Bone Health for Gynaecologists
Abstract
1. Introduction
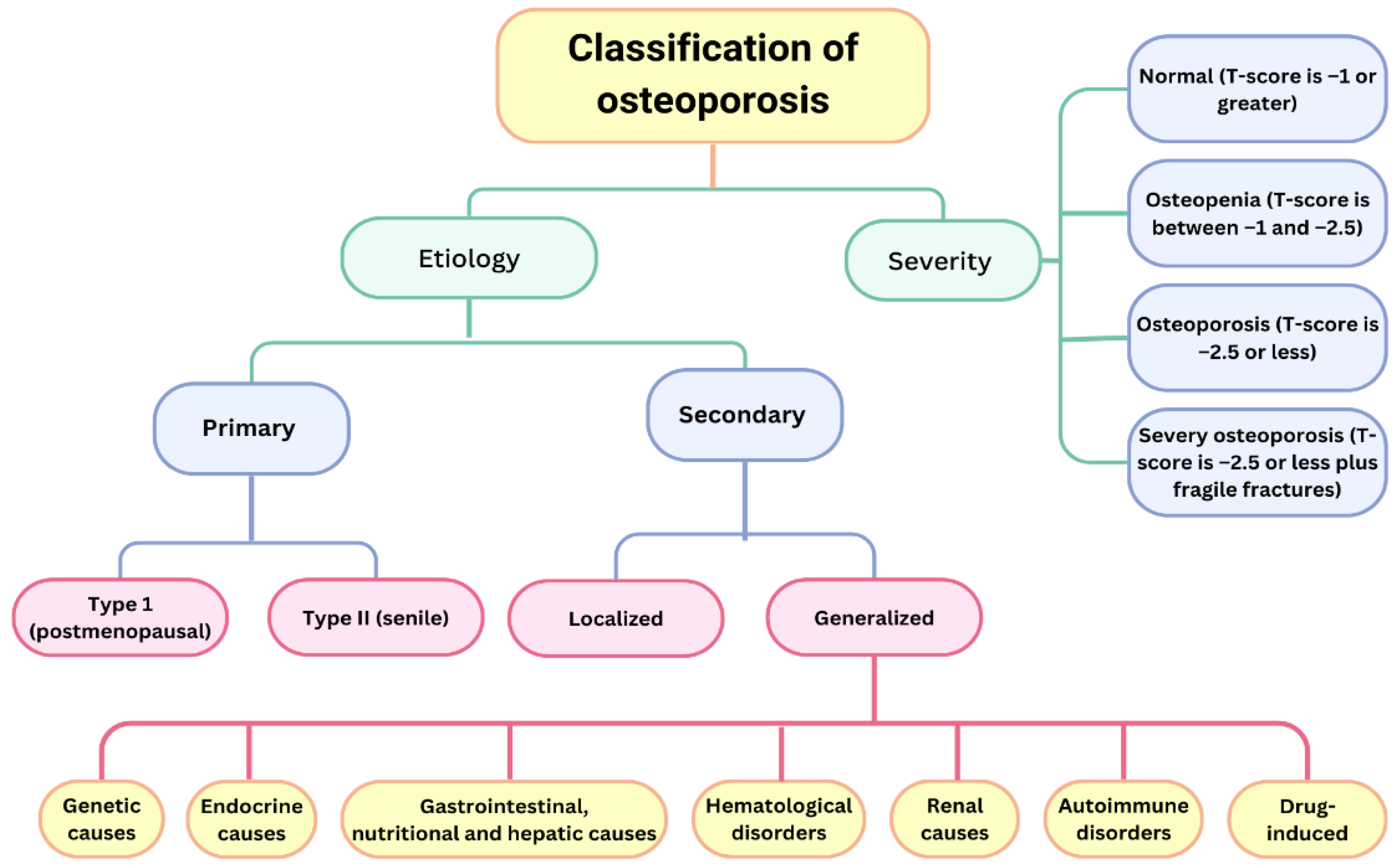
2. Classification
- Type I (postmenopausal osteoporosis)—This is more common in women, due to oestrogen deficiency, and mainly affects trabecular bone. Vertebral and distal end radius fractures are more common. This type of osteoporosis is characterized by low calcium levels (due to reduced intestinal absorption and increased renal excretion) and reduced levels of circulating D3 [14].
- Type II (senile osteoporosis)—This affects both sexes at age >70 years and affects trabecular and cortical bones. Hip and pelvic fractures are more common [14].
- Fracture risk cannot be assessed solely based on BMD values.
- Fractures may also occur in patients with a t-score of ≤−2.5.
- Optimise the treatment of the underlying condition.
- Medications that result in a reduction of BMD should be administered at minimal therapeutic doses.
- Address nutritional deficiencies.
- Address risk factors: smoking, alcohol consumption, caffeine intake, low body mass index (BMI), and decreased dairy product consumption.
3. Bone Loss Periods in a Woman’s Life
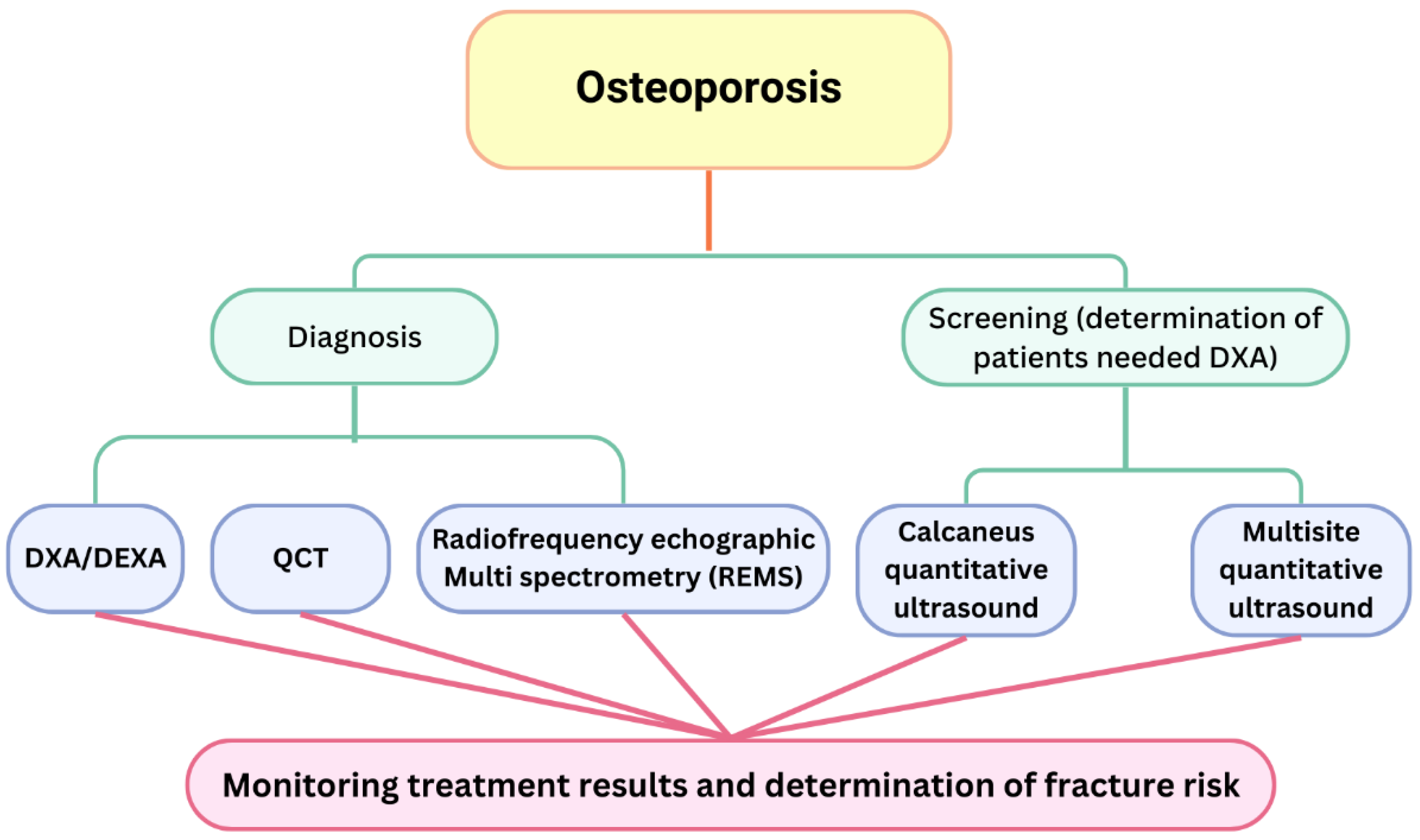
4. Diagnostic and Screening Methods
- Normal bone density: t-score > −1.0 SD.
- Osteopenia: t-score ≥ −1.0 SD < −2.5 SD.
- Osteoporosis: t-score ≤ −2.5 SD.
- Severe osteoporosis: t-score ≤ −2.5 SD and previous osteoporotic fracture
5. Prevention of Osteoporosis
6. Conclusions
- To understand the physiological factors contributing to a reduction in BMD and to identify when these women are at risk for developing osteoporosis.
- To identify risk groups affected by disorders that result in secondary osteoporosis and administer appropriate treatment.
- To assess the risk to bone health in young women receiving hormone therapy for gynecological conditions, particularly in adolescents utilizing oral contraceptives within three years post-menarche.
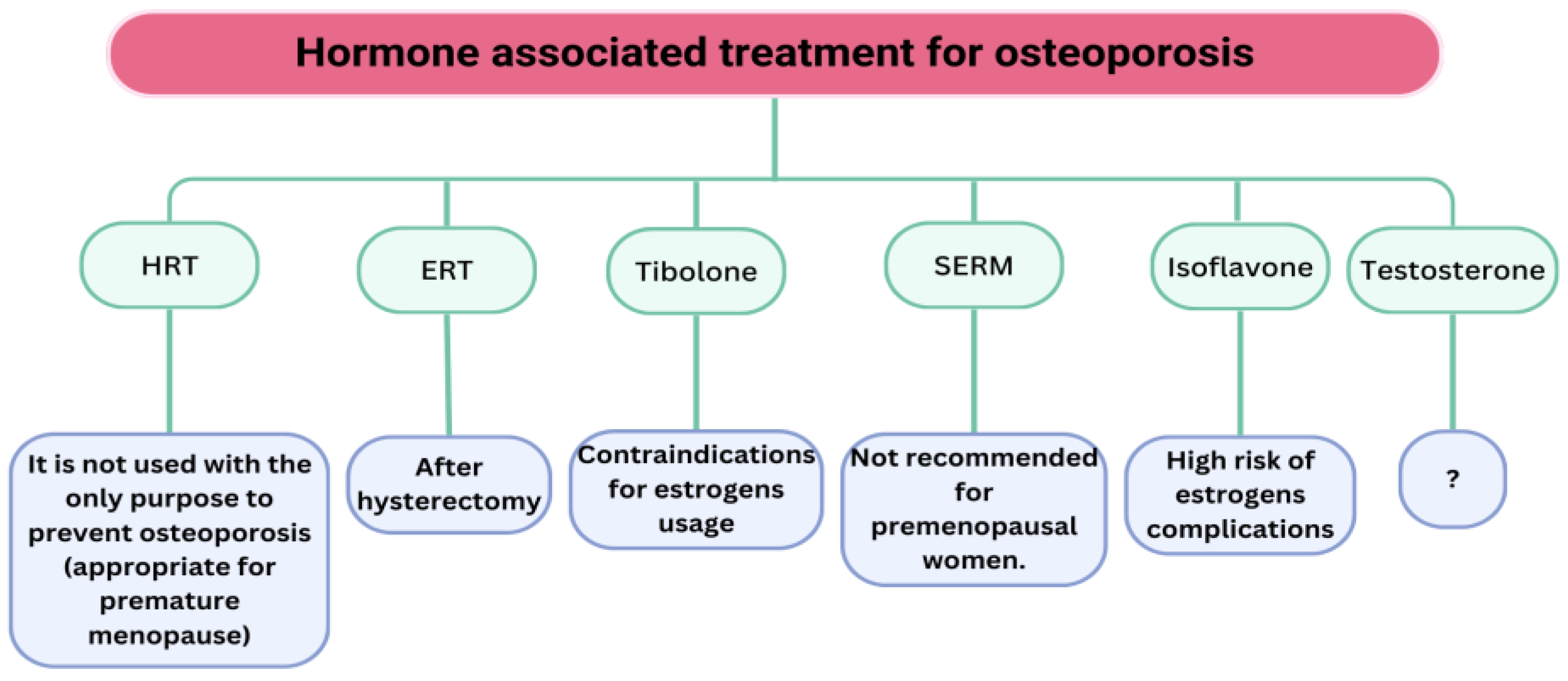
- To effectively manage women with POI and EM, HRT should be administered to women with POI until the age of natural menopause, contingent upon the underlying aetiology.
- To effectively manage women after gynecological procedures, oophorectomy results in accelerated bone mineral density loss, while hysterectomy induces menopause within four years.
- To effectively monitor patients with oncological conditions, particularly breast cancer.
- To understand the capabilities of various imaging modalities employed for diagnosing bone density alterations and their applicability in screening and therapy monitoring (calcaneus and multisite QUS).
- The application of HRT and SERM for preserving bone health in relatively younger women should be limited in duration, and HRT should not be administered to asymptomatic women (lacking climacteric symptoms) as a treatment for osteoporosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melton, L.J., III. How many women have osteoporosis now? J. Bone Miner. Res. 1995, 10, 175–177. [Google Scholar] [CrossRef]
- IOF. IOF Compendium of Osteoporosis, 1st ed.; IOF: Karlstad, Sweden, 2017; Available online: https://www.iofbonehealth.org (accessed on 27 October 2017).
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Schuit, S.C.; Van der Klift, M.; Weel, A.E.; De Laet, C.E.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.G.; Van Leeuwen, J.P.; Pols, H.A. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef]
- Siris, E.S.; Chen, Y.T.; Abbott, T.A.; Barrett-Connor, E.; Miller, P.D.; Wehren, L.E.; Berger, M.L. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch. Intern. Med. 2004, 164, 1108–1112. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, R.E.; Costantini, A.; Kämpe, A.; Alm, J.J.; Mäkitie, O. New Insights into Monogenic Causes of Osteoporosis. Front. Endocrinol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Hannan, F.M.; Newey, P.J.; Whyte, M.P.; Thakker, R.V. Genetic approaches to metabolic bone diseases. Br. J. Clin. Pharmacol. 2019, 85, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Kanis, J.A.; on behalf of the WHO Scientific Group. Assessment of Osteoporosis at the Primary Health-Care Level; Technical Report; WHO Collaborating Centre: Geneva, Switzerland; University of Sheffield: Sheffield, UK, 2008. [Google Scholar]
- Kanis, J.A.; Odén, A.; Johnell, O.; Johansson, H.; De Laet, C.; Brown, J.; Burckhardt, P.; Cooper, C.; Christiansen, C.; Cummings, S.; et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 2007, 18, 1033–1046. [Google Scholar] [CrossRef]
- Amarnath, S.S.; Kumar, V.; Das, S.L. Classification of Osteoporosis. Indian J. Orthop. 2023, 57 (Suppl. S1), 49–54. [Google Scholar] [CrossRef]
- Lu, Y.; Genant, H.K.; Shepherd, J.; Zhao, S.; Mathur, A.; Fuerst, T.P.; Cummings, S.R. Classification of osteoporosis based on bone mineral densities. J. Bone Miner. Res. 2001, 16, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R.; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Dawson-Hughes, B.; Tucker, K.L.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Risk factors for longitudinal bone loss in elderly men and women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2000, 15, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Painter, S.E.; Kleerekoper, M.; Camacho, P.M. Secondary osteoporosis: A review of the recent evidence. Endocr. Pract. 2006, 12, 436–445. [Google Scholar] [CrossRef]
- Harper, K.D.; Weber, T.J. Secondary osteoporosis. Diagnostic considerations. Endocrinol. Metab. Clin. N. Am. 1998, 27, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef]
- Brennan, S.L.; Toomey, L.; Kotowicz, M.A.; Henry, M.J.; Griffiths, H.; Pasco, J.A. Rheumatoid arthritis and incident fracture in women: A case-control study. BMC Musculoskelet. Disord. 2014, 15, 13. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Munoz-Ortego, J.; De Vries, F.; Vosse, D.; Arden, N.K.; Bowness, P.; Cooper, C.; Diez-Perez, A.; Vestergaard, P. Ankylosing spondylitis confers substantially increased risk of clinical spine fractures: A nationwide case-control study. Osteoporos. Int. 2014, 26, 85–91. [Google Scholar] [CrossRef]
- Coleman, R.E.; Banks, L.M.; Girgis, S.I.; Kilburn, L.S.; Vrdoljak, E.; Fox, J.; Cawthorn, S.J.; Patel, A.; Snowdon, C.F.; Hall, E.; et al. Intergroup Exemestane Study g. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): A randomised controlled study. Lancet Oncol. 2007, 8, 119–127. [Google Scholar]
- Eastell, R.; Hannon, R.A.; Cuzick, J.; Dowsett, M.; Clack, G.; Adams, J.E. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J. Bone Miner. Res. 2006, 21, 1215–1223. [Google Scholar] [CrossRef]
- Howell, A.; Cuzick, J.; Baum, M.; Buzdar, A.; Dowsett, M.; Forbes, J.F.; Hoctin-Boes, G.; Houghton, J.; Locker, G.Y.; Tobias, J.S.; et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005, 365, 60–62. [Google Scholar] [PubMed]
- Kaunitz, A.M.; Arias, R.; McClung, M. Bone density recovery after depot medroxyprogesterone acetate injectable contraception use. Contraception 2008, 77, 67–76. [Google Scholar] [CrossRef]
- Hornstein, M.D.; Surrey, E.S.; Weisberg, G.W.; Casino, L.A.; Lupron Add-Back Study Group. Leuprolide acetate depot and hormonal add-back in endometriosis: A 12-month study. Lupron Add-Back Study Group. Obstet. Gynecol. 1998, 91, 16. [Google Scholar] [CrossRef]
- Surrey, E.S.; Judd, H.L. Reduction of vasomotor symptoms and bone mineral density loss with combined norethindrone and long-acting gonadotropin-releasing hormone agonist therapy of symptomatic endometriosis: A prospective randomized trial. J. Clin. Endocrinol. Metab. 1992, 75, 558. [Google Scholar]
- Matsuo, H. Prediction of the change in bone mineral density induced by gonadotropin-releasing hormone agonist treatment for endometriosis. Fertil. Steril. 2004, 81, 149. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Bernatsky, S.; Abrahamowicz, M.; Papaioannou, A.; Bessette, L.; Adachi, J.; Goltzman, D.; Prior, J.; Kreiger, N.; Towheed, T.; et al. Antidepressant use and 10-year incident fracture risk: The population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos. Int. 2014, 25, 1473–1481. [Google Scholar] [CrossRef]
- Triant, V.A.; Brown, T.T.; Lee, H.; Grinspoon, S.K. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J. Clin. Endocrinol. Metab. 2008, 93, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Ginsberg, J.S.; Burrows, R.F.; Duku, E.K.; Webber, C.E.; Brill-Edwards, P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb. Haemost. 1996, 75, 254–257. [Google Scholar]
- Lim, L.S.; Fink, H.A.; Kuskowski, M.A.; Taylor, B.C.; Schousboe, J.T.; Ensrud, K.E. Osteoporotic Fractures in Men Study G. Loop diuretic use and increased rates of hip bone loss in older men: The Osteoporotic Fractures in Men Study. Arch. Intern. Med. 2008, 168, 735–740. [Google Scholar] [CrossRef]
- Seo, J.W.; Lee, D.Y.; Yoon, B.K.; Choi, D. Effects of long-term postoperative dienogest use for treatment of endometriosis on bone mineral density. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 9–12. [Google Scholar] [CrossRef]
- Nappi, C.; Bifulco, G.; Tommaselli, G.A.; Gargano, V.; Di Carlo, C. Hormonal contraception and bone metabolism: A systematic review. Contraception 2012, 86, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, L.K. Hormonal Contraception and Bone Health in Adolescents. Front. Endocrinol. 2020, 11, 603. [Google Scholar] [CrossRef]
- Scholes, D.; Hubbard, R.A.; Ichikawa, L.; LaCroix, A.Z.; Spangler, L.; Beasley, J.M.; Reed, S.; Ott, S.M. Oral contraceptive use and bone density change in adolescent and young adult women: A prospective study of age, hormone dose, and discontinuation. J. Clin. Endocrinol. Metab. 2011, 96, E1380–E1387. [Google Scholar] [CrossRef] [PubMed]
- Harel, Z.; Johnson, C.C.; Gold, M.A.; Cromer, B.; Peterson, E.; Burkman, R.; Stager, M.; Brown, R.; Bruner, A.; Coupey, S.; et al. Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception 2010, 81, 281–291. [Google Scholar] [CrossRef]
- Watts, N.B.; Binkley, N.; Owens, C.D.; Al-Hendy, A.; Puscheck, E.E.; Shebley, M.; Schlaff, W.D.; Simon, J.A. Bone Mineral Density Changes Associated with Pregnancy, Lactation, and Medical Treatments in Premenopausal Women and Effects Later in Life. J. Womens Health 2021, 30, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Charde, S.H.; Joshi, A.; Raut, J. A Comprehensive Review on Postmenopausal Osteoporosis in Women. Cureus 2023, 15, e48582. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Christiansen, C.; Riis, B.J.; Rødbro, P. Prediction of rapid bone loss in postmenopausal women. Lancet 1987, 1, 1105–1108. [Google Scholar] [CrossRef]
- Johnell, O. The socioeconomic burden of fractures: Today and in the 21st century. Am. J. Med. 1997, 103, S20–S26. [Google Scholar] [CrossRef]
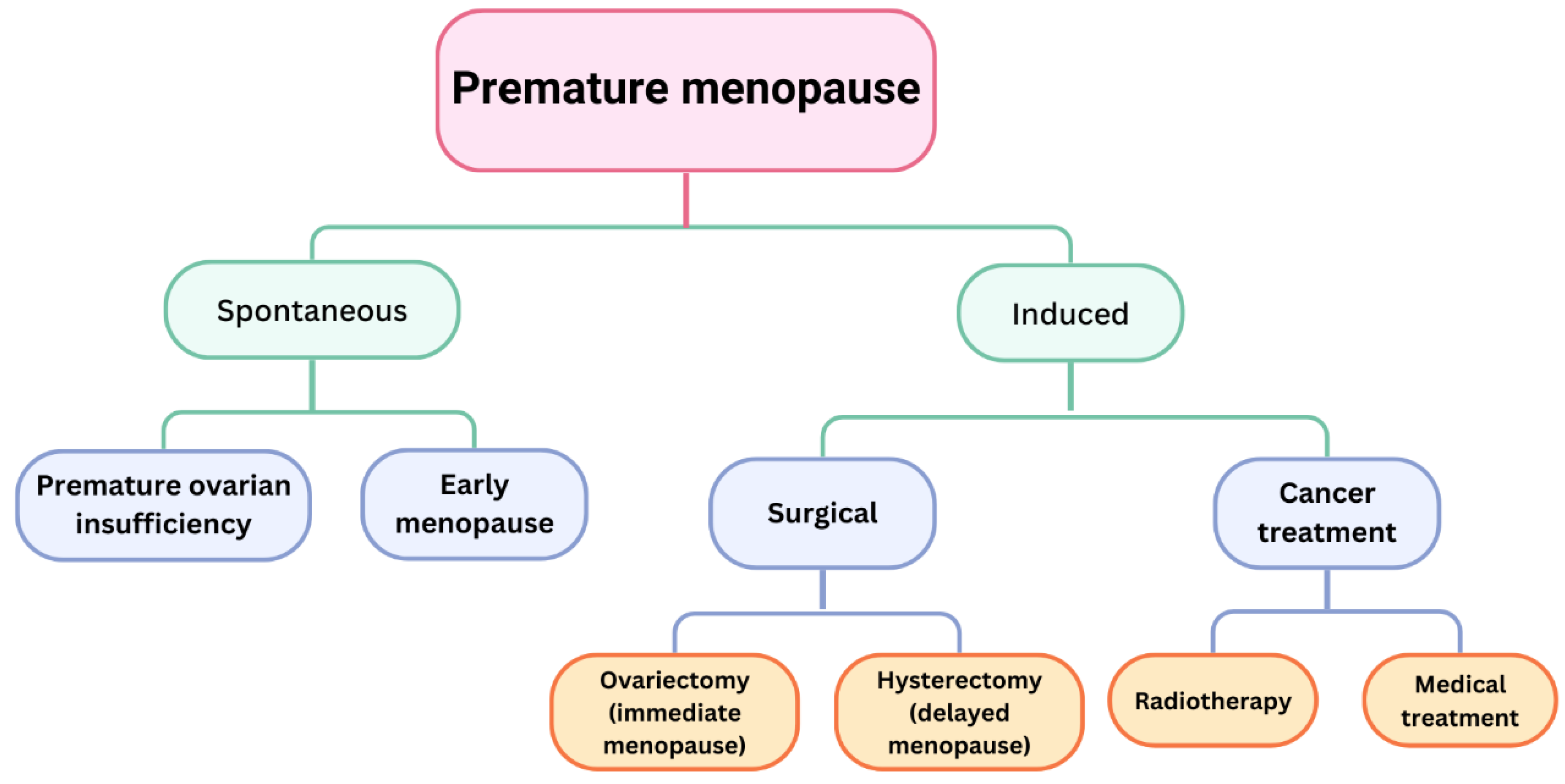
- Hamoda, H.; Sharma, A. Premature ovarian insufficiency, early menopause, and induced menopause. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101823. [Google Scholar] [CrossRef]
- Anagnostis, P.; Lambrinoudaki, I.; Goulis, D.G. Is Early Menopause a Different Entity from Premature Ovarian Insufficiency? Clin. Endocrinol. 2025, 102, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Svejme, O.; Ahlborg, H.G.; Nilsson, J.Å.; Karlsson, M.K. Early menopause and risk of osteoporosis, fracture and mortality: A 34-year prospective observational study in 390 women. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 810–816. [Google Scholar] [CrossRef]
- Anagnostis, P.; Siolos, P.; Gkekas, N.K.; Kosmidou, N.; Artzouchaltzi, A.M.; Christou, K.; Paschou, S.A.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; et al. Association between age at menopause and fracture risk: A systematic review and meta-analysis. Endocrine 2019, 63, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Shieh, A.; Ruppert, K.M.; Greendale, G.A.; Lian, Y.; Cauley, J.A.; Burnett-Bowie, S.A.; Karvonen-Guttierez, C.; Karlamangla, A.S. Associations of Age at Menopause with Postmenopausal Bone Mineral Density and Fracture Risk in Women. J. Clin. Endocrinol. Metab. 2022, 107, e561–e569. [Google Scholar] [CrossRef] [PubMed]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef]
- Available online: https://www.medicalnewstoday.com/articles/317681#causes (accessed on 1 January 2025).
- Langenberg, P.; Kjerulff, K.H.; Stolley, P.D. Hormone replacement and menopausal symptoms following hysterectomy. Am. J. Epidemiol. 1997, 146, 870–880. [Google Scholar] [CrossRef]
- Matsuno, K.; Ueda, K.; Saito, M.; Kamii, M.; Tsuda, A.; Kawabata, A.; Morikawa, A.; Okamoto, A. Pilot study of the effect of surgical menopause on bone mineral density and quality in patients with gynecological malignancies. J. Obstet. Gynaecol. Res. 2025, 51, e16141. [Google Scholar] [CrossRef]
- Hibler, E.A.; Kauderer, J.; Greene, M.H.; Rodriguez, G.C.; Alberts, D.S. Bone loss after oophorectomy among high-risk women: An NRG oncology/gynecologic oncology group study. Menopause 2016, 23, 1228–1232. [Google Scholar] [CrossRef]
- Guise, T.A. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef]
- Alawi, M.; Begum, A.; Harraz, M.; Alawi, H.; Bamagos, S.; Yaghmour, A.; Hafiz, L. Dual-Energy X-Ray Absorptiometry (DEXA) Scan Versus Computed Tomography for Bone Density Assessment. Cureus 2021, 13, e13261. [Google Scholar] [CrossRef]
- Miller, P.D.; Siris, E.S.; Barrett-Connor, E.; Faulkner, K.G.; Wehren, L.E.; Abbott, T.A.; Chen, Y.T.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: Evidence from the National Osteoporosis Risk Assessment. J. Bone Miner. Res. 2002, 17, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Fusco, S.; Gazzotti, S.; Albano, D.; Bonaccorsi, G.; Guglielmi, G.; Bazzocchi, A. DXA beyond bone mineral density and the REMS technique: New insights for current radiologists practice. Radiol. Med. 2024, 129, 1224–1240. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Broy, S.B.; Boutroy, S.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J. Clin. Densitom. 2015, 18, 309–330. [Google Scholar] [CrossRef]
- Chaisen, M.; Sritara, C.; Chitrapazt, N.; Suppasilp, C.; Chamroonrat, W.; Promma, S.; Kositwattanarerk, A.; Sakulpisuti, C.; Thamnirat, K. Opportunistic Screening for Osteoporosis by CT as Compared with DXA. Diagnostics 2024, 14, 2846. [Google Scholar] [CrossRef]
- Tong, X.; Fang, X.; Wang, S.; Fan, Y.; Wei, W.; Xiao, Q.; Chen, A.; Liu, Y.; Liu, L. Opportunistic screening for osteoporosis using enhanced images based on dual-energy computed tomography material decomposition: A comparison with quantitative computed tomography. Quant. Imaging Med. Surg. 2024, 14, 352–364. [Google Scholar] [CrossRef]
- Gazzotti, S.; Aparisi Gómez, M.P.; Schileo, E.; Taddei, F.; Sangiorgi, L.; Fusaro, M.; Miceli, M.; Guglielmi, G.; Bazzocchi, A. High-resolution peripheral quantitative computed tomography: Research or clinical practice? Br. J. Radiol. 2023, 96, 20221016. [Google Scholar] [CrossRef]
- Grimal, Q.; Laugier, P. Quantitative Ultrasound Assessment of Cortical Bone Properties Beyond Bone Mineral Density. IRBM 2019, 40, 16–24. [Google Scholar] [CrossRef]
- Hans, D.B.; Kanis, J.A.; Baim, S.; Bilezikian, J.P.; Binkley, N.; Cauley, J.A.; Compston, J.E.; Cooper, C.; Dawson-Hughes, B.; Fuleihan, G.E.; et al. Joint Official Positions of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. Executive Summary of the 2010 Position Development Conference on Interpretation and use of FRAX® in clinical practice. J. Clin. Densitom. 2011, 14, 171–180. [Google Scholar] [CrossRef]
- Chanprasertpinyo, W.; Punsawad, C.; Khwanchuea, R.; Sukkriang, N.; Yincharoen, P.; Rerkswattavorn, C. Comparison between calcaneus quantitative ultrasound and the gold standard DXA in the ability to detect osteoporosis in chronic obstructive pulmonary disease patients. J. Orthop. Surg. Res. 2023, 18, 778. [Google Scholar] [CrossRef]
- Oral, A.; Esmaeilzadeh, S.; Yalıman, A.; Sindel, D.; Kürsüz Köseoğlu, P.; Aydın, T. The ability of calcaneal and multisite quantitative ultrasound variables in the identification of osteoporosis in women and men. Turk. J. Phys. Med. Rehabil. 2019, 65, 203–215. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status-A Current Picture. Diagnostics 2023, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/staying-healthy-after-menopause (accessed on 5 January 2025).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Available online: https://www.nhs.uk/live-well/bone-health/food-for-strong-bones/ (accessed on 5 January 2025).
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyère, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos. Int. 2017, 28, 447–462. [Google Scholar]
- Farsinejad-Marj, M.; Saneei, P.; Esmaillzadeh, A. Dietary magnesium intake, bone mineral density and risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 1389–1399. [Google Scholar] [CrossRef]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.C.; de Groot, L. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Chevalley, T. Nutrition and Osteoporosis Prevention. Curr. Osteoporos. Rep. 2024, 22, 515–522. [Google Scholar] [CrossRef]
- Ahn, H.; Park, Y.K. Sugar-sweetened beverage consumption and bone health: A systematic review and meta-analysis. Nutr. J. 2021, 20, 41. [Google Scholar] [CrossRef]
- Bose, S.; Sharan, K. Effect of probiotics on postmenopausal bone health: A preclinical meta-analysis. Br. J. Nutr. 2024, 131, 567–580. [Google Scholar] [CrossRef]
- Vanitchanont, M.; Vallibhakara, S.A.; Sophonsritsuk, A.; Vallibhakara, O. Effects of Multispecies Probiotic Supplementation on Serum Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, W.; Liu, P.J. Effects of probiotic supplementation on bone health in postmenopausal women: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1487998. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, M.; Zhang, Q. The effectiveness of combined exercise interventions for preventing postmenopausal Bone loss: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 241–251. [Google Scholar] [CrossRef]
- Bloch-Ibenfeldt, M.; Gates, A.T.; Jørgensen, N.R.; Linneberg, A.; Aadahl, M.; Kjær, M.; Boraxbekk, C.J. Heavy resistance training provides short-term benefits on bone formation in well-functioning older adults. Bone 2025, 193, 117393. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Shojaa, M.; Kohl, M.; von Stengel, S. Effects of different types of exercise on Bone mineral Density in postmenopausal women: A systematic review and meta-analysis. Calcif. Tissue Int. 2020, 107, 409–439. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Chiu, C.Y.; Chen, W.C.; Yang, Y.R.; Wang, R.Y. Effects of exercise on bone density and physical performance in postmenopausal women: A systematic review and meta-analysis. PM R 2024, 16, 1358–1383. [Google Scholar] [CrossRef]
- Gosset, A.; Pouillès, J.M.; Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101551. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, R.; Zhang, L.; Sun, X.; Wang, Y. Effectiveness and safety of hormone replacement therapy in the treatment of menopausal syndrome: A meta-analysis. Am. J. Transl. Res. 2025, 17, 1–15. [Google Scholar] [CrossRef]
- Karim, R.; Dell, R.M.; Greene, D.F.; Mack, W.J.; Gallagher, J.C.; Hodis, H.N. Hip fracture in postmenopausal women after cessation of hormone therapy: Results from a prospective study in a large health management organization. Menopause 2011, 18, 1172–1177. [Google Scholar] [CrossRef]
- Weiss, S.R.; Ellman, H.; Dolker, M. A randomized controlled trial of four doses of transdermal estradiol for preventing postmenopausal bone loss. Transdermal Estradiol Investigator Group. Obstet. Gynecol. 1999, 94, 330–336. [Google Scholar] [CrossRef]
- Delmas, P.D.; Pornel, B.; Felsenberg, D.; Garnero, P.; Hardy, P.; Pilate, C.; Dain, M.P. A dose-ranging trial of a matrix transdermal 17beta-estradiol for the prevention of bone loss in early postmenopausal women. International Study Group. Bone 1999, 24, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, J.; Vaheri, R.; Kainulainen, P.; Timonen, U. Long-term continuous combined hormone replacement therapy in the prevention of postmenopausal bone loss: A comparison of high- and low-dose estrogen-progestin regimens. Osteoporos. Int. 2000, 11, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Gallagher, J.C.; Kleerekoper, M.; Pickar, J.H. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos. Int. 2005, 16, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Gallagher, J.C.; Kleerekoper, M.; Pickar, J.H. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002, 287, 2668–2676. [Google Scholar] [CrossRef]
- Gass, M.; Liu, J.; Rebar, R.W. The effect of low-dose conjugated equine estrogens and cyclic MPA on bone density. Maturitas 2002, 41, 143–147. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, X.; Sun, Y.; Shu, W. Effect of hormone therapy on the risk of bone fractures: A systematic review and meta-analysis of randomized controlled trials. Menopause 2016, 23, 461–470. [Google Scholar] [CrossRef]
- Engel, P.; Fabre, A.; Fournier, A.; Mesrine, S.; Boutron-Ruault, M.C.; Clavel-Chapelon, F. Risk of osteoporotic fractures after discontinuation of menopausal hormone therapy: Results from the E3N cohort. Am. J. Epidemiol. 2011, 174, 12–21. [Google Scholar] [CrossRef]
- Bagger, Y.Z.; Tankó, L.B.; Alexandersen, P.; Hansen, H.B.; Møllgaard, A.; Ravn, P.; Qvist, P.; Kanis, J.A.; Christiansen, C. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: The PERF study. Bone 2004, 34, 728–735. [Google Scholar] [CrossRef]
- Papadakis, G.; Hans, D.; Gonzalez-Rodriguez, E.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.M.; Lamy, O. The Benefit of Menopausal Hormone Therapy on Bone Density and Microarchitecture Persists After its Withdrawal. J. Clin. Endocrinol. Metab. 2016, 101, 5004–5011. [Google Scholar] [CrossRef]
- Anagnostis, P.; Divaris, E.; Bosdou, J.Κ.; Tournis, S.; Stathopoulos, K.; Goulis, D.G. Antiosteoporosis therapy after discontinuation of menopausal hormone therapy: A systematic review. Hormones 2024, 23, 339–344. [Google Scholar] [CrossRef]
- Harper-Harrison, G.; Carlson, K.; Shanahan, M.M. Hormone Replacement Therapy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Crandall, C.J.; Mehta, J.M.; Manson, J.E. Management of Menopausal Symptoms: A Review. JAMA 2023, 329, 405–420. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef] [PubMed]
- Tremollieres, F.A.; Pouilles, J.M.; Ribot, C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos. Int. 2001, 12, 385–390. [Google Scholar] [CrossRef]
- Anagnostis, P.; Bosdou, J.K.; Vaitsi, K.; Goulis, D.G.; Lambrinoudaki, I. Estrogen and bones after menopause: A reappraisal of data and future perspectives. Hormones 2021, 20, 13–21. [Google Scholar] [CrossRef]
- Eriksen, E.F. Hormone replacement therapy or SERMS in the long term treatment of osteoporosis. Minerva Ginecol. 2012, 64, 207–221. [Google Scholar] [PubMed]
- Brown, C. Staying strong. Nature 2017, 550, S15–S17. [Google Scholar] [CrossRef]
- Burr, D.B.; Phipps, R. Selective Estrogen Receptor Modulators (SERMs). In Osteoporotic Fracture and Systemic Skeletal Disorders: Mechanism, Assessment, and Treatment; Takahashi, H.E., Burr, D.B., Yamamoto, N., Eds.; Springer: Singapore, 2022; pp. 399–411. [Google Scholar]
- Guañabens, N.; Moro-Álvarez, M.J.; Casado, E.; Blanch-Rubió, J.; Gómez-Alonso, C.; Díaz-Guerra, G.M.; Del Pino-Montes, J.; Valero Díaz de Lamadrid, C.; Peris, P.; Muñoz-Torres, M.; et al. The next step after anti-osteoporotic drug discontinuation: An up-to-date review of sequential treatment. Endocrine 2019, 64, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Delgado, L.; Castelán-Martínez, O.D.; Clark, P.; Garduño-Espinosa, J.; Mendoza-Núñez, V.M.; Sánchez-Rodríguez, M.A. Effect of Tibolone on Bone Mineral Density in Postmenopausal Women: Systematic Review and Meta-Analysis. Biology 2021, 10, 211. [Google Scholar] [CrossRef]
- Jayasena, C.N.; Alkaabi, F.M.; Liebers, C.S.; Handley, T.; Franks, S.; Dhillo, W.S. A systematic review of randomized controlled trials investigating the efficacy and safety of testosterone therapy for female sexual dysfunction in postmenopausal women. Clin. Endocrinol. 2019, 90, 391–414. [Google Scholar] [CrossRef]
- Elraiyah, T.; Sonbol, M.B.; Wang, Z.; Khairalseed, T.; Asi, N.; Undavalli, C.; Nabhan, M.; Firwana, B.; Altayar, O.; Prokop, L.; et al. Clinical review: The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3543–3550. [Google Scholar] [CrossRef]
- Miller, B.E.; De Souza, M.J.; Slade, K.; Luciano, A.A. Sublingual administration of micronized estradiol and progesterone, with and without micronized testosterone: Effect on biochemical markers of bone metabolism and bone mineral density. Menopause 2000, 7, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Young, R.; Notelovitz, M.; Sullivan, J.; Wiita, B.; Yang, H.M.; Nolan, J. A two-year, double-blind comparison of estrogen-androgen and conjugated estrogens in surgically menopausal women. Effects on bone mineral density, symptoms and lipid profiles. J. Reprod. Med. 1999, 44, 1012–1020. [Google Scholar] [PubMed]
- Davis, S.R.; McCloud, P.; Strauss, B.J.; Burger, H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 1995, 21, 227–236. [Google Scholar] [CrossRef]
- Frazzetta, G. Effect of Testosterone Pellet Therapy on Bone Mineral Density in Postmenopausal Women. J. Clin. Densitom. 2023, 26, 101392. [Google Scholar] [CrossRef]

| Genetic diseases | Idiopathic juvenile osteoporosis Osteogenesis imperfecta Osteoporosis—pseudoglioma syndrome (OPPG) Hereditary enzyme deficiencies Turner syndrome |
| Endocrine diseases | Hyperthyroidism Hypothyroidism Diabetes mellitus Hypogonadism Hyperparathyroidism Cushing syndrome Growth hormone deficiency Acromegalia |
| Gastrointestinal diseases | Celiac disease Inflammatory bowel disease Hemochromatosis Chronic liver diseases Malabsorption syndrome |
| Bariatric surgery | Gastric bypass surgery |
| Eating disorders | Anorexia neurosa Bullimia neurosa |
| Haematological disorders | Monoclonal gammopathy of uncertain significance (MGUS) multiple myeloma Systemic mastocytosis Beta thalassemia major |
| Renal diseases | Idiopathic hypercalciuria Renal tubular acidosis. Chronic kidney disease (CKD) |
| Autoimmune diseases | Rheumatoid arthritis Systemic lupus erythematosus Ankylosing spondylitis Multiple sclerosis |
| Vitamin D3 | Vitamin D3 deficiency |
| Infection diseases | Tuberculosis |
| Glucocorticoids | Up to 3 months use of high doses (the process is reversible) |
| Thyroid hormones | Thyrotoxicosis facticia |
| Aromatase inhibitors | Prolonged use results in diminished oestrogen levels, thereby causing bone loss. [21,22,23] |
| Medroxyprogesterone acetate | Inhibits gonadotropin secretion, suppresses ovarian oestrogen production, and leads to a decrease in BMD (process is reversible) [24] |
| Gonadotropin releasing hormone (GnRH) agonists | Suppress oestrogen levels and thus lead to a decrease in BMD; significant BMD loss can occur after 3–6 months of therapy [25,26,27] (the process is reversible) |
| Antidepressants | More pronounced effect in patients over 50 years of age: they reduce BMD and increase fracture risk [28] |
| Anticonvulsants | Greater decrease in BMD in women aged ≥65 years |
| Thiazolidinediones | Associated with an increased risk of fractures |
| Drugs with actions on the immune system | Calcineurin inhibitors—unknown mechanism Antiretroviral therapy—3- to 7-fold increase in the risk of osteoporosis and increased risk of fractures [29] |
| Anticoagulants | One third of patients taking long-term heparin have reduced BMD [30] |
| Diuretics | Loop diuretics lead to reduced BMD and an increased risk of osteoporotic fractures [31]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Vasileva-Slaveva, M.; Tsoneva, E.; Kostov, S.; Yanachkova, V. Bone Health for Gynaecologists. Medicina 2025, 61, 530. https://doi.org/10.3390/medicina61030530
Yordanov A, Vasileva-Slaveva M, Tsoneva E, Kostov S, Yanachkova V. Bone Health for Gynaecologists. Medicina. 2025; 61(3):530. https://doi.org/10.3390/medicina61030530
Chicago/Turabian StyleYordanov, Angel, Mariela Vasileva-Slaveva, Eva Tsoneva, Stoyan Kostov, and Vesselina Yanachkova. 2025. "Bone Health for Gynaecologists" Medicina 61, no. 3: 530. https://doi.org/10.3390/medicina61030530
APA StyleYordanov, A., Vasileva-Slaveva, M., Tsoneva, E., Kostov, S., & Yanachkova, V. (2025). Bone Health for Gynaecologists. Medicina, 61(3), 530. https://doi.org/10.3390/medicina61030530








