Neuromuscular Response to High-Velocity, Low-Amplitude Spinal Manipulation—An Overview
Abstract
1. Introduction
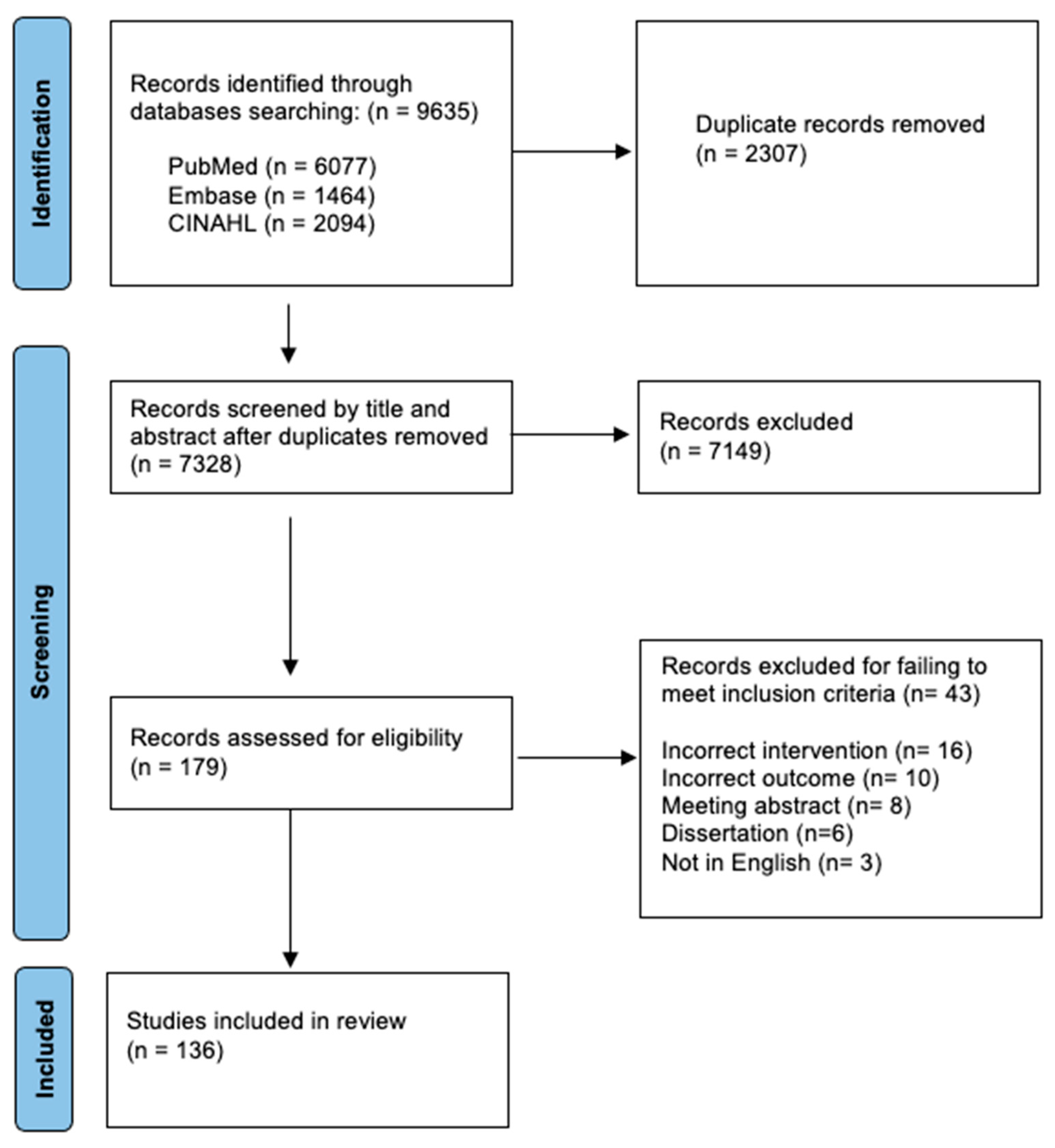
2. Methods
3. Results
3.1. Muscle/Tendon Mechanoreceptors
3.2. Muscle Activation (EMG) and Conduction Velocity
3.3. Muscle Strength
3.4. Muscle Thickness
3.5. M-Wave, H-Reflex, V-Wave, and F-Wave Changes
3.6. MEPs/SEPs/CSPs/MRCPs/CAPs and EEG
4. Discussion
5. Future Directions
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtis, P. Spinal manipulation: Does it work? Occup. Med. 1988, 3, 31–44. [Google Scholar]
- Tuchin, P.J.; Pollard, H.; Bonello, R. A randomized controlled trial of chiropractic spinal manipulative therapy for migraine. J. Manip. Physiol. Ther. 2000, 23, 91–95. [Google Scholar] [CrossRef]
- Young, K.J.; Leboeuf-Yde, C.; Gorrell, L.; Bergström, C.; Evans, D.W.; Axén, I.; Chance-Larsen, K.; Gagey, O.; Georgopoulos, V.; Goncalves, G.; et al. Mechanisms of manipulation: A systematic review of the literature on immediate anatomical structural or positional changes in response to manually delivered high-velocity, low-amplitude spinal manipulation. Chiropr. Man. Ther. 2024, 32, 28. [Google Scholar] [CrossRef] [PubMed]
- Sampath, K.K.; Tumilty, S.; Wooten, L.; Belcher, S.; Farrell, G.; Gisselman, A.S. Effectiveness of spinal manipulation in influencing the autonomic nervous system—A systematic review and meta-analysis. J. Man. Manip. Ther. 2024, 32, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Álvarez, G.; Solà, I.; Cerritelli, F. Do manual therapies have a specific autonomic effect? An overview of systematic reviews. PLoS ONE 2021, 16, e0260642. [Google Scholar] [CrossRef]
- Picchiottino, M.; Leboeuf-Yde, C.; Gagey, O.; Hallman, D.M. The acute effects of joint manipulative techniques on markers of autonomic nervous system activity: A systematic review and meta-analysis of randomized sham-controlled trials. Chiropr. Man. Ther. 2019, 27, 17. [Google Scholar] [CrossRef]
- Araujo, F.X.; Ferreira, G.E.; Angellos, R.F.; Stieven, F.F.; Plentz, R.D.; Silva, M.F. Autonomic Effects of Spinal Manipulative Therapy: Systematic Review of Randomized Controlled Trials. J. Manip. Physiol. Ther. 2019, 42, 623–634. [Google Scholar] [CrossRef]
- Wirth, B.; Gassner, A.; de Bruin, E.D.; Axén, I.; Swanenburg, J.; Humphreys, B.K.; Schweinhardt, P. Neurophysiological Effects of High Velocity and Low Amplitude Spinal Manipulation in Symptomatic and Asymptomatic Humans: A Systematic Literature Review. Spine 2019, 44, E914–E926. [Google Scholar] [CrossRef]
- Lo, C.N.; Ng, J.; Au, C.K.; Lim, E.C.W. The Effectiveness of Spinal Manipulation in Increasing Muscle Strength in Healthy Individuals: A Systematic Review and Meta-Analysis. J. Manip. Physiol. Ther. 2019, 42, 148–158. [Google Scholar] [CrossRef]
- Galindez-Ibarbengoetxea, X.; Setuain, I.; Andersen, L.L.; Ramírez-Velez, R.; González-Izal, M.; Jauregi, A.; Izquierdo, M. Effects of Cervical High-Velocity Low-Amplitude Techniques on Range of Motion, Strength Performance, and Cardiovascular Outcomes: A Review. J. Altern. Complement. Med. 2017, 23, 667–675. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Kumari, N.; Amjad, I.; Duehr, J.; Holt, K. The Potential Mechanisms of High-Velocity, Low-Amplitude, Controlled Vertebral Thrusts on Neuroimmune Function: A Narrative Review. Medicina 2021, 57, 536. [Google Scholar] [CrossRef] [PubMed]
- Gyer, G.; Michael, J.; Inklebarger, J.; Alam, I.I. Effects of biomechanical parameters of spinal manipulation: A critical literature review. J. Integr. Med. 2022, 20, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, M.; Daneau, C.; Marchand, A.-A.; Lardon, A.; Descarreaux, M. Spinal manipulation frequency and dosage effects on clinical and physiological outcomes: A scoping review. Chiropr. Man. Ther. 2019, 27, 23. [Google Scholar] [CrossRef] [PubMed]
- Trager, R.J.; Bejarano, G.; Perfecto, R.-P.T.; Blackwood, E.R.; Goertz, C.M. Chiropractic and spinal manipulation: A review of research trends, evidence gaps, and guideline recommendations. J. Clin. Med. 2024, 13, 5668. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, L.M.; Nyirö, L.; Pasquier, M.; Pagé, I.; Heneghan, N.R.; Schweinhardt, P.; Descarreaux, M. Spinal manipulation characteristics: A scoping literature review of force-time characteristics. Chiropr. Man. Ther. 2023, 31, 36. [Google Scholar] [CrossRef]
- Haavik, H.; Kumari, N.; Holt, K.; Niazi, I.K.; Amjad, I.; Pujari, A.N.; Türker, K.S.; Murphy, B. The contemporary model of vertebral column joint dysfunction and impact of high-velocity, low-amplitude controlled vertebral thrusts on neuromuscular function. Eur. J. Appl. Physiol. 2021, 121, 2675–2720. [Google Scholar] [CrossRef]
- Savva, C.; Giakas, G.; Efstathiou, M. The role of the descending inhibitory pain mechanism in musculoskeletal pain following high-velocity, low amplitude thrust manipulation. A review of the literature. J. Back. Musculoskelet. Rehabil. 2014, 27, 377–382. [Google Scholar] [CrossRef]
- Pickar, J.G. Neurophysiological effects of spinal manipulation. Spine J. 2002, 2, 357–371. [Google Scholar] [CrossRef]
- Clark, B.C.; Thomas, J.S.; Walkowski, S.A.; Howell, J.N. The biology of manual therapies. J. Am. Osteopath. Assoc. 2012, 112, 617–629. [Google Scholar]
- Sun, Y.; Fede, C.; Zhao, X.; Del Felice, A.; Pirri, C.; Stecco, C. Quantity and distribution of muscle spindles in animal and human muscles. Int. J. Mol. Sci. 2024, 25, 7320. [Google Scholar] [CrossRef]
- Lima, C.R.; Martins, D.F.; Reed, W.R. Physiological Responses Induced by Manual Therapy in Animal Models: A Scoping Review. Front. Neurosci. 2020, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez-Juana, D.; Fernández-de-Las-Peñas, C.; Arias-Buría, J.L.; Cleland, J.A.; Plaza-Manzano, G.; Ortega-Santiago, R. Changes in Cervicocephalic Kinesthetic Sensibility, Widespread Pressure Pain Sensitivity, and Neck Pain After Cervical Thrust Manipulation in Patients with Chronic Mechanical Neck Pain: A Randomized Clinical Trial. J. Manip. Physiol. Ther. 2018, 41, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Murphy, B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.R.; Haavik, H.; Lee, A.C.L.; Murphy, B.; Elley, C.R. Effectiveness of Chiropractic Care to Improve Sensorimotor Function Associated With Falls Risk in Older People: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2016, 39, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Learman, K.E.; Myers, J.B.; Lephart, S.M.; Sell, T.C.; Kerns, G.J.; Cook, C.E. Effects of spinal manipulation on trunk proprioception in subjects with chronic low back pain during symptom remission. J. Manip. Physiol. Ther. 2009, 32, 118–126. [Google Scholar] [CrossRef]
- Miranda, I.F.; Facchini, D.; Manfio, E.F. Influence of Cervical Spine Manipulation on Neck Joint Position Sense error in patients with chronic neck pain. Man. Ther. Posturol. Rehabil. J. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Erdem, E.U.; Ünver, B.; Akbas, E.; Kinikli, G.I. Immediate effects of thoracic manipulation on cervical joint position sense in individuals with mechanical neck pain: A randomized controlled trial. J. Back. Musculoskelet. Rehabil. 2021, 34, 735–743. [Google Scholar] [CrossRef]
- Paredes, R.; Crasto, C.; Magalhães, B.; Carvalho, P. Short-Term Effects of Global Pelvic Manipulation on Knee Joint Position Sense in Asymptomatic Participants: A Double-Blind Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 675–682. [Google Scholar] [CrossRef]
- Motealleh, A.; Barzegar, A.; Abbasi, L. The immediate effect of lumbopelvic manipulation on knee pain, knee position sense, and balance in patients with patellofemoral pain: A randomized controlled trial. J. Bodyw. Mov. Ther. 2020, 24, 71–77. [Google Scholar] [CrossRef]
- Lehman, G. Kinesiological research: The use of surface electromyography for assessing the effects of spinal manipulation. J. Electromyogr. Kinesiol. 2012, 22, 692–696. [Google Scholar] [CrossRef]
- Symons, B.P.; Herzog, W.; Leonard, T.; Nguyen, H. Reflex responses associated with activator treatment. J. Manip. Physiol. Ther. 2000, 23, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nougarou, F.; Dugas, C.; Deslauriers, C.; Pagé, I.; Descarreaux, M. Physiological responses to spinal manipulation therapy: Investigation of the relationship between electromyographic responses and peak force. J. Manip. Physiol. Ther. 2013, 36, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Keller, T.S.; Gunzburg, R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part. II. Neurophysiological response. J. Manip. Physiol. Ther. 2003, 26, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Keller, T.S.; Gunzburg, R.; Vandeputte, K.; Fuhr, A.W. Neurophysiologic response to intraoperative lumbosacral spinal manipulation. J. Manip. Physiol. Ther. 2000, 23, 447–457. [Google Scholar] [CrossRef]
- Nougarou, F.; Pagé, I.; Loranger, M.; Dugas, C.; Descarreaux, M. Neuromechanical response to spinal manipulation therapy: Effects of a constant rate of force application. BMC Complement. Altern. Med. 2016, 16, 161. [Google Scholar] [CrossRef]
- Pagé, I.; Nougarou, F.; Dugas, C.; Descarreaux, M. The effect of spinal manipulation impulse duration on spine neuromechanical responses. J. Can. Chiropr. Assoc. 2014, 58, 141–148. [Google Scholar]
- Pagé, I.; Nougarou, F.; Descarreaux, M. Neuromuscular response amplitude to mechanical stimulation using large-array surface electromyography in participants with and without chronic low back pain. J. Electromyogr. Kinesiol. 2016, 27, 24–29. [Google Scholar] [CrossRef]
- Nougarou, F.; Dugas, C.; Loranger, M.; Pagé, I.; Descarreaux, M. The role of preload forces in spinal manipulation: Experimental investigation of kinematic and electromyographic responses in healthy adults. J. Manip. Physiol. Ther. 2014, 37, 287–293. [Google Scholar] [CrossRef][Green Version]
- Colloca, C.J.; Keller, T.S. Electromyographic reflex responses to mechanical force, manually assisted spinal manipulative therapy. Spine 2001, 26, 1117–1124. [Google Scholar] [CrossRef]
- Keller, T.S.; Colloca, C.J. Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: A comparative clinical trial. J. Manip. Physiol. Ther. 2000, 23, 585–595. [Google Scholar] [CrossRef]
- Currie, S.J.; Myers, C.A.; Durso, C.; Enebo, B.A.; Davidson, B.S. The Neuromuscular Response to Spinal Manipulation in the Presence of Pain. J. Manip. Physiol. Ther. 2016, 39, 288–293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pagé, I.; Biner, É.; Descarreaux, M. Vertebral Displacements and Muscle Activity During Manual Therapy: Distinct Behaviors Between Spinal Manipulation and Mobilization. J. Manip. Physiol. Ther. 2018, 41, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Lardon, A.; Pagé, I.; Nougarou, F.; Descarreaux, M. Neuromechanical Responses to Spinal Manipulation and Mobilization: A Crossover Randomized Clinical Trial. J. Manip. Physiol. Ther. 2022, 45, 1–8. [Google Scholar] [CrossRef]
- Gorrell, L.M.; Conway, P.J.; Herzog, W. Differences in force-time parameters and electromyographic characteristics of two high-velocity, low-amplitude spinal manipulations following one another in quick succession. Chiropr. Man. Ther. 2020, 28, 67. [Google Scholar] [CrossRef]
- Gorrell, L.M.; Conway, P.J.; Herzog, W. Reflex Responses of Neck, Back, and Limb Muscles to High-Velocity, Low-Amplitude Manual Cervical and Upper Thoracic Spinal Manipulation of Asymptomatic Individuals—A Descriptive Study. J. Manip. Physiol. Ther. 2019, 42, 572–581. [Google Scholar] [CrossRef]
- Currie, S.J.; Myers, C.A.; Enebo, B.A.; Davidson, B.S. Treatment and response factors in muscle activation during spinal manipulation. J. Clin. Med. 2023, 12, 6377. [Google Scholar] [CrossRef]
- Gorrell, L.M.; Conway, P.J.; Onasch, F.; Herzog, W. Electromyographic responses of neck, back, and limb outlet muscles associated with high-velocity, low-amplitude manual cervical and upper thoracic spinal manipulation of individuals with mild neck disability: A descriptive observational investigation. J. Manip. Physiol. Ther. 2022, 45, 33–44. [Google Scholar] [CrossRef]
- Colloca, C.J.; Keller, T.S. Stiffness and neuromuscular reflex response of the human spine to posteroanterior manipulative thrusts in patients with low back pain. J. Manip. Physiol. Ther. 2001, 24, 489–500. [Google Scholar] [CrossRef]
- Dishman, J.D.; Cunningham, B.M.; Burke, J. Comparison of tibial nerve H-reflex excitability after cervical and lumbar spine manipulation. J. Manip. Physiol. Ther. 2002, 25, 318–325. [Google Scholar] [CrossRef]
- Dunning, J.; Rushton, A. The effects of cervical high-velocity low-amplitude thrust manipulation on resting electromyographic activity of the biceps brachii muscle. Man. Ther. 2009, 14, 508–513. [Google Scholar] [CrossRef]
- de Camargo, V.M.; Alburquerque-Sendín, F.; Bérzin, F.; Stefanelli, V.C.; de Souza, D.P.R.; Fernández-De-Las-Peñas, C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: A randomized controlled Trial. J. Manip. Physiol. Ther. 2011, 34, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, A.K.; Hsu, M.; Roy, J.-S.; Kardouni, J.R.; Kutch, J.J.; Michener, L.A. Evidence for increased neuromuscular drive following spinal manipulation in individuals with subacromial pain syndrome. Clin. Biomech. 2021, 90, 105485. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.W.C.; Silva, A.M.; Silva, A.F.; Martins, F.L.M.; Barbosa, M.C.S.A. Immediate improvements in activation amplitude levels of the deep abdominal muscle following a sacroiliac joint manipulation during rapid upper limb movement. J. Bodyw. Mov. Ther. 2014, 18, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Ferreira, P.H.; Hodges, P.W. Changes in postural activity of the trunk muscles following spinal manipulative therapy. Man. Ther. 2007, 12, 240–248. [Google Scholar] [CrossRef]
- Herzog, W.; Scheele, D.; Conway, P.J. Electromyographic responses of back and limb muscles associated with spinal manipulative therapy. Spine 1999, 24, 146–152. [Google Scholar] [CrossRef]
- Lehman, G.J.; Vernon, H.; McGill, S.M. Effects of a mechanical pain stimulus on erector spinae activity before and after a spinal manipulation in patients with back pain: A preliminary investigation. J. Manip. Physiol. Ther. 2001, 24, 402–406. [Google Scholar] [CrossRef]
- Shambaugh, P. Changes in electrical-activity in muscles resulting from chiropractic adjustment—A pilot-study. J. Manip. Physiol. Ther. 1987, 10, 300–304. [Google Scholar]
- Bicalho, E.; Setti, J.A.P.; Macagnan, J.; Cano, J.L.R.; Manffra, E.F. Immediate effects of a high-velocity spine manipulation in paraspinal muscles activity of nonspecific chronic low-back pain subjects. Man. Ther. 2010, 15, 469–475. [Google Scholar] [CrossRef]
- Lehman, G.J.; McGill, S.M. Spinal manipulation causes variable spine kinematic and trunk muscle electromyographic responses. Clin. Biomech. 2001, 16, 293–299. [Google Scholar] [CrossRef]
- DeVocht, J.W.; Pickar, J.G.; Wilder, D.G. Spinal manipulation alters electromyographic activity of paraspinal muscles: A descriptive study. J. Manip. Physiol. Ther. 2005, 28, 465–471. [Google Scholar] [CrossRef]
- De Carvalho, D.E.; Callaghan, J.P. The effect of lumbar spinal manipulation on biomechanical factors and perceived transient pain during prolonged sitting: A laboratory-controlled cross-sectional study. Chiropr. Man. Ther. 2022, 30, 62. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, X.; Zhang, J.; Wang, Y. Changes in Pressure Pain Thresholds and Basal Electromyographic Activity After Instrument-Assisted Spinal Manipulative Therapy in Asymptomatic Participants: A Randomized, Controlled Trial. J. Manip. Physiol. Ther. 2012, 35, 437–445. [Google Scholar] [CrossRef]
- Suter, E.; McMorland, G.; Herzog, W.; Gray, R. Conservative lower back treatment reduces inhibition in knee-extensor muscles: A randomized controlled trial. J. Manip. Physiol. Ther. 2000, 23, 76–80. [Google Scholar] [CrossRef]
- Fryer, G.; Bird, M.; Robbins, B.; Johnson, J.C. Acute electromyographic responses of deep thoracic paraspinal muscles to spinal manual therapy interventions. An experimental, randomized cross-over study. J. Bodyw. Mov. Ther. 2017, 21, 495–502. [Google Scholar] [CrossRef]
- Cardinale, M.; Boccia, G.; Greenway, T.; Evans, O.; Rainoldi, A. The acute effects of spinal manipulation on neuromuscular function in asymptomatic individuals: A preliminary study. Phys. Ther. Sport. 2015, 16, 121–126. [Google Scholar] [CrossRef]
- Howell, J.N.; Cabell, K.S.; Chila, A.G.; Eland, D.C. Stretch reflex and Hoffmann reflex responses to osteopathic manipulative treatment in subjects with Achilles tendinitis. J. Osteopath. Med. 2006, 106, 537–545. [Google Scholar]
- King, S.L.; Docrat, A.; Abdul-Rasheed, A. Immediate Effects of Cervical Spine Manipulation Compared With Muscle Energy Technique on Neck Muscle Activity and Range of Motion in Asymptomatic Participants: A Randomized Study. J. Chiropr. Med. 2022, 21, 241–248. [Google Scholar] [CrossRef]
- Zhang, J.; Enix, D.; Snyder, B.; Giggey, K.; Tepe, R. Effects of Biofreeze and chiropractic adjustments on acute low back pain: A pilot study. J. Chiropr. Med. 2008, 7, 59–65. [Google Scholar] [CrossRef]
- Sturion, L.A.; Nowotny, A.H.; Barillec, F.; Barette, G.; Santos, G.K.; Teixeira, F.A.; Fernandes, K.P.; da Silva, R. Comparison between high-velocity low-amplitude manipulation and muscle energy technique on pain and trunk neuromuscular postural control in male workers with chronic low back pain: A randomised crossover trial. S. Afr. J. Physiother. 2020, 76, 1420. [Google Scholar] [CrossRef]
- Cholewicki, J.; Lee, A.S.; Reeves, N.P.; Calle, E.A. Trunk muscle response to various protocols of lumbar traction. Man. Ther. 2009, 14, 562–566. [Google Scholar] [CrossRef]
- Pires, P.F.; Packer, A.C.; Dibai-Filho, A.V.; Rodrigues-Bigaton, D. Immediate and short-term effects of upper thoracic manipulation on myoelectric activity of sternocleidomastoid muscles in young women with chronic neck pain: A randomized blind clinical trial. J. Manip. Physiol. Ther. 2015, 38, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Galindez-Ibarbengoetxea, X.; Setuain, I.; Ramirez-Velez, R.; Andersen, L.L.; Gonzalez-Izal, M.; Jauregi, A.; Izquierdo, M. Immediate Effects of Osteopathic Treatment Versus Therapeutic Exercise on Patients with Chronic Cervical Pain. Altern. Ther. Health Med. 2018, 24, 24–32. [Google Scholar] [PubMed]
- Bracht, M.A.; Coan, A.C.B.; Yahya, A.; Dos Santos, M.J. Effects of cervical manipulation on pain, grip force control, and upper extremity muscle activity: A randomized controlled trial. J. Man. Manip. Ther. 2018, 26, 78–88. [Google Scholar] [CrossRef] [PubMed]
- McChesney, B.D.; Haig, L.; Gissane, C. The effect of thoracic spine high-velocity low-amplitude thrust manipulation on myoelectric activity of the lower trapezius and posterior deltoid muscles during treadmill walking. Int. J. Osteopat. Med. 2011, 14, 141–148. [Google Scholar] [CrossRef]
- Zafarian, T.; Taghipour, M.; Khafri, S.; Bahrami, M.; Javanshir, K. The effect of lumbopelvic manipulation on electromyography parameters of gluteus medius and vastus medialis in patients with patellofemoral pain syndrome: A double-blind, placebo-controlled trial. Int. J. Osteopat. Med. 2023, 50, 100667. [Google Scholar] [CrossRef]
- Arcanjo, G.N.; Pires, J.L.V.R.; Jacinto, M.E.M.; Colares, J.M.; Belo, L.M.C.; Lima, P.O.d.P.; Vilaça-Alves, J. Comparison of the Effect of Osteopathic Manipulations and Exercises on the Myoelectric Activity of the Pelvic Floor: A Randomized Controlled Trial. J. Chiropr. Med. 2022, 21, 97–107. [Google Scholar] [CrossRef]
- Haik, M.N.; Alburquerque-Sendín, F.; Camargo, P.R. Short-Term Effects of Thoracic Spine Manipulation on Shoulder Impingement Syndrome: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 1594–1605. [Google Scholar] [CrossRef]
- McCarthy, C.J.; Potter, L.; Oldham, J.A. Comparing targeted thrust manipulation with general thrust manipulation in patients with low back pain. A general approach is as effective as a specific one. A randomised controlled trial. BMJ Open Sport. Exerc. Med. 2019, 5, e000514. [Google Scholar] [CrossRef]
- Lalanne, K.; Lafond, D.; Descarreaux, M. Modulation of the flexion-relaxation response by spinal manipulative therapy: A control group study. J. Manip. Physiol. Ther. 2009, 32, 203–209. [Google Scholar] [CrossRef]
- Jung, S.-H.; Hwang, U.-J.; Ahn, S.-H.; Kim, J.-H.; Kwon, O.-Y. Effects of Manual Therapy and Mechanical Massage on Spinal Alignment, Extension Range of Motion, Back Extensor Electromyographic Activity, and Thoracic Extension Strength in Individuals with Thoracic Hyperkyphosis: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2020, 2020, 6526935. [Google Scholar] [CrossRef]
- Fernandes, W.V.B.; Politti, F.; Lanza, F.C.; Lucareli, P.G.; Corrêa, J.C.F. Immediate effects of spinal manipulation on dynamic electromyographic activity of nonspecific chronic low back pain subjects. Gait Posture 2017, 57, 329. [Google Scholar] [CrossRef]
- Harvey, M.-P.; Descarreaux, M. Short term modulation of trunk neuromuscular responses following spinal manipulation: A control group study. BMC Musculoskelet. Disord. 2013, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Long, C.R.; Vining, R.D.; Gudavalli, M.R.; DeVocht, J.W.; Kawchuk, G.N.; Wilder, D.G.; Goertz, C.M. Association of lumbar spine stiffness and flex-ion-relaxation phenomenon with patient-reported outcomes in adults with chronic low back pain—A single-arm clinical trial investigating the effects of thrust spinal manipulation. BMC Complement. Altern. Med. 2017, 17, 303. [Google Scholar] [CrossRef]
- Galindez-Ibarbengoetxea, X.; Setuain, I.; González-Izal, M.; Jauregi, A.; Ramírez-Velez, R.; Andersen, L.L.; Izquierdo, M. Randomised controlled pilot trial of high-velocity, low-amplitude manipulation on cervical and upper thoracic spine levels in asymptomatic subjects. Int. J. Osteopat. Med. 2017, 25, 6–14. [Google Scholar] [CrossRef]
- Galindez-Ibarbengoetxea, X.; Setuain, I.; Ramírez-Velez, R.; Andersen, L.L.; González-Izal, M.; Jauregi, A.; Izquierdo, M. Short-term effects of manipulative treatment versus a therapeutic home exercise protocol for chronic cervical pain: A randomized clinical trial. J. Back. Musculoskelet. Rehabil. 2018, 31, 133–145. [Google Scholar] [CrossRef]
- Dishman, J.D.; Dougherty, P.E.; Burke, J.R. Evaluation of the effect of postural perturbation on motoneuronal activity following various methods of lumbar spinal manipulation. Spine J. 2005, 5, 650–659. [Google Scholar] [CrossRef]
- Geisser, M.E.; Haig, A.J.; Wallbom, A.S.; Wiggert, E.A. Pain-related fear, lumbar flexion, and dynamic EMG among persons with chronic musculoskeletal low back pain. Clin. J. Pain. 2004, 20, 61–69. [Google Scholar] [CrossRef]
- Neblett, R.; Mayer, T.G.; Gatchel, R.J.; Keeley, J.; Proctor, T.; Anagnostis, C. Quantifying the Lumbar Flexion–Relaxation Phenomenon: Theory, Normative Data, and Clinical Applications. Spine 2003, 28, 1435. [Google Scholar] [CrossRef]
- Lewis, S.; Holmes, P.; Woby, S.; Hindle, J.; Fowler, N. The relationships between measures of stature recovery, muscle activity and psychological factors in patients with chronic low back pain. Man. Ther. 2012, 17, 27–33. [Google Scholar] [CrossRef]
- Niazi, I.K.; Kamavuako, E.N.; Holt, K.; Janjua, T.A.M.; Kumari, N.; Amjad, I.; Haavik, H. The Effect of Spinal Manipulation on the Electrophysiological and Metabolic Properties of the Tibialis Anterior Muscle. Healthcare 2020, 8, 548. [Google Scholar] [CrossRef]
- Robinault, L.; Holobar, A.; Crémoux, S.; Rashid, U.; Niazi, I.K.; Holt, K.; Lauber, J.; Haavik, H. The Effects of Spinal Manipulation on Motor Unit Behavior. Brain Sci. 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Boët, C.; Fugier, S.; Marsault, J.; Toublan, D.; Valot, M.-E.; Cheval, A.; D’Inville, G.A.; Niel, S.; Guihéneuc, P.; Guihard, G. High-velocity low-amplitude thrust manipulation of the lumbar spine immediately modifies soleus T reflex in asymptomatic adults. Int. J. Osteopat. Med. 2013, 16, 131–142. [Google Scholar] [CrossRef]
- Cleland, J.; Selleck, B.; Stowell, T.; Browne, L.; Alberini, S.; Cyr, H.S.; Caron, T. Short-term effects of thoracic manipulation on lower trapezius muscle strength. J. Man. Manip. Ther. 2004, 12, 82–90. [Google Scholar] [CrossRef]
- Botelho, M.B.; Andrade, B.B. Effect of cervical spine manipulative therapy on judo athletes’ grip strength. J. Manip. Physiol. Ther. 2012, 35, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, L.M.; Beath, K.; Engel, R.M. Manual and Instrument Applied Cervical Manipulation for Mechanical Neck Pain: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2016, 39, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carnero, J.; Fernández-De-Las-Peñas, C.; Cleland, J.A. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J. Manip. Physiol. Ther. 2008, 31, 675–681. [Google Scholar] [CrossRef]
- Holt, K.; Niazi, I.K.; Nedergaard, R.W.; Duehr, J.; Amjad, I.; Shafique, M.; Anwar, M.N.; Ndetan, H.; Turker, K.S.; Haavik, H. The effects of a single session of chiropractic care on strength, cortical drive, and spinal excitability in stroke patients. Sci. Rep. 2019, 9, 2673. [Google Scholar] [CrossRef]
- Niazi, I.K.; Türker, K.S.; Flavel, S.; Kinget, M.; Duehr, J.; Haavik, H. Changes in H-reflex and V-waves following spinal manipulation. Exp. Brain Res. 2015, 233, 1165–1173. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Duehr, J.; Allen, K.; Marshall, P.; Türker, K.S.; Hartvigsen, J.; Haavik, H. The effects of a single session of spinal manipulation on strength and cortical drive in athletes. Eur. J. Appl. Physiol. 2018, 118, 737–749. [Google Scholar] [CrossRef]
- Grindstaff, T.L.; Hertel, J.; Beazell, J.R.; Magrum, E.M.; Ingersoll, C.D. Effects of lumbopelvic joint manipulation on quadriceps activation and strength in healthy individuals. Man. Ther. 2009, 14, 415–420. [Google Scholar] [CrossRef]
- Hillermann, B.; Gomes, A.N.; Korporaal, C.; Jackson, D. A pilot study comparing the effects of spinal manipulative therapy with those of extra-spinal manipulative therapy on quadriceps muscle strength. J. Manip. Physiol. Ther. 2006, 29, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, P.D.; Cornish, S.M.; Schulte, A.; Jantz, N.; Magnus, C.R.A.; Schwanbeck, S.; Juurlink, B.H. The effect of spinal manipulation on imbalances in leg strength. J. Can. Chiropr. Assoc. 2011, 55, 183–192. [Google Scholar] [PubMed]
- Kingett, M.; Holt, K.; Niazi, I.K.; Nedergaard, R.W.; Lee, M.; Haavik, H. Increased Voluntary Activation of the Elbow Flexors Following a Single Session of Spinal Manipulation in a Subclinical Neck Pain Population. Brain Sci. 2019, 9, 136. [Google Scholar] [CrossRef]
- de Almeida, B.S.N.; Sabatino, J.H.; Giraldo, P.C. Effects of high-velocity, low-amplitude spinal manipulation on strength and the basal tonus of female pelvic floor muscles. J. Manip. Physiol. Ther. 2010, 33, 109–116. [Google Scholar] [CrossRef]
- Suter, E.; McMorland, G. Decrease in elbow flexor inhibition after cervical spine manipulation in patients with chronic neck pain. Clin. Biomech. 2002, 17, 541–544. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Cleland, J.A.; Arbizu, R.L.T. Examination of motor and hypoalgesic effects of cervical vs thoracic spine manipulation in patients with lateral epicondylalgia: A clinical trial. J. Manip. Physiol. Ther. 2011, 34, 432–440. [Google Scholar] [CrossRef]
- Tahmaz, T.; Genç, H.; Demircioğlu, G. Comparison of the immediate effects of cervical manipulation and foam roller applications in individuals with non-specific neck pain. Adv. Rehabil. 2023, 37, 9–15. [Google Scholar] [CrossRef]
- Metcalfe, S.; Reese, H.; Sydenham, R. Effect of high-velocity low-amplitude manipulation on cervical spine muscle strength: A randomized clinical trial. J. Man. Manip. Ther. 2006, 14, 152–158. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Jochumsen, M.; Sherwin, D.; Flavel, S.; Türker, K.S. Impact of Spinal Manipulation on Cortical Drive to Upper and Lower Limb Muscles. Brain Sci. 2016, 7, 2. [Google Scholar] [CrossRef]
- Navid, M.S.; Niazi, I.K.; Lelic, D.; Amjad, I.; Kumari, N.; Shafique, M.; Holt, K.; Rashid, U.; Drewes, A.M.; Haavik, H. Chiropractic Spinal Adjustment Increases the Cortical Drive to the Lower Limb Muscle in Chronic Stroke Patients. Front. Neurol. 2022, 12, 747261. [Google Scholar] [CrossRef]
- Zunke, P.; Auffarth, A.; Hitzl, W.; Moursy, M. The effect of manual therapy to the thoracic spine on pain-free grip and sympathetic activity in patients with lateral epicondylalgia humeri. A randomized, sample sized planned, placebo-controlled, patient-blinded monocentric trial. BMC Musculoskelet. Disord. 2020, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Ward, J.; Coats, J.; Nobert, J.; Amonette, W.; Dyess, S. Immediate effects of lower cervical spine manipulation on handgrip strength and free-throw accuracy of asymptomatic basketball players: A pilot study. J. Chiropr. Med. 2013, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gevers-Montoro, C.; Deldar, Z.; Mues, A.O.-D. Regional sensorimotor effects of chiropractic spinal manipulation: Preliminary results from an experimental study. J. Manip. Physiol. Ther. 2024, 46, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.S.; Lam, P.Y.; Lau, M.Y.; Siu, W.L.; Yu, K.M.; Lo, C.N.; Ng, J. Changes in Lower Limb Strength and Function Following Lumbar Spinal Mobilization. J. Manip. Physiol. Ther. 2017, 40, 587–596. [Google Scholar] [CrossRef]
- Wong, C.K.; Conway, L.; Fleming, G.; Gopie, C.; Liebeskind, D.; Xue, S. Immediate Effects of a Single Spinal Manipulation on Lower-Limb Strength in Healthy Individuals: A Critically Appraised Topic. J. Sport. Rehabil. 2020, 30, 161–165. [Google Scholar] [CrossRef]
- Vining, R.; Long, C.R.; Minkalis, A.; Gudavalli, M.R.; Xia, T.; Walter, J.; Coulter, I.; Goertz, C.M. Effects of Chiropractic Care on Strength, Balance, and Endurance in Active-Duty U.S. Military Personnel with Low Back Pain: A Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 592–601. [Google Scholar] [CrossRef]
- Scafoglieri, A.; Broeck, J.V.D.; Willems, S.; Tamminga, R.; van der Hoeven, H.; Engelsma, Y.; Haverkamp, S. Effectiveness of local exercise therapy versus spinal manual therapy in patients with patellofemoral pain syndrome: Medium term follow-up results of a randomized controlled trial. BMC Musculoskelet. Disord. 2021, 22, 446. [Google Scholar] [CrossRef]
- Hedlund, S.; Nilsson, H.; Lenz, M.; Sundberg, T. Effect of chiropractic manipulation on vertical jump height in young female athletes with talocrural joint dysfunction: A single-blind randomized clinical pilot trial. J. Manip. Physiol. Ther. 2014, 37, 116–123. [Google Scholar] [CrossRef]
- Haavik, H.; Özyurt, M.G.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Yilmaz, G.; Türker, K.S. Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals. Brain Sci. 2018, 8, 76. [Google Scholar] [CrossRef]
- Sanders, G.D.; Nitz, A.J.; Abel, M.G.; Symons, T.B.; Shapiro, R.; Black, W.S.; Yates, J.W. Effects of Lumbosacral Manipulation on Isokinetic Strength of the Knee Extensors and Flexors in Healthy Subjects: A Randomized, Controlled, Single-Blind Crossover Trial. J. Chiropr. Med. 2015, 14, 240–248. [Google Scholar] [CrossRef][Green Version]
- van der Kolk, H.K.; Scafoglieri, A. Throwing performance after high-velocity low-amplitude thrust manipulation at the cervi-cothoracic and thoracolumbar junction in elite female water polo players: A randomized blind cross-over study. J. Sports Med. Phys. Fit. 2021, 61, 885–891. [Google Scholar]
- Grindstaff, T.L.; Hertel, J.; Beazell, J.R.; Magrum, E.M.; Kerrigan, D.C.; Fan, X.; Ingersoll, C.D. Lumbopelvic Joint Manipulation and Quadriceps Activation of People with Patellofemoral Pain Syndrome. J. Athl. Train. 2012, 47, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ogliari, P.; Debiazi, P.; Pacini, V.C.; Picanço, V.V.; de Carvalho, A.R.; Bertolini, G.R.F. Analysis of the influence of the spinal manipulation in hand strength and pain threshold. Man. Ther. Posturol. Rehabil. J. 2011, 9, 278–283. [Google Scholar]
- Bautista-Aguirre, F.; Oliva-Pascual-Vaca, A.; Heredia-Rizo, A.M.; Boscá-Gandía, J.J.; Ricard, F.; Rodriguez-Blanco, C. Effect of cervical vs. thoracic spinal manipulation on peripheral neural features and grip strength in subjects with chronic mechanical neck pain: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 333–341. [Google Scholar] [CrossRef]
- Fritz, J.M.; Koppenhaver, S.L.; Kawchuk, G.N.; Teyhen, D.S.; Hebert, J.J.; Childs, J.D. Preliminary investigation of the mechanisms underlying the effects of manipulation: Exploration of a multivariate model including spinal stiffness, multifidus recruitment, and clinical findings. Spine 2011, 36, 1772–1781. [Google Scholar] [CrossRef]
- Raney, N.H.; Teyhen, D.S.; Childs, J.D. Observed changes in lateral abdominal muscle thickness after spinal manipulation: A case series using rehabilitative ultrasound imaging. J. Orthop. Sports Phys. Ther. 2007, 37, 472–479. [Google Scholar] [CrossRef]
- Fosberg, K.K.; Puentedura, E.; Schmitz, B.; Jain, T.K.; Cleland, J.A. The Effects of Thrust Joint Manipulation on the Resting and Contraction Thickness of Transversus Abdominis in Patients With Low Back Pain: A Randomized Control Trial. J. Manip. Physiol. Ther. 2020, 43, 339–355. [Google Scholar] [CrossRef]
- Puentedura, E.J.; Landers, M.R.; Hurt, K.; Meissner, M.; Mills, J.; Young, D. Immediate effects of lumbar spine manipulation on the resting and contraction thickness of transversus abdominis in asymptomatic individuals. J. Orthop. Sports Phys. Ther. 2011, 41, 13–21. [Google Scholar] [CrossRef]
- Konitzer, L.N.; Gill, N.W.; Koppenhaver, S.L. Investigation of abdominal muscle thickness changes after spinal manipulation in patients who meet a clinical prediction rule for lumbar stabilization. J. Orthop. Sports Phys. Ther. 2011, 41, 666–674. [Google Scholar] [CrossRef]
- Racinais, S.; Maffiuletti, N.A.; Girard, O. M-wave, H- and V-reflex recruitment curves during maximal voluntary contraction. J. Clin. Neurophysiol. 2013, 30, 415–421. [Google Scholar] [CrossRef]
- Tucker, K.J.; Tuncer, M.; Türker, K.S. A review of the H-reflex and M-wave in the human triceps surae. Hum. Mov. Sci. 2005, 24, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Vila-Chã, C.; Falla, D.; Correia, M.V.; Farina, D. Changes in H reflex and V wave following short-term endurance and strength training. J. Appl. Physiol. 2012, 112, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. Altered sensorimotor integration with cervical spine manipulation. J. Manip. Physiol. Ther. 2008, 31, 115–126. [Google Scholar] [CrossRef]
- Dishman, J.D.; Weber, K.A., 2nd; Corbin, R.L.; Burke, J.R. Understanding inhibitory mechanisms of lumbar spinal manipulation using H-reflex and F-wave responses: A methodological approach. J. Neurosci. Methods 2012, 210, 169–177. [Google Scholar] [CrossRef]
- Dishman, J.D.; Bulbulian, R. Comparison of effects of spinal manipulation and massage on motoneuron excitability. Electromyogr. Clin. Neurophysiol. 2001, 41, 97–106. [Google Scholar] [PubMed]
- Dishman, J.D.; Burke, J. Spinal reflex excitability changes after cervical and lumbar spinal manipulation: A comparative study. Spine J. 2003, 3, 204–212. [Google Scholar] [CrossRef]
- Fryer, G.; Pearce, A.J. The effect of lumbosacral manipulation on corticospinal and spinal reflex excitability on asymptomatic participants. J. Manip. Physiol. Ther. 2012, 35, 86–93. [Google Scholar] [CrossRef]
- Orakifar, N.; Kamali, F.; Pirouzi, S.; Jamshidi, F. Sacroiliac joint manipulation attenuates alpha-motoneuron activity in healthy women: A quasi-experimental study. Arch. Phys. Med. Rehabil. 2012, 93, 56–61. [Google Scholar] [CrossRef]
- Dishman, J.D.; Burke, J.R.; Dougherty, P. Motor Neuron Excitability Attenuation as a Sequel to Lumbosacral Manipulation in Subacute Low Back Pain Patients and Asymptomatic Adults: A Cross-Sectional H-Reflex Study. J. Manip. Physiol. Ther. 2018, 41, 363–371. [Google Scholar] [CrossRef]
- Suter, E.; McMorland, G.; Herzog, W. Short-term effects of spinal manipulation on H-reflex amplitude in healthy and symptomatic subjects. J. Manip. Physiol. Ther. 2005, 28, 667–672. [Google Scholar] [CrossRef]
- Dishman, J.D.; Bulbulian, R. Spinal reflex attenuation associated with spinal manipulation. Spine 2000, 25, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Groisman, S.; Silva, L.; Rocha, N.; Hoff, F.; Rodrigues, M.E.; Ehlers, J.A.; Diniz, L.R. H-reflex responses to High-Velocity Low-Amplitude manipulation in asymptomatic adults. Int. J. Osteopat. Med. 2014, 17, 160–166. [Google Scholar] [CrossRef]
- Grindstaff, T.L.; Pietrosimone, B.G.; Sauer, L.D.; Kerrigan, D.C.; Patrie, J.T.; Hertel, J.; Ingersoll, C.D. Manual therapy directed at the knee or lumbopelvic region does not influence quadriceps spinal reflex excitability. Man. Ther. 2014, 19, 299–305. [Google Scholar] [CrossRef]
- Fragoso, A.; Martínez, B.; Ceballos-Villegas, M.E.; Herrera, E.; Saldaña, J.J.; Gutiérrez-Lozano, A.L.; Manjarrez, E.; Lomelí, J. Absence of Neuroplastic Changes in the Bilateral H-Reflex Amplitude following Spinal Manipulation with Activator IV. Medicina 2022, 58, 1521. [Google Scholar] [CrossRef]
- Cramer, G.D.; Humphreys, C.R.; A Hondras, M.; McGregor, M.; Triano, J.J. The Hmax/Mmax ratio as an outcome measure for acute low back pain. J. Manip. Physiol. Ther. 1993, 16, 7–13. [Google Scholar]
- Haig, A.J. Spinal manipulation can effect the response of the tibial nerve. Spine 2001, 26, 841–842. [Google Scholar] [CrossRef]
- Campbell, D.; Yielder, P.; Ambalavanar, U.; Haavik, H.; Murphy, B. The cervico-ocular reflex changes following treatment in individuals with subclinical neck pain: A randomized control trial. Exp. Brain Res. 2024, 242, 2531–2544. [Google Scholar] [CrossRef]
- Fisher, B.E.; Piraino, A.; Lee, Y.-Y.; Smith, J.A.; Johnson, S.; Davenport, T.E.; Kulig, K. The Effect of Velocity of Joint Mobilization on Corticospinal Excitability in Individuals With a History of Ankle Sprain. J. Orthop. Sports Phys. Ther. 2016, 46, 562–570. [Google Scholar] [CrossRef]
- Ponzo, V.; Cinnera, A.M.; Mommo, F.; Caltagirone, C.; Koch, G.; Tramontano, M. Osteopathic Manipulative Therapy Potentiates Motor Cortical Plasticity. J. Am. Osteopat. Assoc. 2018, 118, 396–402. [Google Scholar] [CrossRef]
- Dishman, J.D.; Greco, D.S.; Burke, J.R. Motor-evoked potentials recorded from lumbar erector spinae muscles: A study of corticospinal excitability changes associated with spinal manipulation. J. Manip. Physiol. Ther. 2008, 31, 258–270. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Transient modulation of intracortical inhibition following spinal manipulation. Chiropr. J. Aust. 2007, 37, 106–116. [Google Scholar]
- Dishman, J.; Ball, K.A.; Burke, J. First Prize: Central motor excitability changes after spinal manipulation: A transcranial magnetic stimulation study. J. Manip. Physiol. Ther. 2002, 25, 1–9. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Amjad, I.; Kumari, N.; Ghani, U.; Ashfaque, M.; Rashid, U.; Navid, M.S.; Kamavuako, E.N.; Pujari, A.N.; et al. Neuroplastic Responses to Chiropractic Care: Broad Impacts on Pain, Mood, Sleep, and Quality of Life. Brain Sci. 2024, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Niazi, I.K.; Jochumsen, M.; Uginčius, P.; Sebik, O.; Yılmaz, G.; Navid, M.S.; Özyurt, M.G.; Türker, K.S. Chiropractic spinal manipulation alters TMS induced I-wave excitability and shortens the cortical silent period. J. Electromyogr. Kinesiol. 2018, 42, 24–35. [Google Scholar] [CrossRef]
- Clark, B.C.; A Goss, D.; Walkowski, S.; Hoffman, R.L.; Ross, A.; Thomas, J.S. Neurophysiologic effects of spinal manipulation in patients with chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Baarbé, J.K.; Yielder, P.; Haavik, H.; Holmes, M.W.R.; Murphy, B.A. Subclinical recurrent neck pain and its treatment impacts motor training-induced plasticity of the cerebellum and motor cortex. PLoS ONE 2018, 13, e0193413. [Google Scholar] [CrossRef]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of Dysfunctional Spinal Joints Affects Sensorimotor Integration in the Prefrontal Cortex: A Brain Source Localization Study. Neural Plast. 2016, 2016, 3704964. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Holt, K.; Murphy, B. Effects of 12 Weeks of Chiropractic Care on Central Integration of Dual Somatosensory Input in Chronic Pain Patients: A Preliminary Study. J. Manip. Physiol. Ther. 2017, 40, 127–138. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B. The effects of spinal manipulation on central integration of dual somatosensory input observed after motor training: A crossover study. J. Manip. Physiol. Ther. 2010, 33, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. Altered central integration of dual somatosensory input after cervical spine manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.K.; Navid, M.S.; Merkle, C.; Amjad, I.; Kumari, N.; Trager, R.J.; Holt, K.; Haavik, H. A randomized controlled trial comparing different sites of high-velocity low amplitude thrust on sensorimotor integration parameters. Sci. Rep. 2024, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- Navid, M.S.; Niazi, I.K.; Holt, K.; Nedergaard, R.B.; Amjad, I.; Ghani, U.; Kumari, N.; Shafique, M.; Duehr, J.; Trager, R.J.; et al. The effects of chiropractic spinal adjustment on EEG in adults with Alzheimer’s and Parkinson’s disease: A pilot randomised cross-over trial. J. Integr. Neurosci. 2024, 23, 98. [Google Scholar] [CrossRef]
- Navid, M.S.; Niazi, I.K.; Lelic, D.; Nedergaard, R.B.; Holt, K.; Amjad, I.; Drewes, A.M.; Haavik, H. Investigating the Effects of Chiropractic Spinal Manipulation on EEG in Stroke Patients. Brain Sci. 2020, 10, 253. [Google Scholar] [CrossRef]
- Kaňovský, P.; Bareš, M.; Rektor, I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: Evidence from intracerebral recordings. Clin. Neurophysiol. 2003, 114, 981–991. [Google Scholar] [CrossRef]
- Cebolla, A.; Palmero-Soler, E.; Dan, B.; Cheron, G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. NeuroImage 2011, 54, 1297–1306. [Google Scholar] [CrossRef]
- Waterstone, T.S.; Niazi, I.K.; Navid, M.S.; Amjad, I.; Shafique, M.; Holt, K.; Haavik, H.; Samani, A. Functional connectivity analysis on resting-state electroencephalography signals following chiropractic spinal manipulation in stroke patients. Brain Sci. 2020, 10, 644. [Google Scholar] [CrossRef]
- Ziloochi, F.; Niazi, I.K.; Amjad, I.; Cade, A.; Duehr, J.; Ghani, U.; Holt, K.; Haavik, H.; Shalchyan, V. Investigating the effects of chiropractic care on resting-state EEG of MCI patients. Front. Aging Neurosci. 2024, 16, 1406664. [Google Scholar] [CrossRef]
- Sillevis, R.; Unum, J.; Weiss, V.; Shamus, E.; Selva-Sarzo, F. The effect of a spinal thrust manipulation’s audible pop on brain wave activity: A quasi-experimental repeated measure design. PeerJ 2024, 12, e17622. [Google Scholar] [CrossRef]
- Colloca, C.J.; Keller, T.S.; Gunzburg, R. Biomechanical and neurophysiological responses to spinal manipulation in patients with lumbar radiculopathy. J. Manip. Physiol. Ther. 2004, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- de Nooij, J.C.; Zampieri, N. The making of a proprioceptor: A tale of two identities. Trends Neurosci. 2023, 46, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.E.; Brockett, C.L.; Morgan, D.L.; Whitehead, N.P.; Proske, U. Effect of eccentric muscle contractions on Golgi tendon organ responses to passive and active tension in the cat. J. Physiol. 2002, 538, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, G.L.; Knutson, L.M. A Guide for use and interpretation of kinesiologic electromyographic data. Phys. Ther. 2000, 80, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.D.; Snodgrass, S.J. The effects of thoracic manipulation on posteroanterior spinal stiffness. J. Orthop. Sports Phys. Ther. 2010, 40, 685–693. [Google Scholar] [CrossRef]
- Colloca, C.J.; Gunzburg, R.; Freeman, B.J.; Szpalski, M.; Afifi, M.; Moore, R.J. Biomechancial quantification of pathologic manipulable spinal lesions: An in vivo ovine model of spondylolysis and intervertebral disc degeneration. J. Manip. Physiol. Ther. 2012, 35, 354–366. [Google Scholar] [CrossRef]
- Lapole, T.; Pérot, C. Hoffmann reflex is increased after 14 days of daily repeated Achilles tendon vibration for the soleus but not for the gastrocnemii muscles. Appl. Physiol. Nutr. Metab. 2012, 37, 14–20. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Cheron, G. Central somatosensory conduction in man: Neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 382–403. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Aminoff, M.; Desmedt, J.; Eisen, A.A.; Goodin, D.; Matsuoka, S.; Mauguière, F.; Shibasaki, H.; Sutherling, W.; Vibert, J.-F. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 6–11. [Google Scholar] [CrossRef]
- Inghilleri, M.; Berardelli, A.; Cruccu, G.; Manfredi, M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J. Physiol. 1993, 466, 521–534. [Google Scholar] [CrossRef]
- Werhahn, K.J.; Kunesch, E.; Noachtar, S.; Benecke, R.; Classen, J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999, 517 Pt 2, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.-L.; Amorim, M.-A.; Schubert, M.; Schweinhardt, P.; Leboeuf-Yde, C. Unravelling functional neurology: Does spinal manipulation have an effect on the brain?—A systematic literature review. Chiropr. Man. Ther. 2019, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Pickar, J.; Bolton, P. Spinal manipulative therapy and somatosensory activation. J. Electromyogr. Kinesiol. 2012, 22, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Simon, C.B.; Bishop, M.D.; George, S.Z. Basis for spinal manipulative therapy: A physical therapist perspective. J. Electromyogr. Kinesiol. 2012, 22, 643–647. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Gillette, R.G. A speculative argument for the coactivation of diverse somatic receptor populations by forceful chiropractic adjustments. Manual Med. 1987, 3, 1–14. [Google Scholar]
- Lima, C.R.; Sozio, R.S.; Law, A.C.; Nelson, A.J.; Singh, H.; Hurt, C.P.; Li, P.; Reed, W.R. Effects of Thrust Magnitude and Duration on Immediate Postspinal Manipulation Trunk Muscle Spindle Responses. J. Manip. Physiol. Ther. 2021, 44, 363–371. [Google Scholar] [CrossRef]
- Lima, C.R.; Martins, D.F.; Avatapally, S.; Cho, M.; Li, P.; Reed, W.R. Influence of Intervertebral Fixation and Segmental Thrust Level on Immediate Post-Spinal Manipulation Trunk Muscle Spindle Response in an Animal Model. Brain Sci. 2021, 11, 1022. [Google Scholar] [CrossRef]
- Nim, C.G.; Downie, A.; O’neill, S.; Kawchuk, G.N.; Perle, S.M.; Leboeuf-Yde, C. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci. Rep. 2021, 11, 23415. [Google Scholar] [CrossRef]
- Keter, D.L.; Bent, J.A.; Bialosky, J.E.; Courtney, C.A.; Esteves, J.E.; Funabashi, M.; Howarth, S.J.; Injeyan, H.S.; Mazzieri, A.M.; Nim, C.G.; et al. An international consensus on gaps in mechanisms of forced-based manipulation research: Findings from a nominal group technique. J. Man. Manip. Ther. 2024, 32, 111–117. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Nim, C.; Hartvigsen, J.; E Cook, C.; Skillgate, E.; Vogel, S.; Hohenschurz-Schmidt, D.; Underwood, M.; Rubinstein, S.M. Waste not, want not: Call to action for spinal manipulative therapy researchers. Chiropr. Man. Ther. 2024, 32, 16. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Barocas, V.H.; Winkelstein, B.A.; Cook, C.E. Uniting disciplines for a modern take: Exploring the science behind manual therapies. J. Man. Manip. Ther. 2024, 32, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, B.; van Dun, P.L.; Jacobson, E.; Fritz, S.; Mettler, P.; Kettner, N.; Franklin, G.; Hensel, K.; Lesondak, D.; Consorti, G.; et al. Profession-based manual therapy nomenclature: Exploring history, limitations, and opportunities. J. Man. Manip. Ther. 2024, 32, 96–110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, M.S.; Degenhardt, B.; Kelley-Franklin, G.; Cox, J.M.; Lipke, L.; Reed, W.R. Neuromuscular Response to High-Velocity, Low-Amplitude Spinal Manipulation—An Overview. Medicina 2025, 61, 187. https://doi.org/10.3390/medicina61020187
Alanazi MS, Degenhardt B, Kelley-Franklin G, Cox JM, Lipke L, Reed WR. Neuromuscular Response to High-Velocity, Low-Amplitude Spinal Manipulation—An Overview. Medicina. 2025; 61(2):187. https://doi.org/10.3390/medicina61020187
Chicago/Turabian StyleAlanazi, Murdi S., Brian Degenhardt, Gwyn Kelley-Franklin, James M. Cox, Laura Lipke, and William R. Reed. 2025. "Neuromuscular Response to High-Velocity, Low-Amplitude Spinal Manipulation—An Overview" Medicina 61, no. 2: 187. https://doi.org/10.3390/medicina61020187
APA StyleAlanazi, M. S., Degenhardt, B., Kelley-Franklin, G., Cox, J. M., Lipke, L., & Reed, W. R. (2025). Neuromuscular Response to High-Velocity, Low-Amplitude Spinal Manipulation—An Overview. Medicina, 61(2), 187. https://doi.org/10.3390/medicina61020187





