Rising Above the Limits of Critical Care ECMO: A Narrative Review
Abstract
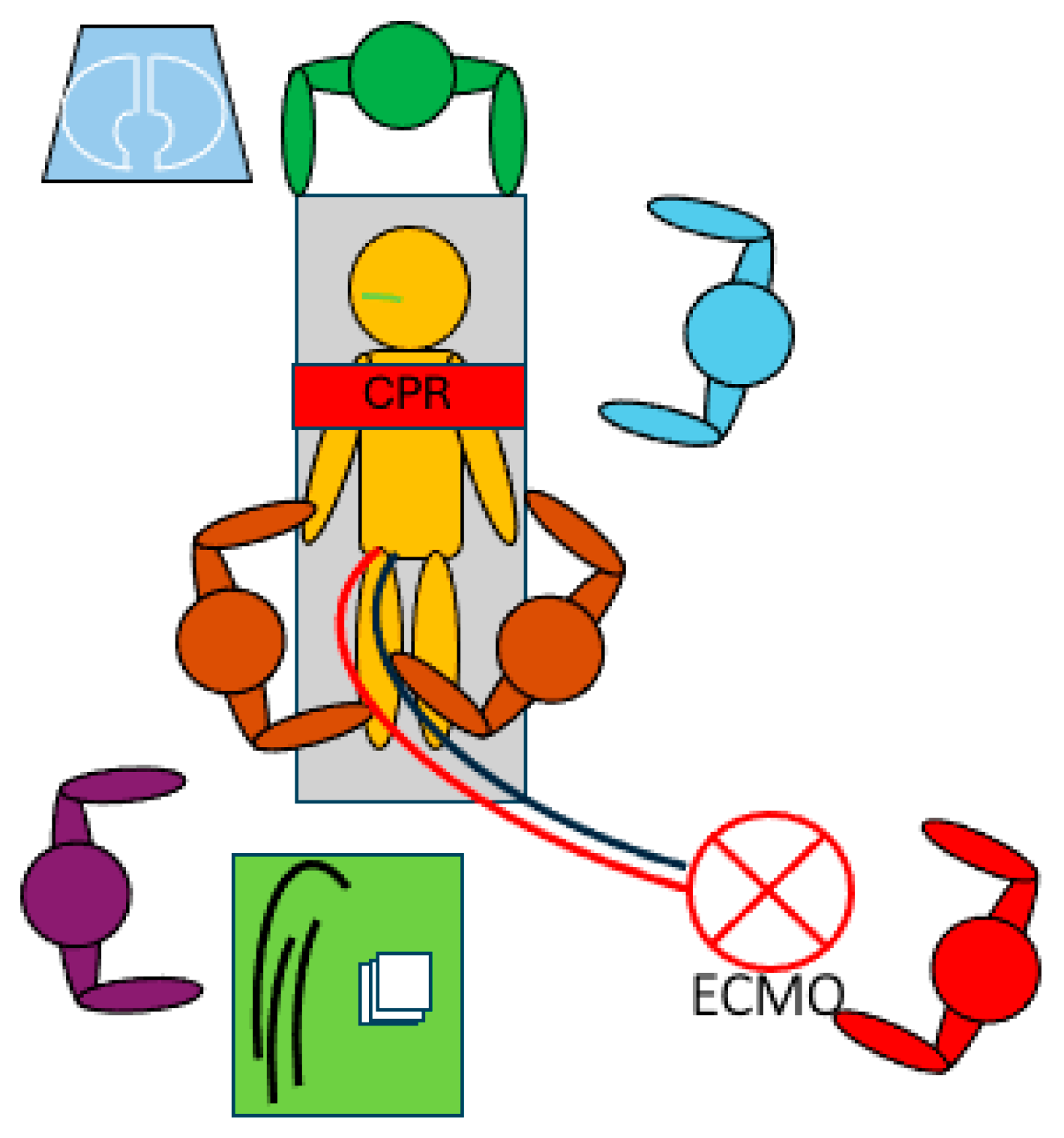
:1. Introduction
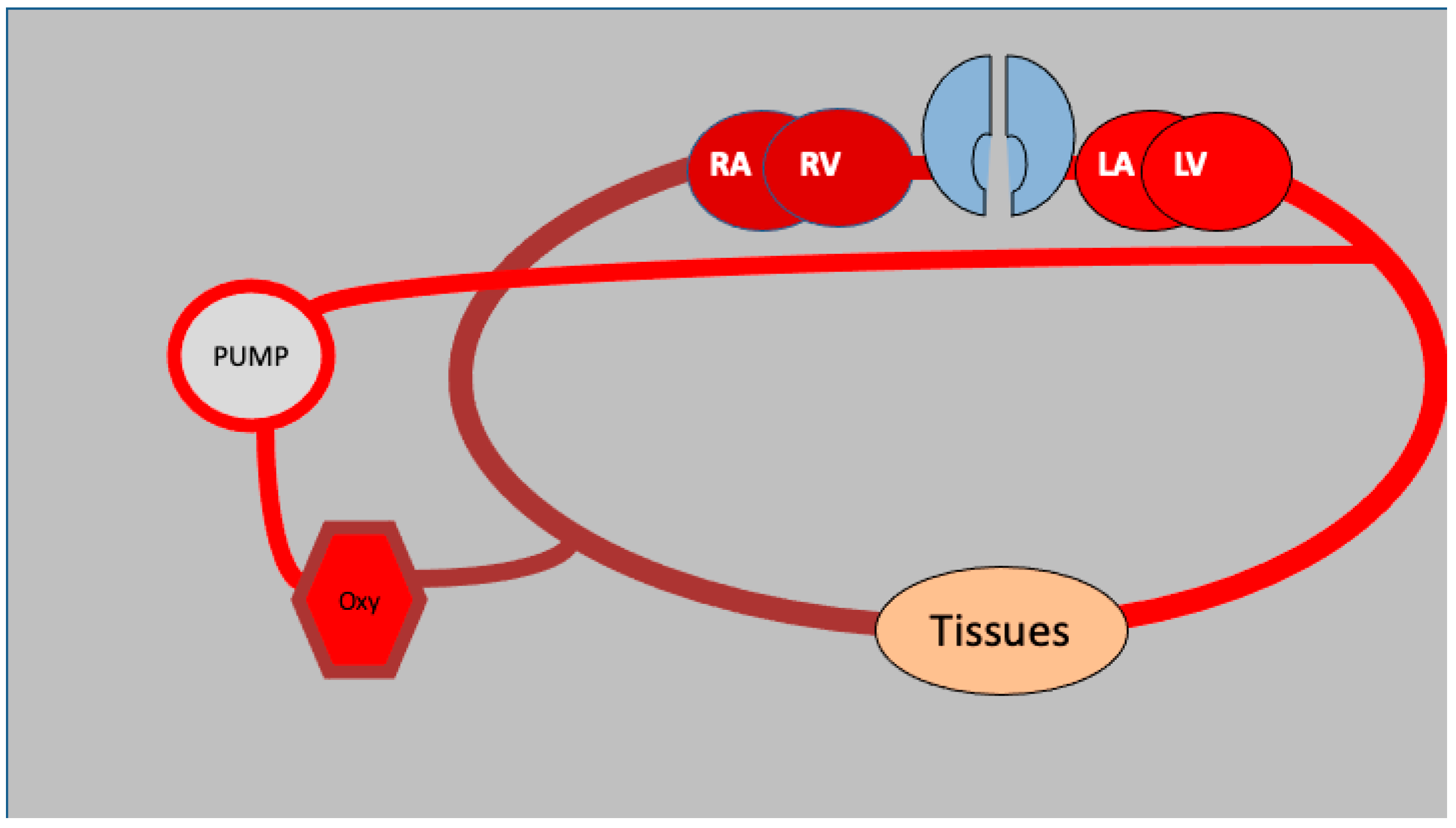
2. The ECMO Circuit and Its Configurations
3. Unconventional Uses of ECMO
3.1. Elective Thoracic Procedures
3.2. High-Risk Cardiac Procedures
3.3. Non-Cardiac Elective Surgical Procedures and Emergency Interventions
4. Factors to Consider and Challenges to Overcome
Success Rate, the Importance of Teamwork and the Advancement of Innovative Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartlett, R.H.; Roloff, D.W.; Cornell, R.G.; Andrews, A.F.; Dillon, P.W.; Zwischenberger, J.B. Extracorporeal circulation in neonatal respiratory failure: A prospective randomized study. Pediatrics 1985, 76, 479–487. [Google Scholar] [CrossRef]
- Combes, A.; Brodie, D.; Bartlett, R.; Brochard, L.; Brower, R.; Conrad, S.; De Backer, D.; Fan, E.; Ferguson, N.; Fortenberry, J.; et al. Position Paper for the Organization of Extracorporeal Membrane Oxygenation Programs for Acute Respiratory Failure in Adult Patients. Am. J. Respir. Crit. Care Med. 2014, 190, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Boonstra, P.S.; MacLaren, G.; Paden, M.; Brodie, D.; Anders, M.; Hoskote, A.; Ramanathan, K.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Life Support Organization Registry International Report 2022: 100,000 Survivors. ASAIO J. 2024, 70, 131–143. [Google Scholar] [CrossRef]
- Bertini, P.; Marabotti, A.; Sangalli, F.; Paternoster, G. Survival difference in patients treated with extracorporeal membrane oxygenation in COVID-19 vs. non-COVID ARDS: A systematic review and meta-analysis. Minerva Anestesiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Taghavi, S.; Aigner, C.; Charchian, R.; Matilla, J.R.; Sano, A.; Klepetko, W. Extracorporeal membrane oxygenation support for resection of locally advanced thoracic tumors. Ann. Thorac. Surg. 2011, 92, 264–270. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef]
- Leng, A.; Shou, B.; Liu, O.; Bachina, P.; Kalra, A.; Bush, E.L.; Whitman, G.J.R.; Cho, S.-M. Machine Learning from Veno-Venous Extracorporeal Membrane Oxygenation Identifies Factors Associated with Neurological Outcomes. Lung 2024, 202, 465–470. [Google Scholar] [CrossRef]
- Bertini, P.; Sangalli, F.; Meani, P.; Marabotti, A.; Rubino, A.; Scolletta, S.; Ajello, V.; Aloisio, T.; Baiocchi, M.; Monaco, F.; et al. Establishing an Extracorporeal Cardiopulmonary Resuscitation Program. Medicina 2024, 60, 1979. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Abouelwafa, M.; Radwan, W.; Abdelfattah, A.; Abdelbary, A.; Khaled, M.; Samy, W.; Yousry, M.; Saeed, A.; Saad, M. The usefulness of Veno-Arterial Extracorporeal Membranous Oxygenation in Patients with Cardiogenic Shock. Open Access Maced. J. Med. Sci. 2019, 7, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, D.; Bartos, J.A.; Martin, C.; Raveendran, G.; Missov, E.; Conterato, M.; Frascone, R.J.; Trembley, A.; Sipprell, K.; John, R.; et al. Minnesota Resuscitation Consortium’s Advanced Perfusion and Reperfusion Cardiac Life Support Strategy for Out-of-Hospital Refractory Ventricular Fibrillation. J. Am. Heart Assoc. 2016, 5, e003732. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Altibi, A.; Prasad, P.; Mukundan, S.; Shekar, K.; Ramanathan, K.; Zakhary, B. Outcomes of Adult Patients With COVID-19 Transitioning From Venovenous to Venoarterial or Hybrid Extracorporeal Membrane Oxygenation in the Extracorporeal Life Support Organization Registry. ASAIO J. 2024, 70, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Cass, S.; Lentz Carvalho, J.; DeAnda, A.; Radhakrishnan, R.S. Extracorporeal Membrane Oxygenation for Patients With Thoracic Neoplasms: An Extracorporeal Life Support Organization (ELSO) Registry Analysis. Ann. Thorac. Surg. 2022, 114, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef]
- Voltolini, L.; Salvicchi, A.; Gonfiotti, A.; Borgianni, S.; Cianchi, G.; Mugnaini, G.; Bongiolatti, S. Veno-venous extra-corporeal membrane oxygenation in complex tracheobronchial resection. J. Thorac. Dis. 2024, 16, 1279–1288. [Google Scholar] [CrossRef]
- Campbell, T.; Bennett, R.G.; Lee, V.; Turnbull, S.; Eslick, A.; Kruit, N.; Pudipeddi, A.; Hing, A.; Kizana, E.; Thomas, S.P.; et al. Ventricular Tachycardia Storm Ablation With Pre-Emptive Circulatory Support by Extracorporeal Membrane Oxygenation: Australian Experience. Heart Lung Circ. 2021, 30, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Chahal, D.; Madathil, R.J.; Kaczorowski, D.; Gupta, A. Percutaneous Coronary Intervention (PCI) Strategies under Hemodynamic Support for Cardiogenic Shock: A Single-Center Experience with Two Patients. Case Rep. Cardiol. 2020, 2020, 6260239. [Google Scholar] [CrossRef]
- Dimarakis, I.; Tennyson, C.; Karatasakis, A.; Macnab, A.; Dobson, L.E.; Kadir, I.; Feddy, L.; Callan, P. Mechanical circulatory support for high-risk surgical aortic valve and ascending aortic replacement in severe bicuspid aortic valve stenosis: A case series. Eur. Heart J. Case Rep. 2024, 8, ytae649. [Google Scholar] [CrossRef]
- Lobo, A.S.; Sandoval, Y.; Henriques, J.P.; Drakos, S.G.; Taleb, I.; Bagai, J.; Cohen, M.G.; Chatzizisis, Y.S.; Sun, B.; Hryniewicz, K.; et al. Cardiogenic Shock Management: International Survey of Contemporary Practices. J. Invasive Cardiol. 2020, 32, 371–374. [Google Scholar] [CrossRef]
- Bertini, P.; Marabotti, A. The anesthetic management and the role of extracorporeal membrane oxygenation for giant mediastinal tumor surgery. Mediastinum 2023, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; De Gala, V.; Lementowski, P.W.; Cvetkovic, D.; Xu, J.L.; Villion, A. Veno-Arterial Extracorporeal Membrane Oxygenation Rescue in a Patient With Pulmonary Hypertension Presenting for Revision Total Hip Arthroplasty: A Case Report and Narrative Review. Cureus 2022, 14, e28234. [Google Scholar] [CrossRef]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef]
- Price, L.C.; Martinez, G.; Brame, A.; Pickworth, T.; Samaranayake, C.; Alexander, D.; Garfield, B.; Aw, T.-C.; McCabe, C.; Mukherjee, B.; et al. Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: A systematic review and expert consensus statement. Br. J. Anaesth. 2021, 126, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Barbas, A.S.; Schroder, J.N.; Borle, D.P.; Suarez, A.; Abraham, N.; Manning, M.W.; Miller, T.E.; Berg, C.L.; Fortin, T.A.; Sudan, D.L.; et al. Planned Initiation of Venoarterial Extracorporeal Membrane Oxygenation Prior to Liver Transplantation in a Patient With Severe Portopulmonary Hypertension. Liver Transpl. 2021, 27, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, C.; Shao, C.; Yang, F.; Xie, H.; Hong, X.; Wang, J.; Li, S.; Li, B.; Chen, D.; et al. Obstetric management strategies for pregnant patients receiving extracorporeal membrane oxygenation and associated maternal-fetal outcomes: A multicentre cohort study. Br. J. Anaesth. 2023, 131, e147–e150. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Helmy, S.; Cardona, J.; Zhao, D.; Rector, R.; Rabin, J.; Mazzeffi, M.; Cho, S.-M.; Parikh, G.; Morris, N.A.; et al. Neurosurgical Procedures in Patients Requiring Extracorporeal Membrane Oxygenation. Crit. Care Explor. 2024, 6, e1166. [Google Scholar] [CrossRef]
- Zagales, R.; Lee, P.; Kumar, S.; Yates, Z.; Awan, M.U.; Cruz, F.; Strause, J.; Schuemann, K.R.; Elkbuli, A. Optimizing Management of Acute Respiratory Distress Syndrome in Critically Ill Surgical Patients: A Systematic Review. J. Surg. Res. 2025, 305, 385–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Huang, X.; Ma, N.; Wang, P.; Li, L.; Chen, X.; Ji, X. ECMO in adult patients with severe trauma: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 412. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Hooft, N.M.; Robinson, B.R.; Todd, E.; Bremner, R.M.; Petersen, S.R.; Smith, M.A. The use of extracorporeal membrane oxygenation in blunt thoracic trauma: A study of the Extracorporeal Life Support Organization database. J. Trauma. Acute Care Surg. 2015, 79, 1049–1053; discussion 1053–1054. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qin, T.; Xi, Z.; Sun, L.; Wu, H.; Li, D. Extracorporeal membrane oxygenation in trauma patients: A systematic review. World J. Emerg. Surg. 2020, 15, 51. [Google Scholar] [CrossRef]
- Upchurch, C.; Blumenberg, A.; Brodie, D.; MacLaren, G.; Zakhary, B.; Hendrickson, R.G. Extracorporeal membrane oxygenation use in poisoning: A narrative review with clinical recommendations. Clin. Toxicol. 2021, 59, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Levitan, R.; Wiegand, T.J.; Lowry, J.; Schult, R.F.; Yin, S. Toxicology Investigators Consortium Extracorporeal Membrane Oxygenation (ECMO) for Severe Toxicological Exposures: Review of the Toxicology Investigators Consortium (ToxIC). J. Med. Toxicol. 2016, 12, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Nazir, M.; Khan, M.K.; Rajendram, R.K.; Shamim, F. Extracorporeal Membrane Oxygenation as Circulatory Support in Adult Patients with Septic Shock: A Systematic Review. J. Crit. Care Med. 2024, 10, 119–129. [Google Scholar] [CrossRef]
- Abdulaziz, S.; Kakar, V.; Kumar, P.G.; Hassan, I.F.; Combes, A.; Brodie, D.; Barrett, N.A.; Tan, J.; Al Ali, S.F. Mechanical Circulatory Support for Massive Pulmonary Embolism. J. Am. Heart Assoc. 2025, 14, e036101. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.S.; Reddy, K.P.; Eberly, L.A.; Fanaroff, A.; Julien, H.M.; Fiorilli, P.; Wald, J.; Mutaawe, S.; Cevasco, M.; Bermudez, C.; et al. Racial, Ethnic, Socioeconomic, and Geographic Inequities in Access to Mechanical Circulatory Support. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101193. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M.; van Diepen, S.; Hollenberg, S.M.; Bernard, S. Extracorporeal Cardiopulmonary Resuscitation: Life-saving or Resource Wasting? US Cardiol. 2024, 18, e12. [Google Scholar] [CrossRef] [PubMed]
- Permenov, B.A.; Zimba, O.; Yessirkepov, M.; Anartayeva, M.; Suigenbayev, D.; Kocyigit, B.F. Extracorporeal membrane oxygenation: Unmet needs and perspectives. Rheumatol. Int. 2024, 44, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Vlok, R.; Buscher, H.; Delaney, A.; Garside, T.; McDonald, G.; Chatoor, R.; Myburgh, J.; Nair, P. Anticoagulation and associated complications in veno-arterial extracorporeal membrane oxygenation in adult patients: A systematic review and meta-analysis. Crit. Care Resusc. 2024, 26, 332–363. [Google Scholar] [CrossRef]
- Peña-López, Y.; Machado, M.C.; Rello, J. Infection in ECMO patients: Changes in epidemiology, diagnosis and prevention. Anaesth. Crit. Care Pain Med. 2024, 43, 101319. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Veronese, G.; Todaro, S.; Marchese, G.; Mondellini, G.M.; Protti, I.; de Arroyabe, B.M.-L.; Epis, F.; Pappalardo, F.; Pedrazzini, G.; et al. Evaluation of Left Ventricular Overload and Use of Unloading Techniques in Venoarterial Extracorporeal Life Support: A Nationwide Survey. ASAIO J. 2024, 70, e57–e60. [Google Scholar] [CrossRef]
- Paternoster, G.; Bertini, P.; Sangalli, F.; Scolletta, S. Awake veno-venous extracorporeal membrane oxygenation: Practical aspects and considerations. Minerva Anestesiol. 2024. [Google Scholar] [CrossRef]
- Nagaoka, E.; Arai, H.; Ugawa, T.; Masuda, T.; Ochiai, K.; Tamaoka, M.; Kurashima, N.; Oi, K.; Fujiwara, T.; Yoshida, M.; et al. Efficacy of multidisciplinary team approach with extracorporeal membrane oxygenation for COVID-19 in a low volume ECMO center. Artif. Organs 2021, 45, 1061–1067. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Bedoya, A.; Bihorac, A.; Celi, L.; Churpek, M.; Economou-Zavlanos, N.J.; Elbers, P.; Saria, S.; Liu, V.; Lyons, P.G.; et al. Use of artificial intelligence in critical care: Opportunities and obstacles. Crit. Care 2024, 28, 113. [Google Scholar] [CrossRef]
- Stephens, A.F.; Šeman, M.; Diehl, A.; Pilcher, D.; Barbaro, R.P.; Brodie, D.; Pellegrino, V.; Kaye, D.M.; Gregory, S.D.; Hodgson, C.; et al. ECMO PAL: Using deep neural networks for survival prediction in venoarterial extracorporeal membrane oxygenation. Intensive Care Med. 2023, 49, 1090–1099. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertini, P.; Marabotti, A.; Meani, P.; Sangalli, F.; Paternoster, G. Rising Above the Limits of Critical Care ECMO: A Narrative Review. Medicina 2025, 61, 174. https://doi.org/10.3390/medicina61020174
Bertini P, Marabotti A, Meani P, Sangalli F, Paternoster G. Rising Above the Limits of Critical Care ECMO: A Narrative Review. Medicina. 2025; 61(2):174. https://doi.org/10.3390/medicina61020174
Chicago/Turabian StyleBertini, Pietro, Alberto Marabotti, Paolo Meani, Fabio Sangalli, and Gianluca Paternoster. 2025. "Rising Above the Limits of Critical Care ECMO: A Narrative Review" Medicina 61, no. 2: 174. https://doi.org/10.3390/medicina61020174
APA StyleBertini, P., Marabotti, A., Meani, P., Sangalli, F., & Paternoster, G. (2025). Rising Above the Limits of Critical Care ECMO: A Narrative Review. Medicina, 61(2), 174. https://doi.org/10.3390/medicina61020174






