationship between bone metabolism, melatonin and other hormones in sham-operated and pinealectomized rats. Endocr. Regul. 2003, 37, 211–224. [Google Scholar]
- Shino, H.; Hasuike, A.; Arai, Y.; Honda, M.; Isokawa, K.; Sato, S. Melatonin enhances vertical bone augmentation in rat calvaria secluded spaces. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e122–e126. [Google Scholar] [CrossRef]
- Dalla-Costa, K.; Yurtsever, F.V.; Penteado, J.; Martinez, E.F.; Sperandio, M.; Peruzzo, D.C. Melatonin has a stimulatory effect on osteoblasts by upregulating col-i and opn expression/secretion. Melatonina tem efeito estimulador em osteoblastos, pela regulação positiva da expressão/secreção de col-i e opn. Acta Odontol. Latinoam. AOL 2020, 33, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, S.; Lu, C.; Yang, W.; Yang, Z.; Hu, W.; Xin, Z.; Yang, Y. Melatonin: Another avenue for treating osteoporosis? J. Pineal Res. 2019, 66, e12548. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Liu, H.D.; Ren, M.X.; Li, Y.; Zhang, R.T.; Ma, N.F.; Li, T.L.; Jiang, W.K.; Zhou, Z.; Yao, X.W.; Liu, Z.Y.; et al. Melatonin alleviates hydrogen peroxide induced oxidative damage in MC3T3-E1 cells and promotes osteogenesis by activating SIRT1. Free. Radic. Res. 2022, 56, 63–76. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, C.; Yang, H.; Li, J.; Feng, Z.; Liao, L.; Li, Y. Melatonin Accelerates Osteoporotic Bone Defect Repair by Promoting Osteogenesis-Angiogenesis Coupling. Front. Endocrinol. 2022, 13, 826660. [Google Scholar] [CrossRef]
- Hazzaa, H.H.A.; El-Kilani, N.S.; Elsayed, S.A.; Abd El Massieh, P.M. Evaluation of Immediate Implants Augmented with Autogenous Bone/Melatonin Composite Graft in the Esthetic Zone: A Randomized Controlled Trial. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2019, 28, e637–e642. [Google Scholar] [CrossRef]
- Igarashi-Migitaka, J.; Seki, A.; Ikegame, M.; Honda, M.; Sekiguchi, T.; Mishima, H.; Shimizu, N.; Matsubara, H.; Srivastav, A.K.; Hirayama, J.; et al. Oral administration of melatonin contained in drinking water increased bone strength in na17turally aged mice. Acta Histochem. 2020, 122, 151596. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Wang, Z.; Li, C.; Li, S.; Li, L.; Fan, Q.; Zheng, L. Melatonin Suppresses Estrogen Deficiency-Induced Osteoporosis and Promotes Osteoblastogenesis by Inactivating the NLRP3 Inflammasome. Calcif. Tissue Int. 2018, 103, 400–410. [Google Scholar] [CrossRef]
- Sun, T.; Li, J.; Xing, H.L.; Tao, Z.S.; Yang, M. Melatonin improves the osseointegration of hydroxyapatite-coated titanium implants in senile female rats. Verbesserung der Osseointegration von hydroxylapatitbeschichteten Titanimplantaten durch Melatonin bei weiblichen Ratten hohen Alters. Z. Gerontol. Geriatr. 2020, 53, 770–777. [Google Scholar] [CrossRef]
- Virto, L.; Haugen, H.J.; Fernández-Mateos, P.; Cano, P.; González, J.; Jiménez-Ortega, V.; Esquifino, A.I.; Sanz, M. Melatonin expression in periodontitis and obesity: An experimental in-vivo investigation. J. Periodontal Res. 2018, 53, 825–831. [Google Scholar] [CrossRef]
- Dong, P.; Gu, X.; Zhu, G.; Li, M.; Ma, B.; Zi, Y. Melatonin Induces Osteoblastic Differentiation of Mesenchymal Stem Cells and Promotes Fracture Healing in a Rat Model of Femoral Fracture via Neuropeptide Y/Neuropeptide Y Receptor Y1 Signaling. Pharmacology 2018, 102, 272–280. [Google Scholar] [CrossRef]
- Altintepe Doğan, S.S.; Toker, H.; Göze, Ö.F. Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study. Biomedicines 2024, 12, 684. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, J.; Chen, R.; Huang, Y.; Liu, Y.; Bai, J.; Ge, G.; Shi, X.; Chen, Y.; Shi, J.; et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 6797154. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J.; Reiter, R.J. Melatonin stimulates osteointegration of dental implants. J. Pineal Res. 2008, 45, 174–179. [Google Scholar] [CrossRef]
- Tresguerres, I.F.; Clemente, C.; Blanco, L.; Khraisat, A.; Tamimi, F.; Tresguerres, J.A. Effects of local melatonin application on implant osseointegration. Clin. Implant. Dent. Relat. Res. 2012, 14, 395–399. [Google Scholar] [CrossRef]
- Hazzaa, H.H.A.; Shawki, N.A.; Abdelaziz, L.M.; Shoshan, H.S. Early Loading of Dental Implant Grafted with Autogenous Bone Alone or Combined with Melatonin Gel: A Randomized Clinical Trial. Austin J. Dent. 2020, 7, 1137. [Google Scholar] [CrossRef]
- El-Gammal, M.Y.; Salem, A.S.; Anees, M.M.; Tawfik, M.A. Clinical and Radiographic Evaluation of Immediate Loaded Dental Implants With Local Application of Melatonin: A Preliminary Randomized Controlled Clinical Trial. J. Oral Implantol. 2016, 42, 119–125. [Google Scholar] [CrossRef]
- Costa, K.L.D.; Abreu, L.F.; Tolomei, C.B.; Eleutério, R.G.; Basting, R.; Balbinot, G.; Collares, F.M.; Lopes, P.; Veiga, N.; Fernandes, G.V.O.; et al. Use of Local Melatonin with Xenogeneic Bone Graft to Treat Critical-Size Bone Defects in Rats with Osteoporosis: A Randomized Study. J. Funct. Biomater. 2024, 15, 124. [Google Scholar] [CrossRef]
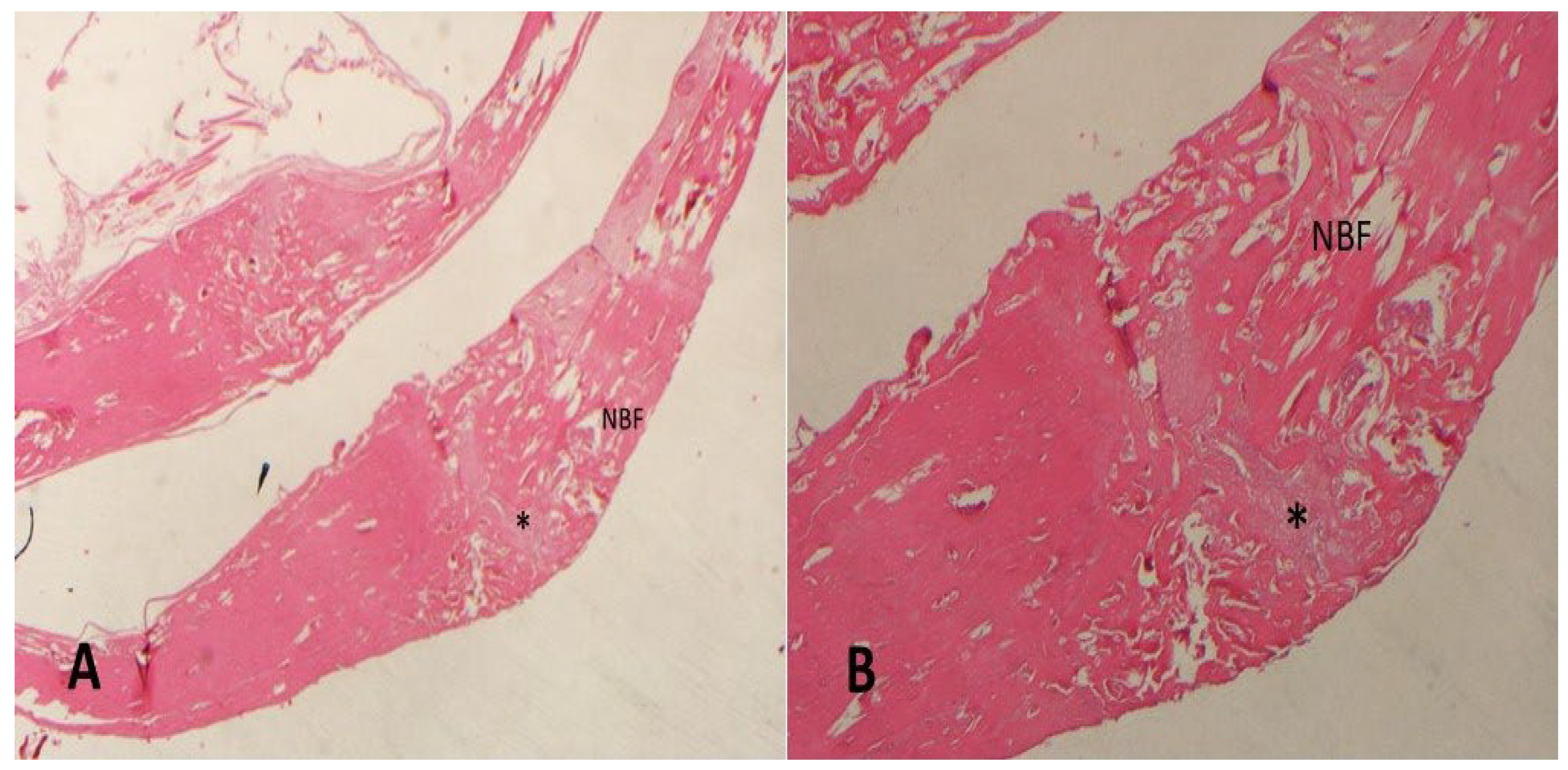
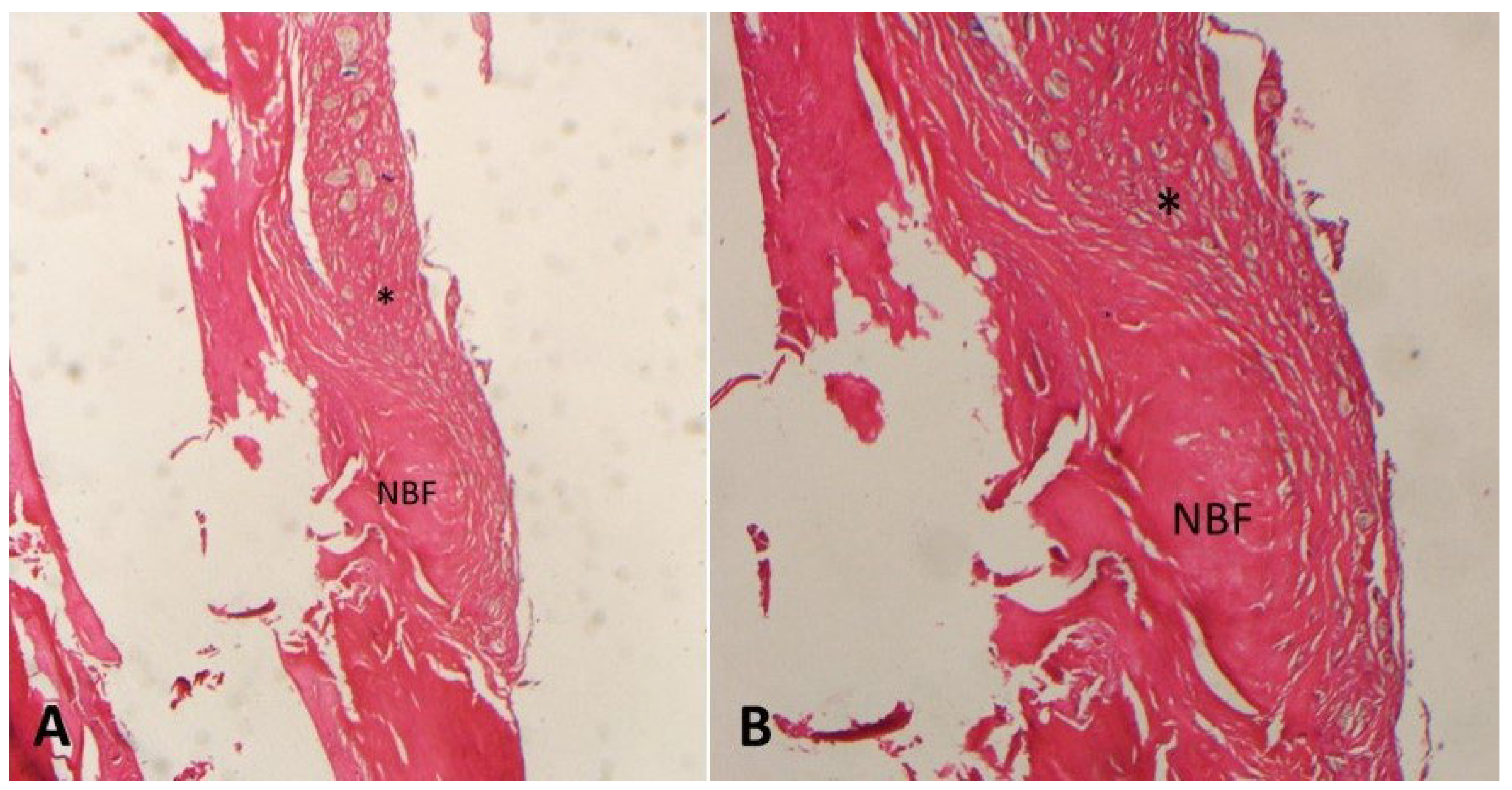
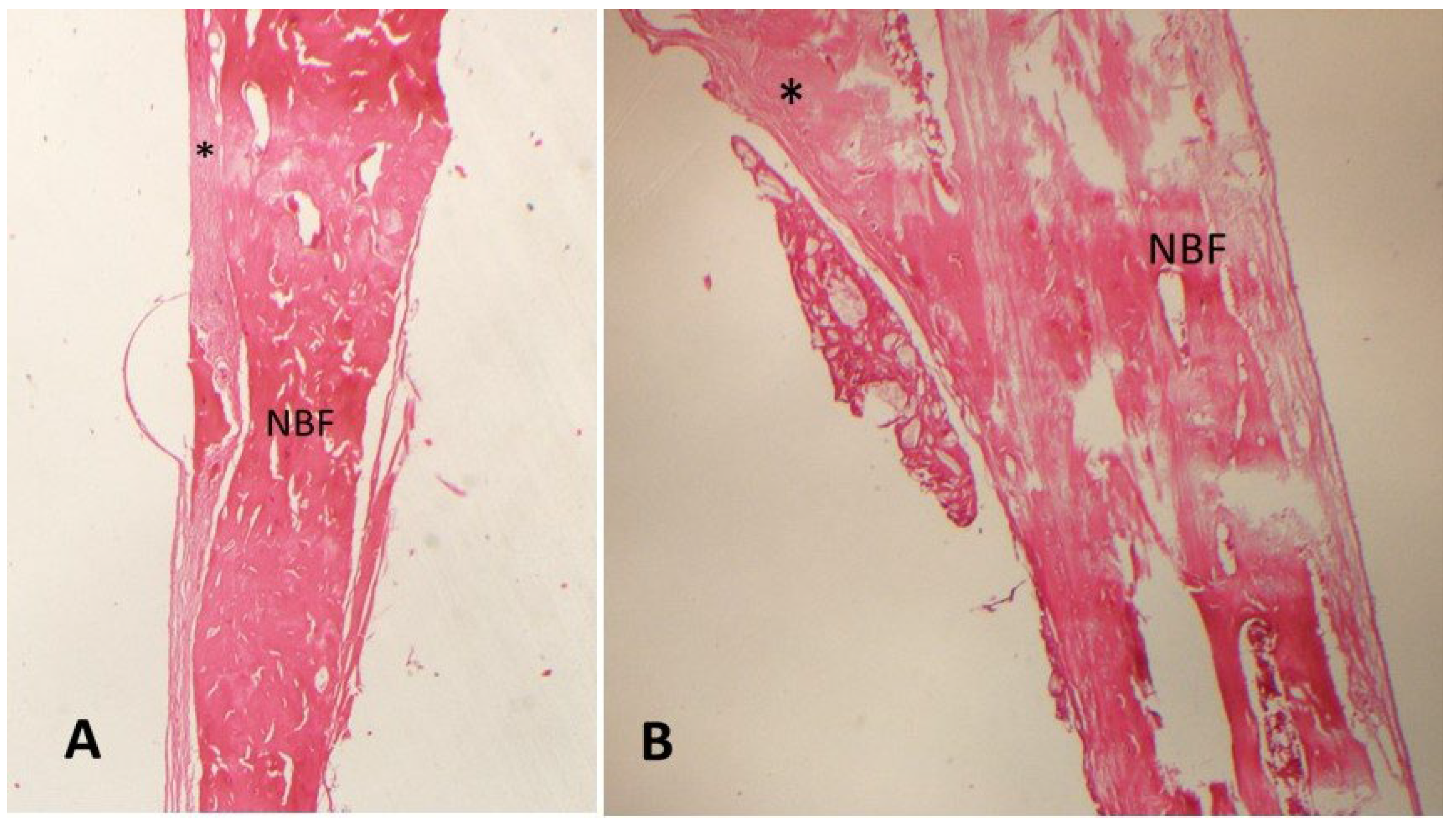
| Groups | N | Mean (NBF) (%) | Std. Dev. | p * |
|---|---|---|---|---|
| Control | 10 | 48.7 | 6.62 | 0.000 |
| MLT D-1 a1 | 10 | 58.1 | 4.82 | |
| MLT D-2 a2 | 10 | 59.2 | 3.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanrisever, M.; Tekin, B.; Can, U.K.; Istek, O.; Ozcan, E.C.; Ozercan, I.H.; Gelic, T.; Dundar, S. The Effect of Local Melatonin Application on Bone Fracture Healing in Rat Tibias. Medicina 2025, 61, 146. https://doi.org/10.3390/medicina61010146
Tanrisever M, Tekin B, Can UK, Istek O, Ozcan EC, Ozercan IH, Gelic T, Dundar S. The Effect of Local Melatonin Application on Bone Fracture Healing in Rat Tibias. Medicina. 2025; 61(1):146. https://doi.org/10.3390/medicina61010146
Chicago/Turabian StyleTanrisever, Murat, Bahar Tekin, Umit Koray Can, Ozmen Istek, Erhan Cahit Ozcan, Ibrahim H. Ozercan, Turker Gelic, and Serkan Dundar. 2025. "The Effect of Local Melatonin Application on Bone Fracture Healing in Rat Tibias" Medicina 61, no. 1: 146. https://doi.org/10.3390/medicina61010146
APA StyleTanrisever, M., Tekin, B., Can, U. K., Istek, O., Ozcan, E. C., Ozercan, I. H., Gelic, T., & Dundar, S. (2025). The Effect of Local Melatonin Application on Bone Fracture Healing in Rat Tibias. Medicina, 61(1), 146. https://doi.org/10.3390/medicina61010146






