The Role of microRNA in Overactive Bladder: Relationship and Clinical Correlation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Healthy Group Selection
2.2. RNA Extraction
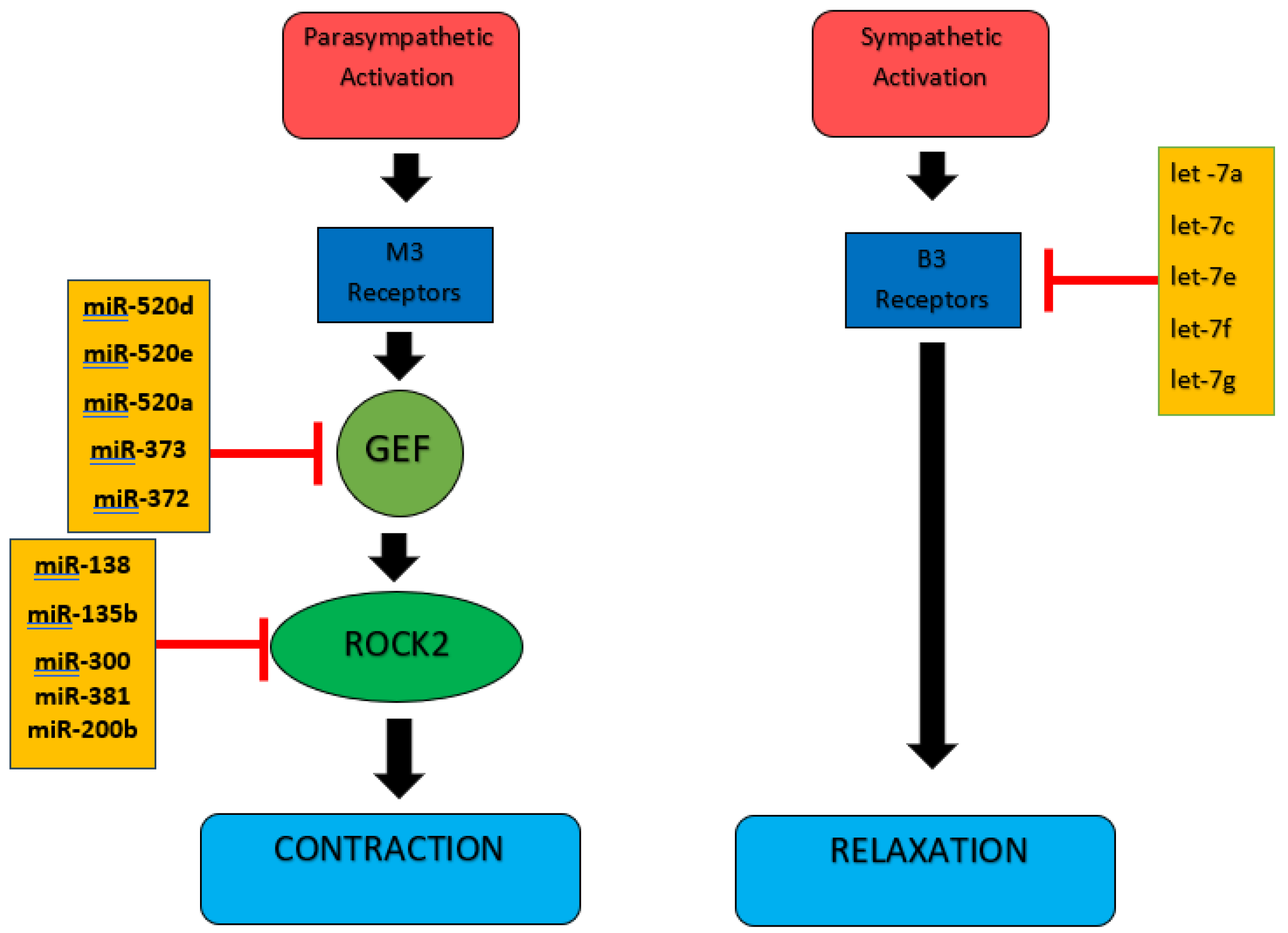
2.3. Selection of miRNAs
2.4. Measurements of miRNA Expression Levels
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAB | Overactive bladder |
| ICS | International Continence Society |
| ROK/ROCK | Rho-related kinase |
| GEFs | Guanine nucleotide exchange factors |
| qPCR | Quantitative polymerase chain reaction |
| ROC | Receiver operator characteristic |
| AUC | Area under curve |
References
- Irwin, D.E.; Kopp, Z.S.; Agatep, B.; Milsom, I.; Abrams, P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011, 108, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Zumrutbas, A.E.; Bozkurt, A.I.; Tas, E.; Acar, C.I.; Alkis, O.; Coban, K.; Cetinel, B.; Aybek, Z. Prevalence of lower urinary tract symptoms, overactive bladder and urinary incontinence in western Turkey: Results of a population-based survey. Int. J. Urol. 2014, 21, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Mostwin, J.L. Pathophysiology: The varieties of bladder overactivity. Urology 2002, 60 (Suppl. 1), 22–26, discussion 27. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Chancellor, M.B. Neurophysiology of lower urinary tract function and dysfunction. Rev. Urol. 2003, 5 (Suppl. 8), S3–S10. [Google Scholar]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Ferreira, C.E.; Fonseca, A.M.; Silva, I.D.; Girão, M.J.; Sartori, M.G.; Castro, R.A. The relationship between the Trp 64 Arg polymorphism of the beta 3-adrenoceptor gene and idiopathic overactive bladder. Am. J. Obstet. Gynecol. 2011, 205, e10–e84. [Google Scholar] [CrossRef]
- Atayi, M.; Mahdavi, N.; Salehi-Pourmehr, H.; Pashazadeh, F.; Kouchakali, G.; Mirzaei, Z.; Barati, T.; Abed, S.; Fattahi, F.; Hajebrahimi, S. The MicroRNAs (miRNAs) Expression in Benign Urological Diseases: A Systematic Review. Urol. J. 2024, 21, 293–306. [Google Scholar] [CrossRef]
- Phelps, C.; Chess-Williams, R.; Moro, C. The role of intracellular calcium and Rho kinase pathways in G protein-coupled receptor-mediated contractions of urinary bladder urothelium and lamina propria. Am. J. Physiol. Cell Physiol. 2023, 324, C787–C797. [Google Scholar] [CrossRef]
- Tarcan, T.; Mangır, N.; Özgür, M.Ö.; Akbal, C. OAB-V8 Aşırı aktif mesane sorgulama formu validasyon çalışması. Urol. Bülteni 2012, 21, 113–116. [Google Scholar]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Chuang, F.C.; Hsiao, S.M.; Kuo, H.C. The Overactive Bladder Symptom Score, International Prostate Symptom Score-Storage Subscore, and Urgency Severity Score in Patients With Overactive Bladder and Hypersensitive Bladder: Which Scoring System is Best? Int. Neurourol. J. 2018, 22, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Arribillaga, L.C.; Ledesma, M.; Montedoro, A.; Pisano, F.; Bengió, R.G. OAB score: A clinical model that predicts the probability of presenting overactive detrusor in the urodynamic study. Int. Braz. J. Urol. 2018, 44, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Igaz, P. Circulating Micrornas in Disease Diagnostics and Their Potential Biological Relevance; Springer: Basel, Switzerland, 2015; Volume 106. [Google Scholar]
- Zhang, S.; Lv, J.W.; Yang, P.; Yu, Q.; Pang, J.; Wang, Z.; Guo, H.; Liu, S.; Hu, J.; Li, J.; et al. Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am. J. Pathol. 2012, 181, 937–946. [Google Scholar] [CrossRef]
- Kashyap, M.; Pore, S.; Chancellor, M.; Yoshimura, N.; Tyagi, P. Bladder overactivity involves overexpression of MicroRNA 132 and nerve growth factor. Life Sci. 2016, 167, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Chermansky, C.J.; Kadow, B.T.; Kashyap, M.; Tyagi, P. MicroRNAs as potential biomarkers to predict the risk of urinary retention following intradetrusor onabotulinumtoxin-A injection. Neurourol. Urodyn. 2018, 37, 99–105. [Google Scholar] [CrossRef]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef]
- Imamura, M.; Sugino, Y.; Long, X.; Slivano, O.J.; Nishikawa, N.; Yoshimura, N.; Miano, J.M. Myocardin and microRNA-1 modulate bladder activity through connexin 43 expression during post-natal development. J. Cell. Physiol. 2013, 228, 1819–1826. [Google Scholar] [CrossRef]
- Fırat, E.; Aybek, Z.; Akgün, Ş.; Küçüker, K.; Akça, H.; Aybek, H. Exploring biomarkers in the overactive bladder: Alterations in miRNA levels of a panel of genes in patients with OAB. Neurourol. Urodyn. 2019, 38, 1571–1578. [Google Scholar] [CrossRef]
- Homma, Y.; Kakizaki, H.; Yamaguchi, O.; Yamanishi, T.; Nishizawa, O.; Yokoyama, O.; Takeda, M.; Seki, N.; Yoshida, M. Assessment of overactive bladder symptoms: Comparison of 3-day bladder diary and the overactive bladder symptoms score. Urology 2011, 77, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Lopes, T.; Carvalho-Barros, S.; Cruz, C.D.; Cruz, F.; Martins-Silva, C. Biomarkers in overactive bladder: A new objective and noninvasive tool? Adv. Urol. 2011, 2011, 382431. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, M.; Silva, I.; Oliveira, O.; Ferreira, S.; Reis, M.J.; Oliveira, J.C.; Correia-de-Sá, P. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS ONE 2013, 8, e64696. [Google Scholar] [CrossRef] [PubMed]
| Patient Group (n = 60) | Control Group (n = 60) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | p | |
| Height (cm) | 156.67 ± 6.26 | 155.5 (139–170) | 158.78 ± 6.74 | 160 (140–172) | 0.079 |
| Weight (kg) | 73.97 ± 16.4 | 73.5 (40–125) | 72.02 ± 13.01 | 70 (50–112) | 0.472 |
| BMI (kg/m2) | 30.04 ± 6.17 | 30.28 (17.78–45.91) | 28.58 ± 5.05 | 28.11 (19.2–43.75) | 0.101 |
| Age (years) | 56.48 ± 15.08 | 60 (23–83) | 55.82 ± 11.83 | 58.5 (27–76) | 0.567 |
| Urinary İncontinence | No Urinary İncontinence | p | |
|---|---|---|---|
| Before treatment | 54 | 6 | 0.0001 * |
| After treatment | 36 | 24 |
| Patient Group (n = 60) | Control Group (n = 60) | p | |
|---|---|---|---|
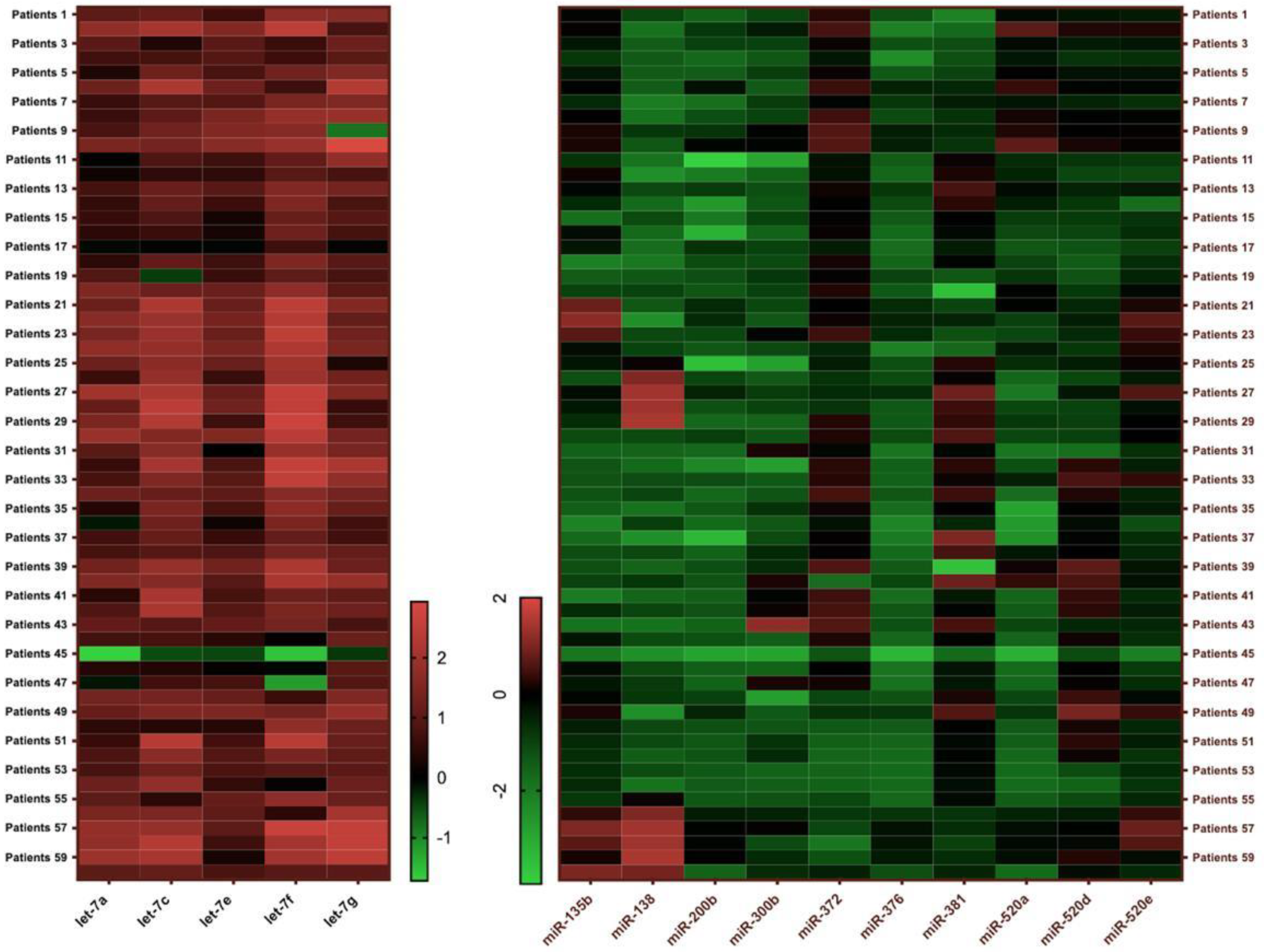
| let-7a | 6.8 (0.02–97.68) (n = 58) | 1.96 (0.11–78.25) (n = 60) | 0.0001 * |
| let-7c | 23.1 (0.31–259.57) (n = 55) | 0.19 (0–2.3) (n = 57) | 0.0001 * |
| let-7e | 7.42 (0.34–44.08) (n = 54) | 0.14 (0–9.61) (n = 58) | 0.0001 * |
| let-7f | 40.93 (0.03–484.38) (n = 54) | 1.5 (0.13–35.75) (n = 58) | 0.0001 * |
| let-7g | 17.75 (0.44–855.13) (n = 55) | 5.37 (0.12–92.41) (n = 56) | 0.0001 * |
| miR-135b | 0.24 (0.01–14.32) (n = 44) | 0.36 (0.04–79.58) (n = 55) | 0.049 * |
| miR-138 | 0.04 (0–29.56) (n = 17) | 0.05 (0–1.8) (n = 11) | 0.557 |
| miR-200b | 0.04 (0–0.97) (n = 44) | 0.07 (0–1.24) (n = 36) | 0.157 |
| miR-300 | 0.06 (0–15.06) (n = 22) | 2.23 (0.14–11.63) (n = 45) | 0.0001 * |
| miR-381 | 2.08 (0–29.74) (n = 54) | 33.18 (5.16–446.39) (n = 58) | 0.0001 * |
| miR-373 | 0.04 (0–0.5) (n = 51) | 4.54 (0.02–61.18) (n = 58) | 0.0001 * |
| miR-372 | 1.89 (0.02–69.36) (n = 57) | 5.06 (0.1–49.77) (n = 59) | 0.0001 * |
| miR-520a | 0.13 (0–5.42) (n = 58) | 0.7 (0.01–9.13) (n = 55) | 0.0001 * |
| miR-520d | 0.45 (0.02–9.21) (n = 58) | 2.59 (0.03–72.86) (n = 58) | 0.0001 * |
| miR-520e | 0.38 (0.01–6.74) (n = 58) | 3.27 (0.14–19.53) (n = 57) | 0.0001 * |
| AUC | p | |
|---|---|---|
| let-7a | 0.767 | 0.0001 * |
| let-7c | 0.985 | 0.0001 * |
| let-7e | 0.939 | 0.0001 * |
| let-7f | 0.876 | 0.0001 * |
| let-7g | 0.714 | 0.0001 * |
| miR-135b | 0.615 | 0.049 * |
| miR-138 | 0.433 | 0.557 |
| miR-200b | 0.592 | 0.157 |
| miR-300 | 0.912 | 0.0001 * |
| miR-372 | 0.709 | 0.0001 * |
| miR-373 | 0.880 | 0.0001 * |
| miR-381 | 0.954 | 0.0001 * |
| miR-520a | 0.727 | 0.0001 * |
| miR-520d | 0.789 | 0.0001 * |
| miR-520e | 0.861 | 0.0001 * |
| let-7c + miR-381 | 1 | 0.0001 * |
| let-7f + let-7c | 0.991 | 0.0001 * |
| let-7f + let-7e | 0.944 | 0.0001 * |
| let-7f + miR-135b | 0.886 | 0.0001 * |
| miR-373 + let-7c | 0.995 | 0.0001 * |
| Treatment Failure (Did Not Respond Clinically to Treatment or Less Than 50% Reduction in OAB V-8 Score After Treatment) | Treatment Success (50% or Greater Reduction in the OAB V-8 Score After Treatment) | p | |
|---|---|---|---|
| n: 60 | 48 | 12 | |
| let-7f expression levels | 32 (0.03–426.91) | 147.86 (0.06–484.38) | 0.045 * |
| miR-135b expression levels | 0.3 (0.01–14.32) | 0.06 (0.03–0.21) | 0.036 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küçüker, K.; Aybek, H.; Akça, H.; Karagür, E.R.; Fırat, E.; Özlülerden, Y.; Çelen, S.; Aybek, Z. The Role of microRNA in Overactive Bladder: Relationship and Clinical Correlation. Medicina 2025, 61, 475. https://doi.org/10.3390/medicina61030475
Küçüker K, Aybek H, Akça H, Karagür ER, Fırat E, Özlülerden Y, Çelen S, Aybek Z. The Role of microRNA in Overactive Bladder: Relationship and Clinical Correlation. Medicina. 2025; 61(3):475. https://doi.org/10.3390/medicina61030475
Chicago/Turabian StyleKüçüker, Kürşat, Hülya Aybek, Hakan Akça, Ege Rıza Karagür, Elif Fırat, Yusuf Özlülerden, Sinan Çelen, and Zafer Aybek. 2025. "The Role of microRNA in Overactive Bladder: Relationship and Clinical Correlation" Medicina 61, no. 3: 475. https://doi.org/10.3390/medicina61030475
APA StyleKüçüker, K., Aybek, H., Akça, H., Karagür, E. R., Fırat, E., Özlülerden, Y., Çelen, S., & Aybek, Z. (2025). The Role of microRNA in Overactive Bladder: Relationship and Clinical Correlation. Medicina, 61(3), 475. https://doi.org/10.3390/medicina61030475







