Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
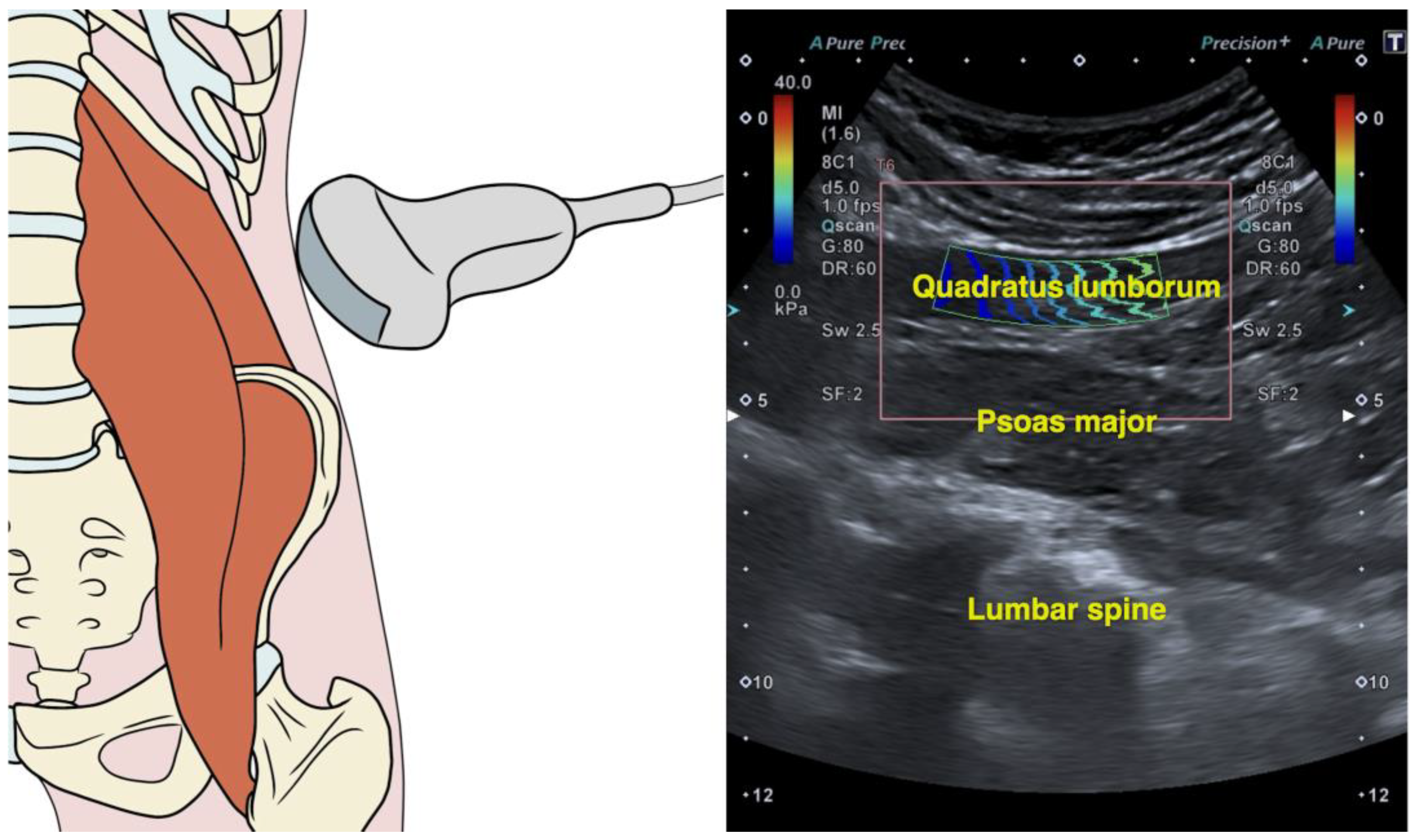
2.4. Quadratus Lumborum Muscle Stiffness
2.5. Clinical Severity Indicators
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Practical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSI | Central Sensitization Inventory |
| LBP | Low back pain |
| MTrP | Myofascial Trigger Point |
| ODI | Oswestry Disability Index |
| QL | Quadratus lumborum |
| STROBE | Strengthening the reporting of observational studies in epidemiology |
| SWE | Shear wave elastography |
| VAS | Visual Analogue Scale |
References
- Delp, S.L.; Suryanarayanan, S.; Murray, W.M.; Uhlir, J.; Triolo, R.J. Architecture of the Rectus Abdominis, Quadratus Lumborum, and Erector Spinae. J. Biomech. 2001, 34, 371–375. [Google Scholar] [CrossRef]
- Phillips, S.; Mercer, S.; Bogduk, N. Anatomy and Biomechanics of Quadratus Lumborum. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 151–159. [Google Scholar] [CrossRef]
- Park, R.J.; Tsao, H.; Claus, A.; Cresswell, A.G.; Hodges, P.W. Recruitment of Discrete Regions of the Psoas Major and Quadratus Lumborum Muscles Is Changed in Specific Sitting Postures in Individuals with Recurrent Low Back Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Park, R.J.; Tsao, H.; Cresswell, A.G.; Hodges, P.W. Changes in Direction-Specific Activity of Psoas Major and Quadratus Lumborum in People with Recurring Back Pain Differ between Muscle Regions and Patient Groups. J. Electromyogr. Kinesiol. 2013, 23, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Ranger, T.A.; Cicuttini, F.M.; Jensen, T.S.; Peiris, W.L.; Hussain, S.M.; Fairley, J.; Urquhart, D.M. Are the Size and Composition of the Paraspinal Muscles Associated with Low Back Pain? A Systematic Review. Spine J. 2017, 17, 1729–1748. [Google Scholar] [CrossRef]
- Kamaz, M.; Kireşi, D.; Oğuz, H.; Emlik, D.; Levendoğlu, F. CT Measurement of Trunk Muscle Areas in Patients with Chronic Low Back Pain. Diagn. Interv. Radiol. 2007, 13, 144–148. [Google Scholar] [PubMed]
- Sions, J.M.; Elliott, J.M.; Pohlig, R.T.; Hicks, G.E. Trunk Muscle Characteristics of the Multifidi, Erector Spinae, Psoas, and Quadratus Lumborum in Older Adults with and Without Chronic Low Back Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 173–179. [Google Scholar] [CrossRef]
- de Franca, G.G.; Levine, L.J. The Quadratus Lumborum and Low Back Pain. J. Manip. Physiol. Ther. 1991, 14, 142–149. [Google Scholar]
- Iglesias-González, J.J.; Muñoz-García, M.T.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F.; Fernández-de-Las-Peñas, C. Myofascial Trigger Points, Pain, Disability, and Sleep Quality in Patients with Chronic Nonspecific Low Back Pain. Pain Med. 2013, 14, 1964–1970. [Google Scholar] [CrossRef]
- Holm-Jensen, A.; Kjaer, P.; Schiøttz-Christensen, B.; Ziegler, D.S.; Andersen, S.; Myburgh, C. The Interexaminer Reproducibility and Prevalence of Lumbar and Gluteal Myofascial Trigger Points in Patients with Radiating Low Back Pain. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Hua, N.K.; Van der Does, E. The Occurrence and Inter-Rater Reliability of Myofascial Trigger Points in the Quadratus Lumborum and Gluteus Medius: A Prospective Study in Non-Specific Low Back Pain Patients and Controls in General Practice. Pain 1994, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sirh, S.-J.; Sirh, S.-W.; Mun, H.-Y.; Sirh, H.-M. Importance of Quadratus Lumborum Muscle Trigger Point Injection and Prolotherapy Technique for Lower Back and Buttock Pain. Front. Pain Res. 2022, 3, 997645. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-de-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef]
- Winn, N.; Lalam, R.; Cassar-Pullicino, V. Sonoelastography in the Musculoskeletal System: Current Role and Future Directions. World J. Radiol. 2016, 8, 868–879. [Google Scholar] [CrossRef]
- Zhou, E.F.M.; Wong, A.Y.L.; Zheng, Y.P.; Lam, K.H.S.; Fu, S.N. Reliability of Ultrasound Shear Wave Elastography for Evaluating Psoas Major and Quadratus Lumborum Stiffness: Gender and Physical Activity Effects. Ultrasound Med. Biol. 2024, 50, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The Strobe Guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M. EQUATOR Reporting Guidelines Should Also Be Used by Clinicians. J. Clin. Epidemiol. 2020, 117, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, C.L.; Sikorskii, A.; Epstein, J. Revisiting Chronic Low Back Pain: Evidence That It Is Not Non-Specific. J. Osteopath. Med. 2022, 123, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Tonosu, J.; Takeshita, K.; Hara, N.; Matsudaira, K.; Kato, S.; Masuda, K.; Chikuda, H. The Normative Score and the Cut-off Value of the Oswestry Disability Index (ODI). Eur. Spine J. 2012, 21, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-Specific Low Back Pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. A Primer of Multivariate Statistics. In A Primer of Multivariate Statistics; Psychology Press: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Wilson Vanvoorhis, C.R.; Morgan, B.L. Understanding Power and Rules of Thumb for Determining Sample Sizes. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Beneciuk, J.M.; Bishop, M.D.; George, S.Z. Clinical Prediction Rules for Physical Therapy Interventions: A Systematic Review. Phys. Ther. 2009, 89, 114–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latash, M.L. Muscle Coactivation: Definitions, Mechanisms, and Functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and Validity of the Visual Analogue Scale for Disability in Patients with Chronic Musculoskeletal Pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R.J. Cross-Cultural Adaptation and Validity of the Spanish Central Sensitization Inventory. Springerplus 2016, 5, 1837. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretation of SF-36 and SF-12 Questionnaires in Spain: Physical and Mental Components. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 6: Correlation and Linear Regression. Indian J. Dermatol. 2016, 61, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.E. Multiple Linear Regression. Methods Mol. Biol. 2007, 404, 165–187. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Asadi, M.; Dommerholt, J.; Yeganeh, A. Changes in the Macroscopic Morphology of Hip Muscles in Low Back Pain. J. Anat. 2020, 236, 3–20. [Google Scholar] [CrossRef]
- Aboufazeli, M.; Akbari, M.; Jamshidi, A.A.; Jafarpisheh, M.S. Comparison of Selective Local and Global Muscle Thicknesses in Females with and without Chronic Low Back Pain. Ortop. Traumatol. Rehabil. 2018, 20, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.E.; Hides, J.A.; Hodges, P.W. Size and Symmetry of Trunk Muscles in Ballet Dancers with and without Low Back Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Lin, C.; Li, X.; Zeng, W.; Ma, C. MRI Assessment of Paraspinal Muscles in Patients with Acute and Chronic Unilateral Low Back Pain. Br. J. Radiol. 2015, 88, 20140546. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.A.; Grundström, H.; Thorstensson, A. Diverging Intramuscular Activity Patterns in Back and Abdominal Muscles during Trunk Rotation. Spine 2002, 27, E152–E160. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Hinrichs, R.N. The Biomechanical and Clinical Significance of the Lumbar Erector Spinae Flexion-Relaxation Phenomenon: A Review of Literature. J. Manip. Physiol. Ther. 2005, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.W.; Peng, B.G.; Wang, L.; Huang, Y.Q.; Jia, D.L.; Jiang, H.; Lv, Y.; Liu, X.G.; Liu, R.G.; Li, Y.; et al. Expert Consensus on the Diagnosis and Treatment of Myofascial Pain Syndrome. World J. Clin. Cases 2021, 9, 2077–2089. [Google Scholar] [CrossRef]
- Lucas, N.; MacAskill, P.; Irwig, L.; Moran, R.; Bogduk, N. Reliability of Physical Examination for Diagnosis of Myofascial Trigger Points: A Systematic Review of the Literature. Clin. J. Pain 2009, 25, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, A.V.; Yavuz, U.Ş.; Petzke, F.; Nordez, A.; Falla, D. Neck Muscle Stiffness Measured with Shear Wave Elastography in Women with Chronic Nonspecific Neck Pain. J. Orthop. Sports Phys. Ther. 2020, 50, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Koppenhaver, S.L.; Timo-Dondoyano, R.M.; Baumann, K.; Scheirer, V.F.; Wolff, A.; Sutlive, T.G.; Elliott, J.M. Ultrasound Shear Wave Elastography Measurement of the Deep Posterior Cervical Muscles: Reliability and Ability to Differentiate between Muscle Contraction States. J. Electromyogr. Kinesiol. 2021, 6, 102488. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Jorge, S.; Buffet-García, J.; Varol, U.; Gallego-Sendarrubias, G.M.; Álvarez-González, J. Is Shear-Wave Elastography a Clinical Severity Indicator of Myofascial Pain Syndrome? An Observational Study. J. Clin. Med. 2021, 10, 2895. [Google Scholar] [CrossRef]
- Xie, Y.; Thomas, L.; Johnston, V.; Coombes, B.K. Cervical and Axioscapular Muscle Stiffness Measured with Shear Wave Elastography: A Comparison between Different Levels of Work-Related Neck Disability. J. Electromyogr. Kinesiol. 2023, 69, 102754. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Sánchez-Jorge, S.; Buffet-García, J.; Varol, U.; Fernández-de-Las-Peñas, C.; Álvarez-González, J. Changes in Stiffness at Active Myofascial Trigger Points of the Upper Trapezius after Dry Needling in Patients with Chronic Neck Pain: A Randomized Controlled Trial. Acupunct. Med. J. Br. Med. Acupunct. Soc. 2023, 41, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, A.; Maffulli, N.; Schäfer, L.; Manocchio, N.; Bossa, M.; Foti, C.; Klimuch, A.; Migliorini, F. Physiotherapeutic and Non-Conventional Approaches in Patients with Chronic Low-Back Pain: A Level I Bayesian Network Meta-Analysis. Sci. Rep. 2024, 14, 11546. [Google Scholar] [CrossRef]
| Variables | Subjects with Low Back Pain (n = 76) | Difference (95% Confidence Interval) | |
|---|---|---|---|
| Females (n = 37) | Males (n = 39) | ||
| Demographics | |||
| Age, years | 30.8 ± 13.7 | 26.1 ± 8.6 | 4.6 (−2.9; 12.1) p = 0.221 |
| Weight, kg | 66.6 ± 15.0 | 81.9 ± 12.6 | −15.3 (−24.0; −6.6) p < 0.001 |
| Height, m | 1.65 ± 0.04 | 1.76 ± 0.08 | −0.11 (−0.15; −0.08) p < 0.001 |
| BMI, kg/m2 | 24.8 ± 5.7 | 26.5 ± 2.7 | −1.6 (−4.6; 1.4) p = 0.287 |
| Clinical Characteristics | |||
| Recurrence (episodes last year, n) | 12.1 ± 1.4 | 9.6 ± 1.3 | 2.5 (1.89; 3.11) p < 0.001 |
| Chronicity (months, n) | 65.8 ± 50.0 | 58.9 ± 46.3 | 6.9 (−14.7; 28.5) p = 0.525 |
| Pain Intensity (VAS, 0–10) | 4.9 ± 1.5 | 4.7 ± 1.4 | 0.2 (−0.4; 0.8) p = 0.522 |
| Related Disability (ODI, 0–100) | 13.4 ± 7.1 | 9.0 ± 4.9 | 4.4 (1.6; 7.3) p = 0.003 |
| Central Sensitization (CSI, 0–100) | 42.6 ± 13.3 | 29.3 ± 12.6 | 13.3 (7.5; 19.1) p < 0.001 |
| Physical Quality of Life (SF-12, 0–100) | 45.4 ± 10.0 | 47.0 ± 8.9 | −1.60 (−5.9; 2.7) p = 0.460 |
| Mental Quality of Life (SF-12, 0–100) | 42.1 ± 10.3 | 46.3 ± 11.3 | −4.2 (−8.9; 0.5) p = 0.081 |
| Quadratus Lumborum Stiffness (SWS, m/s) | 2.41 ± 0.65 | 2.47 ± 0.54 | −0.06 (−0.33; 0.21) p = 0.669 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Duration (months) | |||||||
| 2. Recurrence (episodes) | n.s. | ||||||
| 3. Pain Intensity (VAS) | −0.372 ** | 0.231 * | |||||
| 4. Disability (ODI) | n.s. | 0.290 * | n.s. | ||||
| 5. Central Sensitization (CSI) | n.s. | 0.483 ** | 0.576 ** | 0.283 * | |||
| 6. Physical Quality of Life (SF-12) | n.s. | n.s. | −0.650 ** | −0.570 ** | −0.536 ** | ||
| 7. Psychological Quality of Life (SF-12) | n.s. | n.s. | n.s. | n.s. | −0.470 ** | n.s. | |
| 8. Muscle Stiffness (SWS) | n.s. | −0.260 * | −0.507 ** | n.s. | −0.441 ** | 0.403 ** | n.s. |
| Predictor Outcome | R2 Adj | B | SE B | 95% CI | β | t | P | |
|---|---|---|---|---|---|---|---|---|
| Pain Intensity | Step 1 Physical Quality of Life | 0.326 | −0.085 | 0.013 | −0.111, −0.059 | −0.578 | −6.486 | <0.001 |
| Step 2 Physical Quality of Life CSI | 0.399 | −0.063 0.031 | 0.014 0.009 | −0.091, −0.036 0.013, 0.049 | −0.431 0.319 | −4.545 3.362 | <0.001 0.001 | |
| Step 3 Physical Quality of Life CSI QL Stiffness | 0.439 | −0.053 0.027 −0.542 | 0.014 0.009 0.206 | −0.081, −0.025 0.009, 0.045 −0.952, −0.131 | −0.361 0.28 −0.233 | −3.785 3.026 −2.625 | <0.001 0.003 0.010 | |
| Step 4 Physical Quality of Life CSI QL Stiffness Chronicity | 0.491 | −0.053 0.029 −0.381 −0.007 | 0.013 0.009 0.204 0.002 | −0.080, −0.027 0.012, 0.046 −0.786, 0.024 −0.012, −0.002 | −0.363 0.296 −0.164 −0.245 | −3.994 3.345 −1.872 −3.044 | <0.001 0.001 0.048 0.003 | |
| Central Sensitization Inventory | Step 1 Pain Intensity | 0.259 | 5.299 | 0.957 | 3.397, 7.201 | 0.517 | 5.539 | <0.001 |
| Step 2 Pain Intensity Physical Quality of Life | 0.291 | 3.855 −0.368 | 1.147 0.169 | 1.574, 6.135 −0.704, −0.032 | 0.376 −0.244 | 3.362 −2.181 | 0.001 0.032 | |
| Step 3 Pain Intensity Physical Quality of Life Recurrence | 0.342 | 3.000 −0.433 0.027 | 1.147 0.164 0.010 | 0.717, 5.282 −0.760, −0.106 0.007, 0.047 | 0.293 −0.287 0.250 | 2.614 −2.635 2.738 | 0.011 0.010 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Redondo, M.; Vicente-Campos, D.; Álvarez-González, J.; Roldán-Ruiz, A.; Sánchez-Jorge, S.; Buffet-García, J.; Rabanal-Rodríguez, G.; Valera-Calero, J.A. Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study. Medicina 2025, 61, 270. https://doi.org/10.3390/medicina61020270
López-Redondo M, Vicente-Campos D, Álvarez-González J, Roldán-Ruiz A, Sánchez-Jorge S, Buffet-García J, Rabanal-Rodríguez G, Valera-Calero JA. Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study. Medicina. 2025; 61(2):270. https://doi.org/10.3390/medicina61020270
Chicago/Turabian StyleLópez-Redondo, Mónica, Davinia Vicente-Campos, Javier Álvarez-González, Alberto Roldán-Ruiz, Sandra Sánchez-Jorge, Jorge Buffet-García, Gabriel Rabanal-Rodríguez, and Juan Antonio Valera-Calero. 2025. "Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study" Medicina 61, no. 2: 270. https://doi.org/10.3390/medicina61020270
APA StyleLópez-Redondo, M., Vicente-Campos, D., Álvarez-González, J., Roldán-Ruiz, A., Sánchez-Jorge, S., Buffet-García, J., Rabanal-Rodríguez, G., & Valera-Calero, J. A. (2025). Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study. Medicina, 61(2), 270. https://doi.org/10.3390/medicina61020270







