The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Cultıre
2.3. Cell Culture for HBECs
2.4. Cell Culture for HEK293T
2.5. The ASL Height Measurements
2.6. The Ussing Chamber Measurements
2.7. Cytotoxicity Tests
2.8. Statistical Analysis
3. Results
3.1. The Effects of the JUUL Components on the ASL Height
3.2. The Effects of the JUUL Components on the Ion Transportation
3.3. The Effects of the JUUL Components on the Transepithelial Electrical Resistance
3.4. Cytotoxic Effects of the JUUL Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann-Boyce, J.; McRobbie, H.; Bullen, C.; Begh, R.; Stead, L.F.; Hajek, P. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2016, 9, CD010216. [Google Scholar] [CrossRef] [PubMed]
- Walley, S.C.; Wilson, K.M.; Winickoff, J.P.; Groner, J. A public health crisis: Electronic cigarettes, vape, and JUUL. Pediatrics 2019, 143, e20182741. [Google Scholar] [CrossRef] [PubMed]
- Gholap, V.V.; Heyder, R.S.; Kosmider, L.; Halquist, M.S. An analytical perspective on determination of free base nicotine in e-Liquids. J. Anal. Methods Chem. 2020, 2020, 6178570. [Google Scholar] [CrossRef] [PubMed]
- Voos, N.; Goniewicz, M.L.; Eissenberg, T. What is the nicotine delivery profile of electronic cigarettes? Expert Opin. Drug Deliv. 2019, 16, 1193–1203. [Google Scholar] [CrossRef]
- Gray, T.; Coakley, R.; Hirsh, A.; Thornton, D.; Kirkham, S.; Koo, J.-S.; Burch, L.; Boucher, R.; Nettesheim, P.; Physiology, M. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1β in human bronchial epithelia. Am. J. Physiol. Cell. Mol. Physiol. 2004, 286, L320–L330. [Google Scholar] [CrossRef]
- Tarran, R.; Trout, L.; Donaldson, S.H.; Boucher, R.C. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J. Gen. Physiol. 2006, 127, 591–604. [Google Scholar] [CrossRef]
- LTai, T.; Ma, H.-P.; Eaton, D.C. Epithelial Sodium Channels (ENaCs). In Ion Channels and Transporters of Epithelia in Health and Disease; Hamilton, K.L., Devor, D.C., Eds.; Springer: New York, NY, USA, 2016; pp. 569–641. [Google Scholar]
- Meng, X.; Clews, J.; Kargas, V.; Wang, X.; Ford, R.C. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell. Mol. Life Sci. 2017, 74, 23–38. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef]
- Hartzell, C.; Putzier, I.; Arreola, J. Calcium-activated chloride channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Ghosh, A.; Beyazcicek, O.; Davis, E.S.; Onyenwoke, R.U.; Tarran, R. Cellular effects of nicotine salt-containing e-liquids. J. Appl. Toxicol. 2021, 41, 493–505. [Google Scholar] [CrossRef]
- Woodall, M.; Jacob, J.; Kalsi, K.; Schroeder, V.; Davis, E.; Kenyon, B.; Khan, I.; Garnett, J.; Tarran, R.; Baines, D.L.; et al. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L957–L967. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.W.; Stanford, D.; LaFontaine, J.; Allen, A.D.; Raju, S.V. Nicotine aerosols diminish airway CFTR function and mucociliary clearance. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L557–L570. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Baumlin, N.; Dennis, J.S.; Moore, R.; Salathe, S.F.; Whitney, P.L.; Sabater, J.; Abraham, W.M.; Kim, M.D.; Salathe, M. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am. J. Respir. Crit. Care Med. 2019, 200, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coakley, R.D.; Alexis, N.E.; Tarran, R. Vaping-Induced Proteolysis Causes Airway Surface Dehydration. Int. J. Mol. Sci. 2023, 24, 15348. [Google Scholar] [CrossRef]
- Garcia-Arcos, I.; Geraghty, P.; Baumlin, N.; Campos, M.; Dabo, A.J.; Jundi, B.; Cummins, N.; Eden, E.; Grosche, A.; Salathe, M. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016, 71, 1119–1129. [Google Scholar] [CrossRef]
- Kim, M.D.; Chung, S.; Baumlin, N.; Qian, J.; Montgomery, R.N.; Sabater, J.; Berkland, C.; Salathe, M. The combination of propylene glycol and vegetable glycerin e-cigarette aerosols induces airway inflammation and mucus hyperconcentration. Sci. Rep. 2024, 14, 1942. [Google Scholar] [CrossRef]
- Higham, A.; Bostock, D.; Booth, G.; Dungwa, J.V.; Singh, D. The effect of electronic cigarette and tobacco smoke exposure on COPD bronchial epithelial cell inflammatory responses. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 989–1000. [Google Scholar] [CrossRef]
- Tyrrell, J.; Ghosh, A.; Manzo, N.D.; Randell, S.H.; Tarran, R. Evaluation of chronic cigarette smoke exposure in human bronchial epithelial cultures. J. Appl. Toxicol. 2023, 43, 862–873. [Google Scholar] [CrossRef]
- Terryah, S.T.; Fellner, R.C.; Ahmad, S.; Moore, P.J.; Reidel, B.; Sesma, J.I.; Kim, C.S.; Garland, A.L.; Scott, D.W.; Sabater, J.R.; et al. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L192–L205. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Gabriel, S.E.; Olsen, J.C.; Tatreau, J.R.; Gentzsch, M.; Livanos, E.; Saavedra, M.T.; Salmon, P.; Randell, S.H. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L82–L91. [Google Scholar] [CrossRef]
- Sloane, P.A.; Shastry, S.; Wilhelm, A.; Courville, C.; Tang, L.P.; Backer, K.; Levin, E.; Raju, S.V.; Li, Y.; Mazur, M. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE 2012, 7, e39809. [Google Scholar] [CrossRef] [PubMed]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]
- Donaldson, S.H.; Bennett, W.D.; Zeman, K.L.; Knowles, M.R.; Tarran, R.; Boucher, R.C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 2006, 354, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Boucher, R.C.; Button, B.; Elston, T.; Lin, C.-L. An integrated mathematical epithelial cell model for airway surface liquid regulation by mechanical forces. J. Theor. Biol. 2018, 438, 34–45. [Google Scholar] [CrossRef]
- Chambers, L.A.; Rollins, B.M.; Tarran, R. Liquid movement across the surface epithelium of large airways. Respir. Physiol. Neurobiol. 2007, 159, 256–270. [Google Scholar] [CrossRef]
- Wu, T.; Wrennall, J.A.; Dang, H.; Baines, D.L.; Tarran, R. Passaging Primary Human Bronchial Epithelia Reduces CFTR-Mediated Fluid Transport and Alters mRNA Expression. Cells 2023, 12, 997. [Google Scholar] [CrossRef]
- Blank, U.; Rückes, C.; Clauss, W.; Weber, W.-M. Effects of nicotine on human nasal epithelium: Evidence for nicotinic receptors in non-excitable cells. Pflug. Archit. 1997, 434, 581–586. [Google Scholar] [CrossRef]
- Lin, V.Y.; Fain, M.D.; Jackson, P.L.; Berryhill, T.F.; Wilson, L.S.; Mazur, M.; Barnes, S.J.; Blalock, J.E.; Raju, S.V.; Rowe, S.M. Vaporized E-cigarette liquids induce ion transport dysfunction in airway epithelia. Am. J. Respir. Cell Mol. Biol. 2019, 61, 162–173. [Google Scholar] [CrossRef]
- Kim, M.D.; Chung, S.; Dennis, J.S.; Yoshida, M.; Aguiar, C.; Aller, S.P.; Mendes, E.S.; Schmid, A.; Sabater, J.; Baumlin, N. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front. Pharmacol. 2022, 13, 1012723. [Google Scholar] [CrossRef]
- Chu, M.; Deng, J.; Hu, H.; Wang, R.; Li, D.; Chen, Z.; Liu, X.-A.; Lu, J. Nicotine transport across calu-3 cell monolayer: Effect of nicotine salts and flavored e-liquids. Drug Dev. Ind. Pharm. 2023, 49, 628–636. [Google Scholar] [CrossRef]
- Pinkston, R.; Zaman, H.; Hossain, E.; Penn, A.L.; Noël, A. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air–liquid interface. Respir. Res. 2020, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Brune, K.A.; Putcha, N.; Mandke, P.; O’Neal, W.K.; Shade, D.; Srivastava, V.; Wang, M.; Lam, H.; An, S.S. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L581–L591. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, J.; Sundar, I.K.; Freter, R.; Sekera, E.R.; Friedman, A.E.; Robinson, R.; Pagano, T.; Rahman, I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography–mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl. Vitr. Toxicol. 2017, 3, 28–40. [Google Scholar] [CrossRef]
- Raduka, A.; Gao, N.; Chatburn, R.L.; Rezaee, F. Electronic cigarette exposure disrupts airway epithelial barrier function and exacerbates viral infection. Am. J. Physiol. Cell. Mol. Physiol. 2023, 325, L580–L593. [Google Scholar] [CrossRef]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Pankow, J.F.; Talbot, P. High-nicotine electronic cigarette products: Toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem. Res. Toxicol. 2019, 32, 1058–1069. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control. 2019, 28, 678–680. [Google Scholar] [CrossRef]
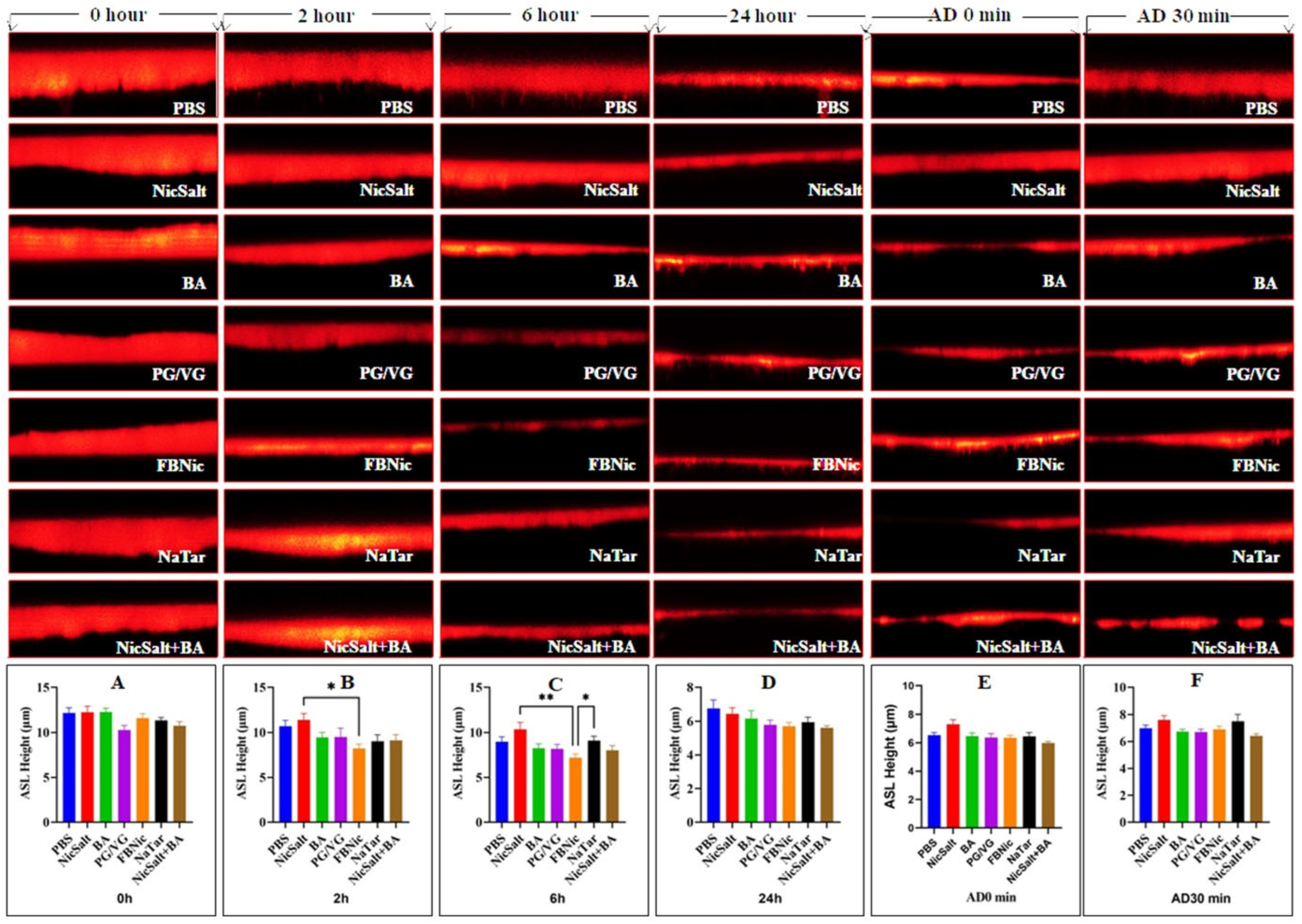
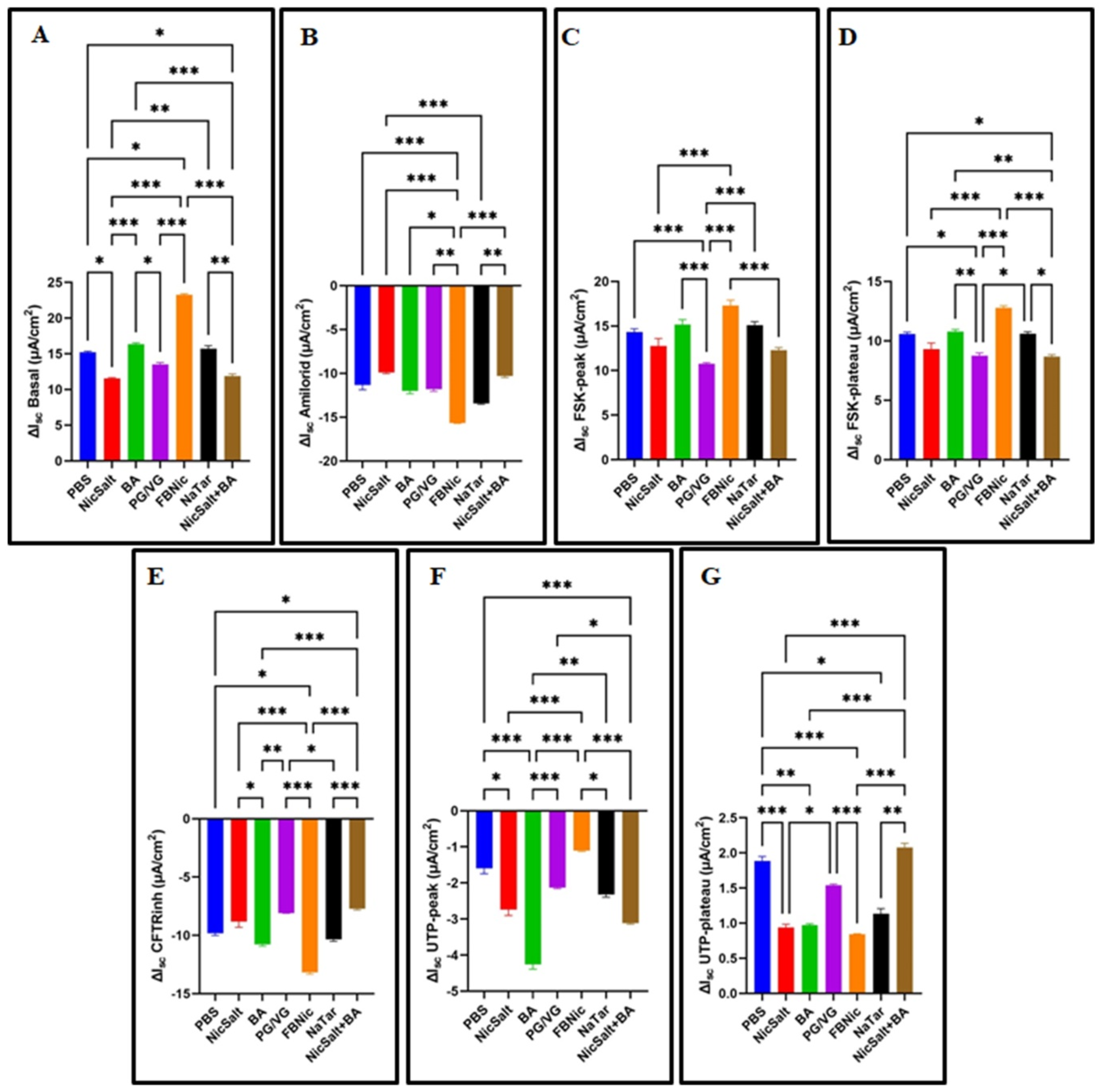

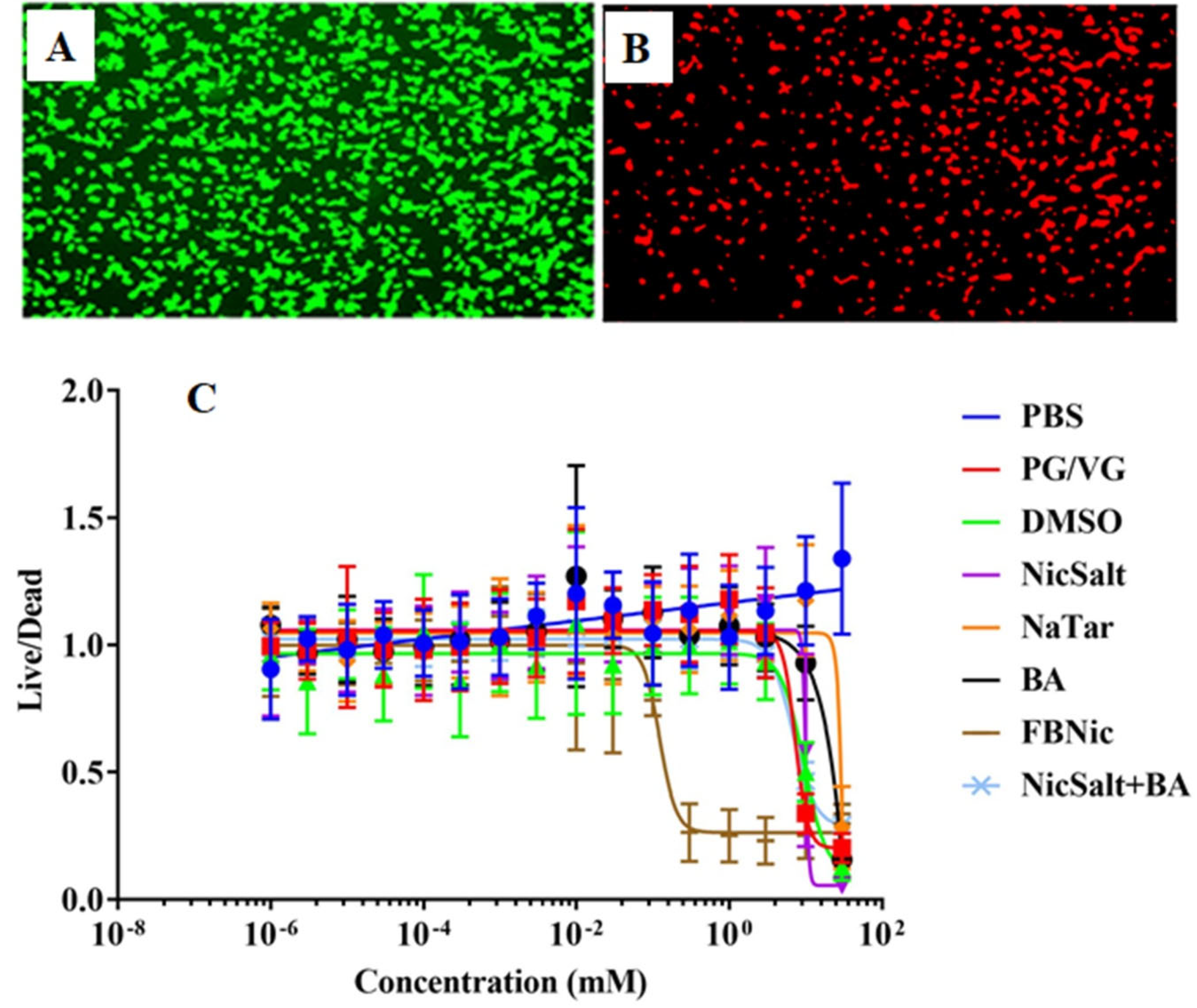
| Cell Types | Exposure Substance | Key Findings | References |
|---|---|---|---|
| HBECs | 3% PG/VG | 3% PG/VG mixture initially increased ASL height transiently, followed by a significant decrease, suggesting barrier disruption and potential cellular stress. | [12] |
| HBECs | E-cig aerosols | Aerosolized nicotine e-liquids significantly reduced ASL height by impairing ENaC and CFTR ion channel functions, compromising airway epithelial integrity. | [13] |
| HBECs | E-cig vapor | E-cig vapor significantly reduced ASL height by impairing CFTR ion channel functions and compromising airway epithelial integrity. | [14] |
| HBECs | Bronchoalveolar lavage fluid (BALF) from non-smokers, smokers, and vapers | JUUL e-liquids increased ENaC channel activity, reduced ASL height, and compromised airway mucociliary clearance mechanisms. | [15] |
| NHBECs | E-cigarette vapours or nicotine solutions | E-cigarette vapours or nicotine solutions reduced ASL height, and compromised airway mucociliary clearance mechanisms. | [16] |
| HBECs | 50% PG/VG | PG/VG aerosols significantly decreased mucus hydration by impairing CFTR ion channel functions, compromising airway epithelial integrity. | [17] |
| BEC, Calu-3 cells | E-cig vapor extract | E-cig vapor extract caused toxicity in BECs and Calu-3 cells. | [18] |
| Groups | Substance | Dissolvent | Amount (Per Culture with Dex) | Dose | Duration of Exposure (h) | |
|---|---|---|---|---|---|---|
| The determination of the effect of e-cig components on ASL Height | PBS | PBS | - | 14 µL | - | 0–24 |
| Nicotine Salt (NicSalt) | Nicotine Salt | PBS | 14 µL | 100 µM | 0–24 | |
| Freebase Nicotine (FBNic) | Freebase Nicotine | PBS | 14 µL | 100 µM | 0–24 | |
| Benzoic Acid (BA) | Benzoic Acid | 1 M stock solution in DMSO diluted in media | 14 µL | 100 µM | 0–24 | |
| Sodium Hydrogen Tartrate (NaTar) | Sodium Hydrogen Tartrate | PBS | 14 µL | 100 µM | 0–24 | |
| PG/VG | Propylene Glycerol + Vegetable Glycerine (55/45%) | PBS | 14 µL | 100 µM | 0–24 | |
| Nicotine Salt +Benzoic Acid (NicSalt+BA) | Nicotine Salt+ Benzoic Acid Mix | PBS | 14 µL | 100 µM | 0–24 | |
| The determination of the effects of e-cig on ion transport and TEER | PBS | PBS | - | 20 µL | - | 24 |
| Nicotine Salt (NicSalt) | Nicotine Salt | PBS | 20 µL | 100 µM | 24 | |
| Freebase Nicotine (FBNic) | Freebase Nicotine | PBS | 20 µL | 100 µM | 24 | |
| Benzoic Acid (BA) | Benzoic Acid | 1 M stock solution in DMSO diluted in media | 20 µL | 100 µM | 24 | |
| Sodium Hydrogen Tartrate (NaTar) | Sodium Hydrogen Tartrate | PBS | 20 µL | 100 µM | 24 | |
| PG/VG | Propylene Glycerol + Vegetable Glycerine (55/45%) | PBS | 20 µL | 100 µM | 24 | |
| Nicotine Salt +Benzoic Acid (NicSalt+BA) | Nicotine Salt+ Benzoic Acid Mix | PBS | 20 µL | 100 µM | 24 | |
| The determination of cytotoxic effects of E-Cig | PBS | PBS | - | 20 µL | 999.10−7–30 mM | 24 |
| Nicotine Salt (NicSalt) | Nicotine Salt | PBS | 20 µL | 999.10−7–30 mM | 24 | |
| Freebase Nicotine (FBNic) | Freebase Nicotine | PBS | 20 µL | 999.10−7–30 mM | 24 | |
| Benzoic Acid (BA) | Benzoic Acid | 1 M stock solution in DMSO Diluted in Media | 20 µL | 999.10−7–30 mM | 24 | |
| Sodium Hydrogen Tartrate (NaTar) | Sodium Hydrogen Tartrate | PBS | 20 µL | 999.10−7–30 mM | 24 | |
| PG/VG | Propylene Glycerol + Vegetable Glycerine (55/45%) | PBS | 20 µL | 999.10−7–30 mM | 24 | |
| DMSO | Dimethyl Sulfoxide | - | 20 µL | 999.10−7–30 mM | 24 | |
| Nicotine Salt + Benzoic Acid (NicSalt+BA) | Nicotine Salt+ Benzoic Acid Mix | PBS | 20 µL | 999.10−7–30 mM | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyazcicek, O.; Tarran, R.; Ozmerdivenli, R.; Beyazcicek, E. The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells. Medicina 2025, 61, 526. https://doi.org/10.3390/medicina61030526
Beyazcicek O, Tarran R, Ozmerdivenli R, Beyazcicek E. The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells. Medicina. 2025; 61(3):526. https://doi.org/10.3390/medicina61030526
Chicago/Turabian StyleBeyazcicek, Ozge, Robert Tarran, Recep Ozmerdivenli, and Ersin Beyazcicek. 2025. "The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells" Medicina 61, no. 3: 526. https://doi.org/10.3390/medicina61030526
APA StyleBeyazcicek, O., Tarran, R., Ozmerdivenli, R., & Beyazcicek, E. (2025). The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells. Medicina, 61(3), 526. https://doi.org/10.3390/medicina61030526






