Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements
Abstract
1. Introduction
2. Materials and Methods
3. Results
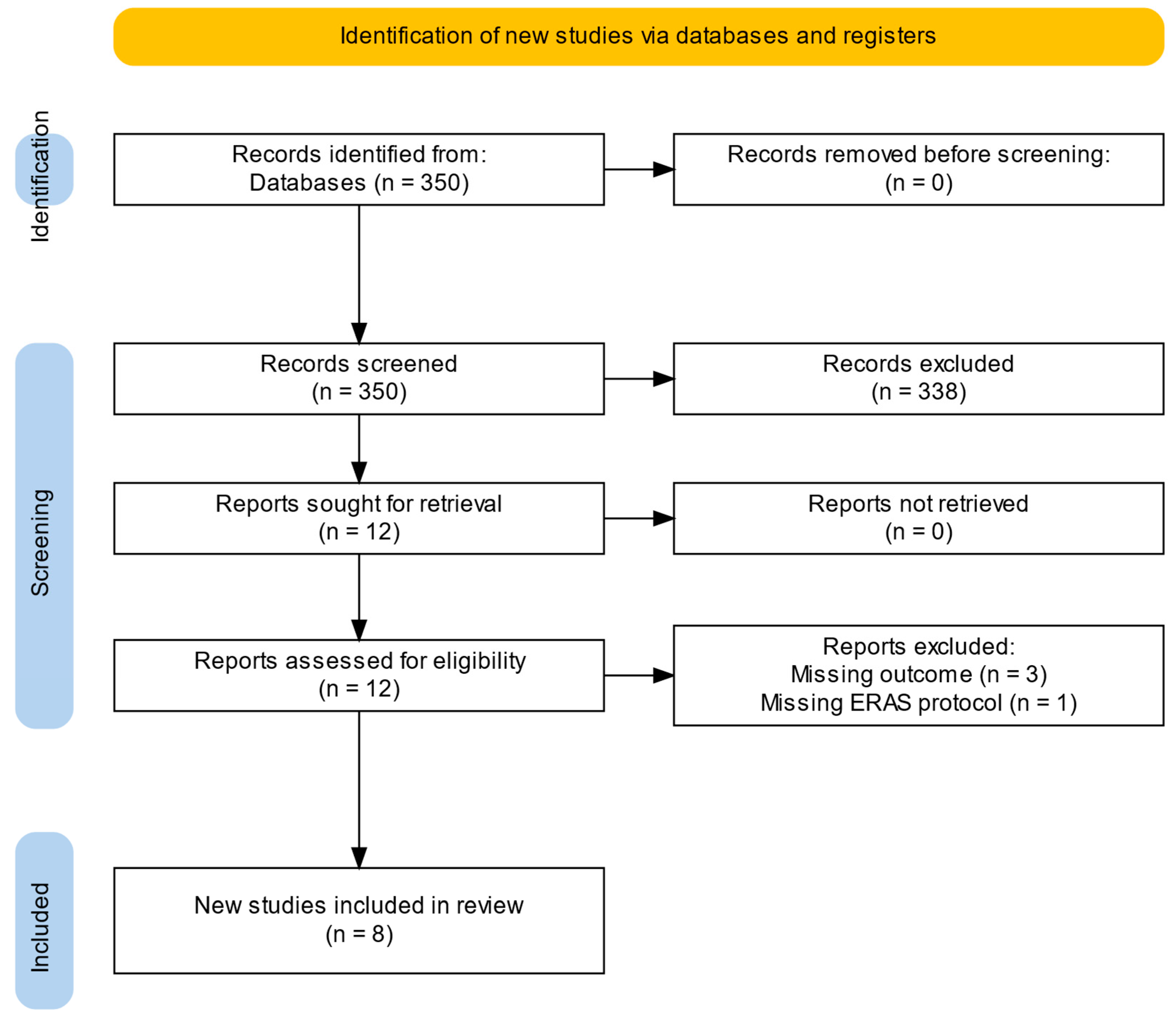
3.1. Study Selection
3.2. General Elements
3.2.1. Shared Decision Making, Patient Engagement, and Education
3.2.2. Establishment of a Multidisciplinary Team (MDT)
3.2.3. Compliance and Outcomes Auditing
3.3. Preoperative Process Measures
3.3.1. Preoperative Screening and Risk Assessment
3.3.2. Prehabilitation
3.3.3. Limiting Nil per Os Status
3.4. Intraoperative Process Measures
3.4.1. Transesophageal Echocardiography
3.4.2. Protective Lung Ventilation
3.4.3. Ventilation on Cardiopulmonary Bypass
3.4.4. Use of Pulmonary Artery Catheters
3.4.5. Central Nervous System Monitoring
3.4.6. Postoperative Nausea and Vomiting Prevention
3.4.7. Goal-Directed Perfusion
3.5. Postoperative Process Measures
3.5.1. Early Extubation Strategies
3.5.2. Intraoperative Extubation
3.5.3. Acute Kidney Injury Prevention and Management
3.5.4. Postoperative Activity and Sternal Precautions
3.6. Multiphase Process Measures
3.6.1. Goal-Directed Therapy
3.6.2. Opioid-Sparing Pain Management
3.6.3. Regional Analgesia
3.6.4. Patient Blood Management Program
3.6.5. Postoperative Atrial Fibrillation Prevention
3.6.6. Systematic Delirium Screening and Prevention
3.6.7. Surgical Site Infection Prevention Bundle
3.7. Process Measures Not Graded by ERAS Guidelines
3.7.1. Minimally Invasive Surgery Approach
3.7.2. Removal of Chest Tubes, Catheters, and Pacemaker Wires
3.8. Promising Process Measures Not Included in ERAS Guidelines
3.8.1. Shortening ICU Stay
3.8.2. Minimally Invasive Extracorporeal Circulation
3.8.3. Posterior Pericardiotomy
3.8.4. Interpersonal Advancements
3.8.5. Anticoagulation After MIVS
3.8.6. Nutritional Intake
3.9. Non-Promising Process Measures Not Included in ERAS Guidelines
3.9.1. Hemofiltration
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| CPB | Cardiopulmonary Bypass |
| DO2I | Oxygen Delivery Index |
| ERAS | Enhanced Recovery After Surgery |
| GDT | Goal-Directed Therapy |
| ICU | Intensive Care Unit |
| MDT | Multidisciplinary Team |
| MiECC | Minimally Invasive Extracorporeal Circulation |
| MIVS | Minimally Invasive Valve Surgery |
| PACU | Post-Anesthesia Care Unit |
| PEEP | Positive End-Expiratory Pressure |
| PONV | Postoperative Nausea and Vomiting |
| SSI | Surgical Site Infection |
| TEE | Transesophageal Echocardiography |
References
- Carpentier, A.; Loulmet, D.; Carpentier, A.; Le Bret, E.; Haugades, B.; Dassier, P.; Guibourt, P. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. Comptes Rendus Acad. Sci. 1996, 319, 219–223. [Google Scholar]
- Cosgrove, D.M.; Sabik, J.F. Minimally Invasive Approach for Aortic Valve Operations. Ann. Thorac. Surg. J. Soc. Thorac. Surg. South. Thorac. Surg. Assoc. 1996, 62, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Jones, S.G.; Muir, A.; Malaisrie, S.C.; Chung, D.A.; Williams, R.J.; Akowuah, E. Limited versus Full Sternotomy for Aortic Valve Replacement. Cochrane Database Syst. Rev. 2023, 12, CD011793. [Google Scholar] [CrossRef]
- Al Shamry, A.; Jegaden, M.; Ashafy, S.; Eker, A.; Jegaden, O. Minithoracotomy versus Sternotomy in Mitral Valve Surgery: Meta-Analysis from Recent Matched and Randomized Studies. J. Cardiothorac. Surg. 2023, 18, 101. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. Engl. Ed. 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Vollroth, M.; Seeburger, J.; Garbade, J.; Borger, M.A.; Misfeld, M.; Mohr, F.W. Conversion Rate and Contraindications for Minimally Invasive Mitral Valve Surgery. Ann. Cardiothorac. Surg. 2013, 2, 853–854. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Höfer, D.; Holfeld, J.; Hangler, H.; Bonaros, N.; Grimm, M. Indications and Contra-Indications for Minimally Invasive Mitral Valve Surgery. J. Vis. Surg. 2018, 4, 255. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery after Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Maj, G.; Regesta, T.; Campanella, A.; Cavozza, C.; Parodi, G.; Audo, A. Optimal Management of Patients Treated With Minimally Invasive Cardiac Surgery in the Era of Enhanced Recovery After Surgery and Fast-Track Protocols: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 766–775. [Google Scholar] [CrossRef]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef]
- Zaouter, C.; Oses, P.; Assatourian, S.; Labrousse, L.; Rémy, A.; Ouattara, A. Reduced Length of Hospital Stay for Cardiac Surgery—Implementing an Optimized Perioperative Pathway: Prospective Evaluation of an Enhanced Recovery After Surgery Program Designed for Mini-Invasive Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Kubitz, J.C.; Schulte-Uentrop, L.; Zoellner, C.; Lemke, M.; Messner-Schmitt, A.; Kalbacher, D.; Sill, B.; Reichenspurner, H.; Koell, B.; Girdauskas, E. Establishment of an Enhanced Recovery after Surgery Protocol in Minimally Invasive Heart Valve Surgery. PLoS ONE 2020, 15, e0231378. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Kloth, B.; Konertz, J.; Kubitz, J.; Schulte-Uentrop, L.; Ketels, G.; Reichenspurner, H.; Girdauskas, E. Economic Impact of Enhanced Recovery after Surgery Protocol in Minimally Invasive Cardiac Surgery. BMC Health Serv. Res. 2021, 21, 254. [Google Scholar] [CrossRef]
- Berretta, P.; De Angelis, V.; Alfonsi, J.; Pierri, M.D.; Malvindi, P.G.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. Enhanced Recovery after Minimally Invasive Heart Valve Surgery: Early and Midterm Outcomes. Int. J. Cardiol. 2023, 370, 98–104. [Google Scholar] [CrossRef]
- Gebauer, A.; Konertz, J.; Petersen, J.; Brickwedel, J.; Köster, D.; Schulte-Uentrop, L.; Reichenspurner, H.; Girdauskas, E. The Impact of a Standardized Enhanced Recovery After Surgery (ERAS) Protocol in Patients Undergoing Minimally Invasive Heart Valve Surgery. PLoS ONE 2023, 18, e0283652. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.; Berger Veith, S.; Holst, T.; Erfani, S.; Pochert, J.; Dumps, C.; Girdauskas, E. Feasibility of Deescalating Postoperative Care in Enhanced Recovery after Cardiac Surgery. Front. Cardiovasc. Med. 2024, 11, 1412869. [Google Scholar] [CrossRef] [PubMed]
- Ertugay, S.; Karaca, S.; Engin, A.Y.; Kahraman, Ü.; Ünlü, Z.; Kocabaş, S.; Çalkavur, T.; Özbaran, M. Fine Tuning for Totally Endoscopic Mitral Valve Surgery: ERAS Applications. Front. Cardiovasc. Med. 2024, 11, 1398438. [Google Scholar] [CrossRef]
- Pitts, L.; Dini, M.; Goecke, S.; Kofler, M.; Ott, S.; Stoppe, C.; O’Brien, B.; Jacobs, S.; Falk, V.; Hommel, M.; et al. Enhanced Recovery after Minimally Invasive Cardiac Surgery Following a Zero ICU Concept—A Propensity Score Matched Analysis. Eur. J. Cardiothorac. Surg. 2024, 66, ezae439. [Google Scholar] [CrossRef]
- Klotz, S.G.R.; Ketels, G.; Behrendt, C.A.; König, H.-H.; Kohlmann, S.; Löwe, B.; Petersen, J.; Stock, S.; Vettorazzi, E.; Zapf, A.; et al. Interdisciplinary and Cross-Sectoral Perioperative Care Model in Cardiac Surgery: Implementation in the Setting of Minimally Invasive Heart Valve Surgery (INCREASE)—Study Protocol for a Randomized Controlled Trial. Trials 2022, 23, 528. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Vessella, W.; Carozza, R.; Capestro, F.; D’Alfonso, A.; Zingaro, C.; Munch, C.; Berretta, P. Ultra Fast-Track Minimally Invasive Aortic Valve Replacement: Going beyond Reduced Incisions. Eur. J. Cardiothorac. Surg. 2018, 53, ii14–ii18. [Google Scholar] [CrossRef]
- Schmid, M.E.; Stock, S.; Girdauskas, E. Implementation of an Innovative ERAS Protocol in Cardiac Surgery: A Qualitative Evaluation from Patients’ Perspective. PLoS ONE 2024, 19, e0303399. [Google Scholar] [CrossRef] [PubMed]
- Gaweda, B.; Kolowca, M.; Olszowka, P.; Trojnar, B.; Widenka, K. Implementation ERAS Protocol in Minimally Invasive Cardiac Surgery. Clin. Nutr. ESPEN 2019, 31, 108. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Brown, J.K.; Singh, K.; Dumitru, R.; Chan, E.; Kim, M.P. The Benefits of Enhanced Recovery After Surgery Programs and Their Application in Cardiothoracic SurgeryCME. Methodist. DeBakey Cardiovasc. J. 2018, 14, 77. [Google Scholar] [CrossRef]
- Stone, A.B.; Yuan, C.T.; Rosen, M.A.; Grant, M.C.; Benishek, L.E.; Hanahan, E.; Lubomski, L.H.; Ko, C.; Wick, E.C. Barriers to and Facilitators of Implementing Enhanced Recovery Pathways Using an Implementation Framework: A Systematic Review. JAMA Surg. 2018, 153, 270. [Google Scholar] [CrossRef] [PubMed]
- Jawitz, O.K.; Bradford, W.T.; McConnell, G.; Engel, J.; Allender, J.E.; Williams, J.B. How to Start an Enhanced Recovery After Surgery Cardiac Program. Crit. Care Clin. 2020, 36, 571–579. [Google Scholar] [CrossRef]
- Ltaief, Z.; Verdugo-Marchese, M.; Carel, D.; Gunga, Z.; Nowacka, A.; Melly, V.; Addor, V.; Botteau, C.; Hennemann, M.; Lavanchy, L.; et al. Implementation of Cardiac Enhanced Recovery after Surgery at Lausanne University Hospital, Our Roadbook to Certification. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae118. [Google Scholar] [CrossRef]
- Zorrilla-Vaca, A.; Stone, A.B.; Ripolles-Melchor, J.; Abad-Motos, A.; Ramirez-Rodriguez, J.M.; Galan-Menendez, P.; Mena, G.E.; Grant, M.C.; Garcia-Perez, C.; Higuera-Míguelez, E.; et al. Institutional Factors Associated with Adherence to Enhanced Recovery Protocols for Colorectal Surgery: Secondary Analysis of a Multicenter Study. J. Clin. Anesth. 2021, 74, 110378. [Google Scholar] [CrossRef]
- Lee, J.A.; Yanagawa, B.; An, K.R.; Arora, R.C.; Verma, S.; Friedrich, J.O. Frailty and Pre-Frailty in Cardiac Surgery: A Systematic Review and Meta-Analysis of 66,448 Patients. J. Cardiothorac. Surg. 2021, 16, 184. [Google Scholar] [CrossRef]
- Pozzi, M.; Mariani, S.; Scanziani, M.; Passolunghi, D.; Bruni, A.; Finazzi, A.; Lettino, M.; Foti, G.; Bellelli, G.; Marchetto, G. The Frail Patient Undergoing Cardiac Surgery: Lessons Learned and Future Perspectives. Front. Cardiovasc. Med. 2023, 10, 1295108. [Google Scholar] [CrossRef]
- Niebauer, J.; Bäck, C.; Bischoff-Ferrari, H.A.; Dehbi, H.-M.; Szekely, A.; Völler, H.; Sündermann, S.H. Preinterventional Frailty Assessment in Patients Scheduled for Cardiac Surgery or Transcatheter Aortic Valve Implantation: A Consensus Statement of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Preventive Cardiology (EAPC) of the European Society of Cardiology (ESC). Eur. J. Prev. Cardiol. 2023, 31, 146–181. [Google Scholar] [CrossRef]
- Studniarek, A.; Borsuk, D.J.; Marecik, S.J.; Park, J.J.; Kochar, K. Enhanced Recovery After Surgery Protocols. Does Frailty Play a Role? Am. Surg. 2021, 87, 1054–1061. [Google Scholar] [CrossRef]
- Zaouter, C.; Damphousse, R.; Moore, A.; Stevens, L.-M.; Gauthier, A.; Carrier, F.M. Elements Not Graded in the Cardiac Enhanced Recovery After Surgery Guidelines Might Improve Postoperative Outcome: A Comprehensive Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 746–765. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.A.; Smith, M.D.; Herbison, G.P.; Plank, L.D.; McCall, J.L. Network Meta-Analysis of the Effect of Preoperative Carbohydrate Loading on Recovery after Elective Surgery. Br. J. Surg. 2017, 104, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.Z.; Ahmed, Z. Does a Preoperative Carbohydrate Drink Reduce Postoperative Inflammation? A Systematic Review and Meta-Analysis. Trauma. Care 2023, 3, 294–307. [Google Scholar] [CrossRef]
- Tong, E.; Chen, Y.; Ren, Y.; Zhou, Y.; Di, C.; Zhou, Y.; Shao, S.; Qiu, S.; Hong, Y.; Yang, L.; et al. Effects of Preoperative Carbohydrate Loading on Recovery after Elective Surgery: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 951676. [Google Scholar] [CrossRef]
- Nicoara, A.; Skubas, N.; Ad, N.; Finley, A.; Hahn, R.T.; Mahmood, F.; Mankad, S.; Nyman, C.B.; Pagani, F.; Porter, T.R.; et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach. J. Am. Soc. Echocardiogr. 2020, 33, 692–734. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Sündermann, S.H.; Kempfert, J. Endoaortic Balloon Occlusion During Minimally Invasive Mitral Valve Surgery. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2022, 17, 83–87. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Sündermann, S.H.; Montagner, M.; Heck, R.; Starck, C.; Stamm, C.; Jacobs, S.; Kempfert, J.; Falk, V. Minimally Invasive Approach for Infective Mitral Valve Endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 702–704. [Google Scholar] [CrossRef]
- Ender, J.; Sgouropoulou, S. Value of Transesophageal Echocardiography (TEE) Guidance in Minimally Invasive Mitral Valve Surgery. Ann. Cardiothorac. Surg. 2013, 2, 796–802. [Google Scholar]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Baek, S.-H.; Je, H.G.; Kim, T.K.; Kim, H.J.; Ahn, J.H.; Park, S.J. Comparison of the Single-Lumen Endotracheal Tube and Double-Lumen Endobronchial Tube Used in Minimally Invasive Cardiac Surgery for the Fast Track Protocol. J. Thorac. Dis. 2016, 8, 778–783. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Pratomo, B.Y.; Sudadi, S.; Setianto, B.Y.; Novenanto, T.T.; Raksawardana, Y.K.; Rayhan, A.; Kurniawaty, J. Intraoperative Goal-Directed Perfusion in Cardiac Surgery with Cardiopulmonary Bypass: The Roles of Delivery Oxygen Index and Cardiac Index. Ann. Thorac. Cardiovasc. Surg. 2024, 30, 23-00189. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Johnson, I.; Willcox, T.; Baker, R.A.; Boer, C.; Baumann, A.; Justison, G.A.; De Somer, F.; Exton, P.; Agarwal, S.; et al. Goal-Directed Perfusion to Reduce Acute Kidney Injury: A Randomized Trial. J. Thorac. Cardiovasc. Surg. 2018, 156, 1918–1927.e2. [Google Scholar] [CrossRef]
- Srey, R.; Rance, G.; Shapeton, A.D.; Leissner, K.B.; Zenati, M.A. A Quick Reference Tool for Goal-Directed Perfusion in Cardiac Surgery. J. Extracorpor. Technol. 2019, 51, 172–174. [Google Scholar] [CrossRef]
- Ball, L.; Costantino, F.; Pelosi, P. Postoperative Complications of Patients Undergoing Cardiac Surgery. Curr. Opin. Crit. Care 2016, 22, 386–392. [Google Scholar] [CrossRef]
- Bojar, R.M. Respiratory Management. In Manual of Perioperative Care in Adult Cardiac Surgery; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 457–512. ISBN 978-1-119-58254-0. [Google Scholar]
- Zakhary, W.; Lindner, J.; Sgouropoulou, S.; Eibel, S.; Probst, S.; Scholz, M.; Ender, J. Independent Risk Factors for Fast-Track Failure Using a Predefined Fast-Track Protocol in Preselected Cardiac Surgery Patients. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1461–1465. [Google Scholar] [CrossRef]
- Badhwar, V.; Esper, S.; Brooks, M.; Mulukutla, S.; Hardison, R.; Mallios, D.; Chu, D.; Wei, L.; Subramaniam, K. Extubating in the Operating Room after Adult Cardiac Surgery Safely Improves Outcomes and Lowers Costs. J. Thorac. Cardiovasc. Surg. 2014, 148, 3101–3109.e1. [Google Scholar] [CrossRef]
- Wang, Y.; Bellomo, R. Cardiac Surgery-Associated Acute Kidney Injury: Risk Factors, Pathophysiology and Treatment. Nat. Rev. Nephrol. 2017, 13, 697–711. [Google Scholar] [CrossRef]
- Xie, C.; Yao, Y.; Yang, K.; Shen, M.; He, L.; Dai, Z.; the Evidence in Cardiovascular Anesthesia (EICA) Group. Furosemide Does Not Reduce the Incidence of Postoperative Acute Kidney Injury in Adult Patients Undergoing Cardiac Surgery: A PRISMA-compliant Systematic Review and Meta-analysis. J. Card. Surg. 2022, 37, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery–Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Wainwright, T.W.; McDonald, D.A.; Burgess, L.C. The Role of Physiotherapy in Enhanced Recovery After Surgery in The intensive care unit. ICU Manag. Pract. 2017, 17, 144–147. [Google Scholar]
- Ahmad, A.M. Essentials of Physiotherapy after Thoracic Surgery: What Physiotherapists Need to Know. A Narrative Review. Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 293–307. [Google Scholar] [CrossRef]
- Onan, B. Minimal Access in Cardiac Surgery. Turk. J. Thorac. Cardiovasc. Surg. 2020, 28, 708–724. [Google Scholar] [CrossRef]
- Yayla, A.; Özer, N. Effects of Early Mobilization Protocol Performed after Cardiac Surgery on Patient Care Outcomes. Int. J. Nurs. Pract. 2019, 25, e12784. [Google Scholar] [CrossRef]
- Kapoor, P.; Magoon, R.; Rawat, R.; Mehta, Y. Perioperative Utility of Goal-Directed Therapy in High-Risk Cardiac Patients Undergoing Coronary Artery Bypass Grafting: “A Clinical Outcome and Biomarker-Based Study”. Ann. Card. Anaesth. 2016, 19, 638. [Google Scholar] [CrossRef]
- Aya, H.D.; Cecconi, M.; Hamilton, M.; Rhodes, A. Goal-Directed Therapy in Cardiac Surgery: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2013, 110, 510–517. [Google Scholar] [CrossRef]
- Huang, J.; Firestone, S.; Moffatt-Bruce, S.; Tibi, P.; Shore-Lesserson, L. 2021 Clinical Practice Guidelines for Anesthesiologists on Patient Blood Management in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3493–3495. [Google Scholar] [CrossRef]
- Callum, J.; Skubas, N.J.; Bathla, A.; Keshavarz, H.; Clark, E.G.; Rochwerg, B.; Fergusson, D.; Arbous, S.; Bauer, S.R.; China, L.; et al. Use of Intravenous Albumin. CHEST 2024, 166, 321–338. [Google Scholar] [CrossRef]
- Xiang, F.; Huang, F.; Huang, J.; Li, X.; Dong, N.; Xiao, Y.; Zhao, Q.; Xiao, L.; Zhang, H.; Zhang, C.; et al. Expert Consensus on the Use of Human Serum Albumin in Adult Cardiac Surgery. Chin. Med. J. 2023, 136, 1135–1143. [Google Scholar] [CrossRef]
- Jacob, M.; Fellahi, J.-L.; Chappell, D.; Kurz, A. The Impact of Hydroxyethyl Starches in Cardiac Surgery: A Meta-Analysis. Crit. Care 2014, 18, 656. [Google Scholar] [CrossRef]
- Navickis, R.J.; Haynes, G.R.; Wilkes, M.M. Effect of Hydroxyethyl Starch on Bleeding after Cardiopulmonary Bypass: A Meta-Analysis of Randomized Trials. J. Thorac. Cardiovasc. Surg. 2012, 144, 223–230.e5. [Google Scholar] [CrossRef]
- Grant, M.C.; Chappell, D.; Gan, T.J.; Manning, M.W.; Miller, T.E.; Brodt, J.L.; Shaw, A.D.; Engelman, D.; Mythen, M.; Guinn, N.R.; et al. Pain Management and Opioid Stewardship in Adult Cardiac Surgery: Joint Consensus Report of the PeriOperative Quality Initiative and the Enhanced Recovery After Surgery Cardiac Society. J. Thorac. Cardiovasc. Surg. 2023, 166, 1695–1706.e2. [Google Scholar] [CrossRef]
- Loria, C.M.; Zborek, K.; Millward, J.B.; Anderson, M.P.; Richardson, C.M.; Namburi, N.; Faiza, Z.; Timsina, L.R.; Lee, L.S. Enhanced Recovery after Cardiac Surgery Protocol Reduces Perioperative Opioid Use. JTCVS Open 2022, 12, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Kumar, R.; Mokhtassi, S.S.; Alassiri, A.K.; Odaman, A.; Khan, M.A.R.; Lakshmana, S.; Din, Z.U.; Acharya, P.; Cheema, H.A.; et al. Minimally Invasive vs. Conventional Mitral Valve Surgery: A Meta-Analysis of Randomised Controlled Trials. Front. Cardiovasc. Med. 2024, 11, 1437524. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.J.; Arora, R.C.; Chatterjee, S.; Crisafi, C.; Morton-Bailey, V.; Rea, A.; Salenger, R.; Engelman, D.T.; Grant, M.C.; Cangut, B.; et al. Enhanced Recovery After Surgery (ERAS) Cardiac Turnkey Order Set for Perioperative Pain Management in Cardiac Surgery: Proceedings from the American Association for Thoracic Surgery (AATS) ERAS Conclave 2023. JTCVS Open 2024, 22, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.S.; Samad, K.; Ahmed, S.S.; Siddiqui, K.M.; Ullah, H. Cardiac Surgery and Blood-Saving Techniques: An Update. Cureus 2022, 14, e21222. [Google Scholar] [CrossRef]
- Berretta, P.; Cefarelli, M.; Montecchiani, L.; Alfonsi, J.; Vessella, W.; Zahedi, M.H.; Carozza, R.; Munch, C.; Di Eusanio, M. Minimally Invasive versus Standard Extracorporeal Circulation System in Minimally Invasive Aortic Valve Surgery: A Propensity Score-Matched Study. Eur. J. Cardiothorac. Surg. 2020, 57, 717–723. [Google Scholar] [CrossRef]
- Anastasiadis, K.; Antonitsis, P.; Deliopoulos, A.; Argiriadou, H. From Less Invasive to Minimal Invasive Extracorporeal Circulation. J. Thorac. Dis. 2021, 13, 1909–1921. [Google Scholar] [CrossRef]
- Arsenault, K.A.; Yusuf, A.M.; Crystal, E.; Healey, J.S.; Morillo, C.A.; Nair, G.M.; Whitlock, R.P. Interventions for Preventing Post-Operative Atrial Fibrillation in Patients Undergoing Heart Surgery. Cochrane Database Syst. Rev. 2013, 2013, CD003611. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, A.; Rocca, B.; Hansson, E.C.; Gudbjartsson, T.; James, S.; Kaski, J.K.; Magro, P.; Pan, E.; Ravn, H.B.; Sandner, S.; et al. 2024 EACTS Guidelines on Perioperative Medication in Adult Cardiac Surgery. Eur. J. Cardiothorac. Surg. 2024, 67, ezae355. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.D.; Reeves, B.C.; Culliford, L.A.; Maishman, R.; Rogers, C.A.; Anastasiadis, K.; Antonitsis, P.; Argiriadou, H.; Carrel, T.; Keller, D.; et al. Conventional versus Minimally Invasive Extra-Corporeal Circulation in Patients Undergoing Cardiac Surgery: A Randomized Controlled Trial (COMICS). Perfusion 2024, 02676591241258054. [Google Scholar] [CrossRef]
- Soletti, G.J.; Perezgrovas-Olaria, R.; Harik, L.; Rahouma, M.; Dimagli, A.; Alzghari, T.; Demetres, M.; Bratton, B.A.; Yaghmour, M.; Satija, D.; et al. Effect of Posterior Pericardiotomy in Cardiac Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2022, 9, 1090102. [Google Scholar] [CrossRef]
- O’Brien, B.; Campbell, N.G.; Allen, E.; Jamal, Z.; Sturgess, J.; Sanders, J.; Opondo, C.; Roberts, N.; Aron, J.; Maccaroni, M.R.; et al. Potassium Supplementation and Prevention of Atrial Fibrillation After Cardiac Surgery: The TIGHT K Randomized Clinical Trial. JAMA 2024, 332, 979. [Google Scholar] [CrossRef] [PubMed]
- Wyler Von Ballmoos, M.C.; Hui, D.S.; Mehaffey, J.H.; Malaisrie, S.C.; Vardas, P.N.; Gillinov, A.M.; Sundt, T.M.; Badhwar, V. The Society of Thoracic Surgeons 2023 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2024, 118, 291–310. [Google Scholar] [CrossRef]
- Chen, H.; Mo, L.; Hu, H.; Ou, Y.; Luo, J. Risk Factors of Postoperative Delirium after Cardiac Surgery: A Meta-Analysis. J. Cardiothorac. Surg. 2021, 16, 113. [Google Scholar] [CrossRef]
- Bojar, R.M. Post-ICU Care and Other Complications. In Manual of Perioperative Care in Adult Cardiac Surgery; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 737–842. ISBN 978-1-119-58254-0. [Google Scholar]
- Andrade, L.S.D.; Siliprandi, E.M.O.; Karsburg, L.L.; Berlesi, F.P.; Carvalho, O.L.D.F.; Rosa, D.S.D.; Santos, R.P.D. Surgical Site Infection Prevention Bundle in Cardiac Surgery. Arq. Bras. Cardiol. 2019, 112, 769–774. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Stamm, C.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Montagner, M.; Nazari Shafti, T.Z.; Jacobs, S.; Falk, V.; Kempfert, J. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interv. Cardiol. Rev. 2017, 13, 14. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Akansel, S.; Montagner, M.; Meyer, A.; Sündermann, S.H.; Falk, V.; Kempfert, J. Periareolar Endoscopic Minimally Invasive Cardiac Surgery: Postoperative Scar Assessment Analysis. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac200. [Google Scholar] [CrossRef]
- Sündermann, S.H.; Sromicki, J.; Rodriguez Cetina Biefer, H.; Seifert, B.; Holubec, T.; Falk, V.; Jacobs, S. Mitral Valve Surgery: Right Lateral Minithoracotomy or Sternotomy? A Systematic Review and Meta-Analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1989–1995.e4. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D.A.; Melisa, S.; Andrianto, G.A.; Suwatri, W.T.; Sugisman. Outcomes of Minimally Invasive versus Conventional Sternotomy for Redo Mitral Valve Surgery According to Mitral Valve Academic Research Consortium: A Systematic Review and Meta-Analysis. Asian J. Surg. 2024, 47, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Daemen, J.H.T.; Heuts, S.; Olsthoorn, J.R.; Maessen, J.G.; Sardari Nia, P. Right Minithoracotomy versus Median Sternotomy for Reoperative Mitral Valve Surgery: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Cardiothorac. Surg. 2018, 54, 817–825. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kampen, A.; Kofler, M.; Richter, G.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Kurz, S.; Jacobs, S.; Falk, V.; et al. Minimally Invasive Surgical Aortic Valve Replacement: The RALT Approach. J. Card. Surg. 2020, 35, 2341–2346. [Google Scholar] [CrossRef]
- Yost, C.C.; Rosen, J.L.; Mandel, J.L.; Wong, D.H.; Prochno, K.W.; Komlo, C.M.; Ott, N.; Goldhammer, J.E.; Guy, T.S. Feasibility of Postoperative Day One or Day Two Discharge After Robotic Cardiac Surgery. J. Surg. Res. 2023, 289, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Hwang, B.; Huang, L.; Wilson-Smith, A.; Brookes, J.; Eranki, A.; Yan, T.D.; Guy, T.S.; Bonatti, J. Robotic versus Conventional Sternotomy Mitral Valve Surgery: A Systematic Review and Meta-Analysis. Ann. Cardiothorac. Surg. 2022, 11, 490–503. [Google Scholar] [CrossRef]
- Dittrich, T.; Tschudin-Sutter, S.; Widmer, A.F.; Rüegg, S.; Marsch, S.; Sutter, R. Risk Factors for New-Onset Delirium in Patients with Bloodstream Infections: Independent and Quantitative Effect of Catheters and Drainages—A Four-Year Cohort Study. Ann. Intensive Care 2016, 6, 104. [Google Scholar] [CrossRef]
- Darras, M.; Schneider, C.; Marguerite, S.; Oulehri, W.; Collange, O.; Mertes, P.-M.; Mazzucotelli, J.-P.; Kindo, M. Early Chest Tube Removal on the 1st Postoperative Day Protocol of an Enhanced Recovery after Cardiac Surgery Programme Is Safe. Eur. J. Cardiothorac. Surg. 2024, 65, ezae092. [Google Scholar] [CrossRef]
- Mubarak, F.S.; Ellepola, Y.; Chamba, K.N.; Agrawal, S.; Makhoul, M. Complications of Epicardial Pacing Wire Removal Following Adult Cardiac Surgery: A Systematic Review. Cureus 2023, 15, e49076. [Google Scholar] [CrossRef]
- Bojar, R.M. Cardiovascular Management. In Manual of Perioperative Care in Adult Cardiac Surgery; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 513–671. ISBN 978-1-119-58254-0. [Google Scholar]
- Farina, J.; Biffi, M.; Folesani, G.; Di Marco, L.; Martin, S.; Zenesini, C.; Savini, C.; Ziacchi, M.; Diemberger, I.; Martignani, C.; et al. Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors. J. Clin. Med. 2024, 13, 538. [Google Scholar] [CrossRef]
- Haanschoten, M.C.; Van Straten, A.H.M.; Ter Woorst, J.F.; Stepaniak, P.S.; Van Der Meer, A.-D.; Van Zundert, A.A.J.; Soliman Hamad, M.A. Fast-Track Practice in Cardiac Surgery: Results and Predictors of Outcome. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Kirov, H.; Caldonazo, T.; Runkel, A.; Fischer, J.; Tasoudis, P.; Mukharyamov, M.; Cancelli, G.; Dell’Aquila, M.; Doenst, T. Percutaneous Versus Surgical Femoral Cannulation in Minimally Invasive Cardiac Surgery: A Systematic Review and Meta-Analysis. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2024, 19, 247–253. [Google Scholar] [CrossRef]
- Gupta, S.; McEwen, C.; Basha, A.; Panchal, P.; Eqbal, A.; Wu, N.; Belley-Cote, E.P.; Whitlock, R. Retrograde Autologous Priming in Cardiac Surgery: A Systematic Review and Meta-Analysis. Eur. J. Cardiothorac. Surg. 2021, 60, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.; Hafez, A.H.; Elaraby, A.; Roshdy, M.R.; Abdelaziz, M.; Eltobgy, M.A.; Elsayed, H.; El-Samahy, M.; Elbehbeh, N.A.; Philip, K.G.; et al. Posterior Pericardiotomy for the Prevention of Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis of 25 Randomised Controlled Trials. EuroInterv. J. Eur. Collab. Work. Group. Interv. Cardiol. Eur. Soc. Cardiol. 2023, 19, e305–e317. [Google Scholar] [CrossRef] [PubMed]
- Niburski, K.; Guadagno, E.; Abbasgholizadeh-Rahimi, S.; Poenaru, D. Shared Decision Making in Surgery: A Meta-Analysis of Existing Literature. Patient Patient-Centered Outcomes Res. 2020, 13, 667–681. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Rev. Esp. Cardiol. 2018, 71, 110. [Google Scholar]
- Hill, A.; Nesterova, E.; Lomivorotov, V.; Efremov, S.; Goetzenich, A.; Benstoem, C.; Zamyatin, M.; Chourdakis, M.; Heyland, D.; Stoppe, C. Current Evidence about Nutrition Support in Cardiac Surgery Patients—What Do We Know? Nutrients 2018, 10, 597. [Google Scholar] [CrossRef]
- Stoppe, C.; Dresen, E.; Wendt, S.; Elke, G.; Patel, J.J.; McKeever, L.; Chourdakis, M.; McDonald, B.; Meybohm, P.; Lindner, M.; et al. Current Practices in Nutrition Therapy in Cardiac Surgery Patients: An International Multicenter Observational Study. J. Parenter. Enter. Nutr. 2023, 47, 604–613. [Google Scholar] [CrossRef]
- Soliman, R.; Fouad, E.; Belghith, M.; Abdelmageed, T. Conventional Hemofiltration during Cardiopulmonary Bypass Increases the Serum Lactate Level in Adult Cardiac Surgery. Ann. Card. Anaesth. 2016, 19, 45. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Bifulco, O.; Berretta, P.; Galeazzi, M.; Alfonsi, J.; Cefarelli, M.; Zingaro, C.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. The Enhanced Recovery after Surgery Approach in Heart Valve Surgery: A Systematic Review of Clinical Studies. J. Clin. Med. 2024, 13, 2903. [Google Scholar] [CrossRef]
- Sá, M.P.B.O.; Van Den Eynde, J.; Cavalcanti, L.R.P.; Kadyraliev, B.; Enginoev, S.; Zhigalov, K.; Ruhparwar, A.; Weymann, A.; Dreyfus, G. Mitral Valve Repair with Minimally Invasive Approaches vs Sternotomy: A Meta-analysis of Early and Late Results in Randomized and Matched Observational Studies. J. Card. Surg. 2020, 35, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, M.E.; Okoh, A.K.; Chao, J.C.; Soto, C.; Baxi, J.; Salgueiro, L.A.; Olds, A.; Ikegami, H.; Lemaire, A.; Russo, M.J.; et al. Early Discharge After Minimally Invasive Aortic and Mitral Valve Surgery. Ann. Thorac. Surg. 2022, 114, 91–97. [Google Scholar] [CrossRef] [PubMed]
| Author | Short Description | Patient Characteristics (ERAS/Control) | Inclusion/Exclusion Criteria | Study Goals | Primary and Secondary Endpoints (ERAS vs. Control) |
|---|---|---|---|---|---|
| Zaouter et al.—2019 (Bordeaux, FRA) [11] |
After: 05/2015–11/2015 ERAS: n = 23 Control: n = 23 | Age: 80 (74–82)/73 (68–82); p = 0.038 Sex (M/F): 14/9/16/7; p = 0.038 BMI: 26 (23–27)/28 (26–32); p = 0.022 CPB Time (min): 81 (75–85)/80 (73–90); p = 0.667 |
|
|
|
| Kubitz et al.—2020 (Hamburg, GER) [12] |
ERAS: n = 50 control: n = 0 | Age: 51.9 ± 11.9/NA; p = NA Sex (M/F): 38/12/NA; p = NA BMI: 26.1 ± 3.1/NA; p = NA CPB Time (min): 137.8 ± 47.9/NA; p = NA |
|
|
|
| Petersen et al.—2021 (Hamburg, GER) [13] |
ERAS: n = 61 Control: n = 69 | Age: 50.7 ± 12.9/54.1 ± 9.5; p = 0.096 Sex (M/F): 47/14/52/17; p = 0.256 BMI: 26 (23–27)/28 (26–32); p = 0.022 CPB Time (min): 87 (73–108)/94 (77–112); p = 0.23 |
|
|
|
| Beretta et al.—2023 (Ancona, ITA) [14] |
ERAS: n = 152 Control: n = 152 (after propensity matching) | Age: 69.6 ± 11.1/70 ± 11.9; p = 0.813 Sex (M/F): 78/74/84/68; p = 0.813 BMI: 26.2 ± 3.8/26.2 ± 4.5; p = NA CPB Time (min): 77 (64–96)/77 (63–101); p = NA |
|
|
|
| Gebauer et al.—2023 (Hamburg, GER) [15] |
ERAS: n = 101 Control: n = 111 | Age: 56 ± 17/57.5 ± 13; p = 0.015 Sex (M/F): 74/27/79/32; p = 0.734 BMI: 25.7 ± 3.4/26.2 ± 3.3; p = 0.271 CPB Time (min): 130.5 ± 61/147 ± 81; p = 0.076 |
|
|
|
| Stock et al.—2024 (Augsburg, GER) [16] |
ERAS: n = 297 Control: n = 0 Abort: n = 61 (mainly due to prolonged mechanical ventilation) | Age: 63 (55–70)/NA; p = NA Sex (M/F): 193/104/NA; p = NA BMI: 25 (23–28)/NA; p = NA CPB Time (min): NA/NA; p = NA |
|
|
|
| Ertugay et al. 2024 (İzmir, TUR) [17] |
ERAS: n = 113 Control: n = 0 Abort: n = 4 (conversion to sternotomy) | Age: 54.7 ± 11.6/NA; p = NA Sex (M/F): 51/62/NA; p = NA BMI: 25.2 ± 4.2/NA; p = NA CPB Time (min): 149.9 ± 30.4/NA; p = NA |
|
|
|
| Pitts et al.—2024 (Berlin, GER) [18] |
ERAS: n = 45 Control: n = 90 (after propensity matching) | Age: 55 (46–61)/54 (46–60); p = 1.0 Sex (M/F): 39/6/90/12; p = 1.0 BMI: 25.0 (22.4–27.1)/24.9 (23.2–27.1); p = 0.74 CPB Time (min): 87 (73–108)/94 (77–112); p = 0.23 |
|
|
|
| ERAS Element | Zaouter et al. [11] | Kubitz et al. [12]/Gebauer et al. [15] | Petersen et al. [13] | Berretta et al. [14] | Stock et al. [16] | Ertugay et al. [17] | Pitts et al. [18] |
|---|---|---|---|---|---|---|---|
| Shared Decision Making, Patient Engagement, and Education | Meeting with surgeon, physiotherapist, nursing staff, psychologist; video on operating room arrival | Meeting with MDT 2–3 weeks before | Meeting with MDT 2–3 weeks before | Unknown | Unknown | Preoperative education and operative course | ERMICS patient education |
| Establishment of a Multidisciplinary Team (MDT) | Yes, but MDT not specified | Cardiac surgeon, anesthetist, perfusionist, physiotherapist | Cardiac surgeons, anesthesiologists, cardiologists, perfusionists, physiotherapist | Surgeons, anesthesiologists, perfusionists, physiotherapists, nurses | ERAS nurse (advanced practice nurse), physiotherapist, psychotherapist, anesthesiologist, cardiac surgeon | Cardiac surgeon, nurses, anesthesiologists, perfusionists, dietitians, physiotherapy | ERAS coordinator, MDT (team not specified) |
| Compliance and Outcomes Auditing | End-of-study monitoring | Pain self-assessments; PONV protocol | Unknown | Unknown | Unknown | Unknown | ERAS coordinator monitoring and troubleshooting |
| Preoperative Screening and Risk Assessment | Pre-op meeting; screening for tobacco, comorbidities, malnutrition | Frailty scoring; formal physical condition assessment | Frailty scoring; formal physical condition assessment, 6 min walk test | Nutritional correction (if necessary), HbA1c measurement | Individualized risk assessment by senior surgeon | Laboratory analysis, HbA1c, frailty screening | Standardized risk assessment |
| Prehabilitation | Tailored diet (if necessary) | Daily exercises and nutritional supplementation for 2–3 weeks | Daily exercises and nutritional supplementation for 2–3 weeks | Unknown | Interdisciplinary pre-op clinic visit | Pulmonary/physical rehabilitation, anxiety support, nutritional support | Unknown |
| Limiting Nil Per Os (NPO) Status | No (Future: carb drink 2 h pre-op, shorten fasting | Maltose carb drink 2 h pre-op | Unknown | NPO after midnight, clear liquid 2–4 h pre-op | Unknown | Unknown | NPO after midnight, clear liquid 2 h pre-op |
| Transesophageal Echocardiography | Yes | Yes | Unknown | Unknown | Unknown | Yes | Yes |
| Protective Lung Ventilation | Ventilation on CPB | Unknown | Unknown | Alveolar recruitment by PEEP 10 cm H2O | Unknown | Ventilation on CPB | Unknown |
| Ventilation on Cardiopulmonary Bypass | Tidal volume 3 mL/kg, PEEP 5 cm H2O | Unknown | Unknown | Unknown | Unknown | Tidal volume 4–6 mL/kg, PEEP 5–10 cm H2O | Unknown |
| Use of Pulmonary Artery Catheters | Excluded usage | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Central Nervous System Monitoring | Unknown | NIRS, bispectral monitoring | NIRS, bispectral monitoring | Unknown | Unknown | NIRS, bispectral monitoring | NIRS, bispectral monitoring |
| Postoperative Nausea and Vomiting Prevention | Pre-op dexamethasone | Dexamethasone and ondansetron; droperidol (if needed) | Antiemetic prophylaxis (not specified) | Unknown | Dexamethasone; granisetrone and dimenhydrinate | Dexamethasone and ondansetron | Dexamethasone and ondansetron; avoidance of benzodiazepines |
| Goal-Directed Perfusion | Not in protocol (Future: GDP-strategy on CPB) | Flow > 3.2 L/m2 BSA; restrictive vasopressors | Flow > 3.2 L/m2 BSA | Unknown | Unknown | Not specified | DO2I ≥ 280 mL/min/m2, hypothermia |
| Early Extubation Strategies/IntraOP Extubation | Remifentanil use; extubation criteria met in ICU | Early NIV, on-table extubation | On-table extubation | On-table extubation | On-table extubation; no NIV | Extubation within 6 h post-op | Remifentanil use; extubation after normothermia |
| Aki Prevention and Management | Fluid loading; vena cava variability monitoring | Unknown | Furosemide, CPB hypothermia | Use of biomarkers | Unknown | Unknown | DO2I ≥ 280 mL/min/m2, hypothermia |
| Postoperative Activity and Sternal Precautions | Sitting in chair after 4 h | first physio after 2–3 h; extended physio afterward | first physio after 2–3 h; second physio in the evening by nursing, extended physio afterward | respiratory therapy after 3–6 h after; early mobilization after 6–12 h | Unknown | Sitting outside bed morning; ambulation evening POD 1 | Physio in PACU to bedside, sometimes standing; respiratory; individualized physiotherapy |
| Goal-Directed Therapy (GDT) | GDT algorithm | Restrictive fluid therapy; hemofiltration on CPB | Restrictive fluid therapy | GDT with fluids, vasopressors, inotropes (not specified) | Unknown | Vasopressors vs. fluids (not specified) | GDT by ERAS anesthesiologist |
| Opioid-Sparing Pain Management | Multimodal: up to 8 agents; acetaminophen, nefopam, magnesium, pregabalin | Focus on PACU: metamizole and piritramide | Unknown | No (morphine and tramadol infusion) | Structured tapering; POD 3 opioid cessation | Escalating analgesic regimen: acetaminophen, dextromethorphan, tramadol | Structured tapering; opioid cessation after drain removal |
| Regional Analgesia | Wound infiltration (ropivacaine 0.75%) | Intercostal catheter + ropivacaine | Regional anesthesia (not further specified) | Serratus block, lidocaine-ropivacaine infiltration | Parasternal/serratus block pre-surgery | Cryotherapy, local neuroblocker/perfusion catheter | Serratus block at surgery end (ropivacaine 0.375%) |
| Patient Blood Management Program | Transfusion trigger Hb < 7.2 | Transfusion trigger Hb < 7.5 | Unknown | MiECC | Unknown | Anemia diagnostics (iron supplementation if necessary), retrograde priming, normovolemic hemodilution, antifibrinolytics | MiECC, Retrograde priming |
| Postoperative Atrial Fibrillation Prevention | Unknown | Amiodarone infusion (for high-risk patients), AF ablation, LAAC | Low-dose amiodarone prophylaxis | Unknown | Unknown | AF ablation, LAAC | AF ablation, LAAC |
| Systematic Delirium Screening and Prevention | Benzodiazepines avoided pre-op; AGS statement followed | Unknown | Unknown | Early family contact, delirium screening 1x/shift | Unknown | Alcohol cessation focus | Early family contact (in-person or video) |
| Surgical Site Infection Prevention Bundle | Unknown | Normothermia post-op | Unknown | Glycemic control, antibiotics, smoking cessation | Glycemic control, antibiotics | Glycemic control, antibiotics | Glycemic control, antibiotics |
| Category | Element | Zaouter et al. [11] | Kubitz et al. [12]/Gebauer et al. [15] | Petersen et al. [13] | Berretta et al. [14] | Stock et al. [16] | Ertugay et al. [17] | Pitts et al. [18] | QoE |
|---|---|---|---|---|---|---|---|---|---|
| General |
| Y | Y | Y | N | N | Y | Y | Low |
| Y | Y | Y | Y | Y | Y | Y | Moderate | |
| Y | Y | N | N | N | N | Y | Moderate | |
| Pre-OP |
| Y | Y | Y | Y | Y | Y | Y | Moderate |
| Y | Y | Y | N | Y | Y | N | Low | |
| N | Y | N | Y | N | N | Y | Low | |
| Intraoperative |
| Y | Y | N | N | N | Y | Y | Moderate |
| Y * | N | N | Y | N | Y * | N | High | |
| Y | N | N | N | N | Y | N | Moderate | |
| Y | N | N | N | N | N | N | Moderate | |
| N | Y | Y | N | N | Y | Y | Moderate | |
| Y | Y | Y | N | Y | Y | Y | Moderate | |
| N | Y | Y | N | N | N/S | Y | Low | |
| Post-OP |
| Y | Y | Y | Y | Y | Y | Y | Moderate |
| Y | Y | Y | Y | Y | N | N | Low | |
| Y | N | Y | Y | N | N | Y | Moderate | |
| Y | Y | Y | Y | N | Y | Y | Moderate | |
| Multiphase |
| Y | Y | Y | Y | N | N/S | N/S | Moderate |
| Y | N/S | N | N | Y | Y | Y | Moderate | |
| Y | Y | Y | Y | Y | Y | Y | Moderate | |
| Y | Y | N | Y | N | Y | Y | Moderate | |
| N | Y | Y | N | N | Y | Y | Moderate | |
| Y | N | N | Y | N | Y | Y | High | |
| N | Y | N | Y | Y | Y | Y | High | |
| Total elements implemented (out of 24 elements) | 18 | 18 | 14 | 12 | 9 | 16 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goecke, S.; Pitts, L.; Dini, M.; Montagner, M.; Wert, L.; Akansel, S.; Kofler, M.; Stoppe, C.; Ott, S.; Jacobs, S.; et al. Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements. Medicina 2025, 61, 495. https://doi.org/10.3390/medicina61030495
Goecke S, Pitts L, Dini M, Montagner M, Wert L, Akansel S, Kofler M, Stoppe C, Ott S, Jacobs S, et al. Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements. Medicina. 2025; 61(3):495. https://doi.org/10.3390/medicina61030495
Chicago/Turabian StyleGoecke, Simon, Leonard Pitts, Martina Dini, Matteo Montagner, Leonhard Wert, Serdar Akansel, Markus Kofler, Christian Stoppe, Sascha Ott, Stephan Jacobs, and et al. 2025. "Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements" Medicina 61, no. 3: 495. https://doi.org/10.3390/medicina61030495
APA StyleGoecke, S., Pitts, L., Dini, M., Montagner, M., Wert, L., Akansel, S., Kofler, M., Stoppe, C., Ott, S., Jacobs, S., O’Brien, B., Falk, V., Hommel, M., & Kempfert, J. (2025). Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements. Medicina, 61(3), 495. https://doi.org/10.3390/medicina61030495








