Continuous Non-Invasive Hemodynamic Monitoring in Cirrhotic Patients—Friend or Foe?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minimally Invasive Monitoring
2.2. Continuous Non-Invasive Monitoring
2.3. Statistics
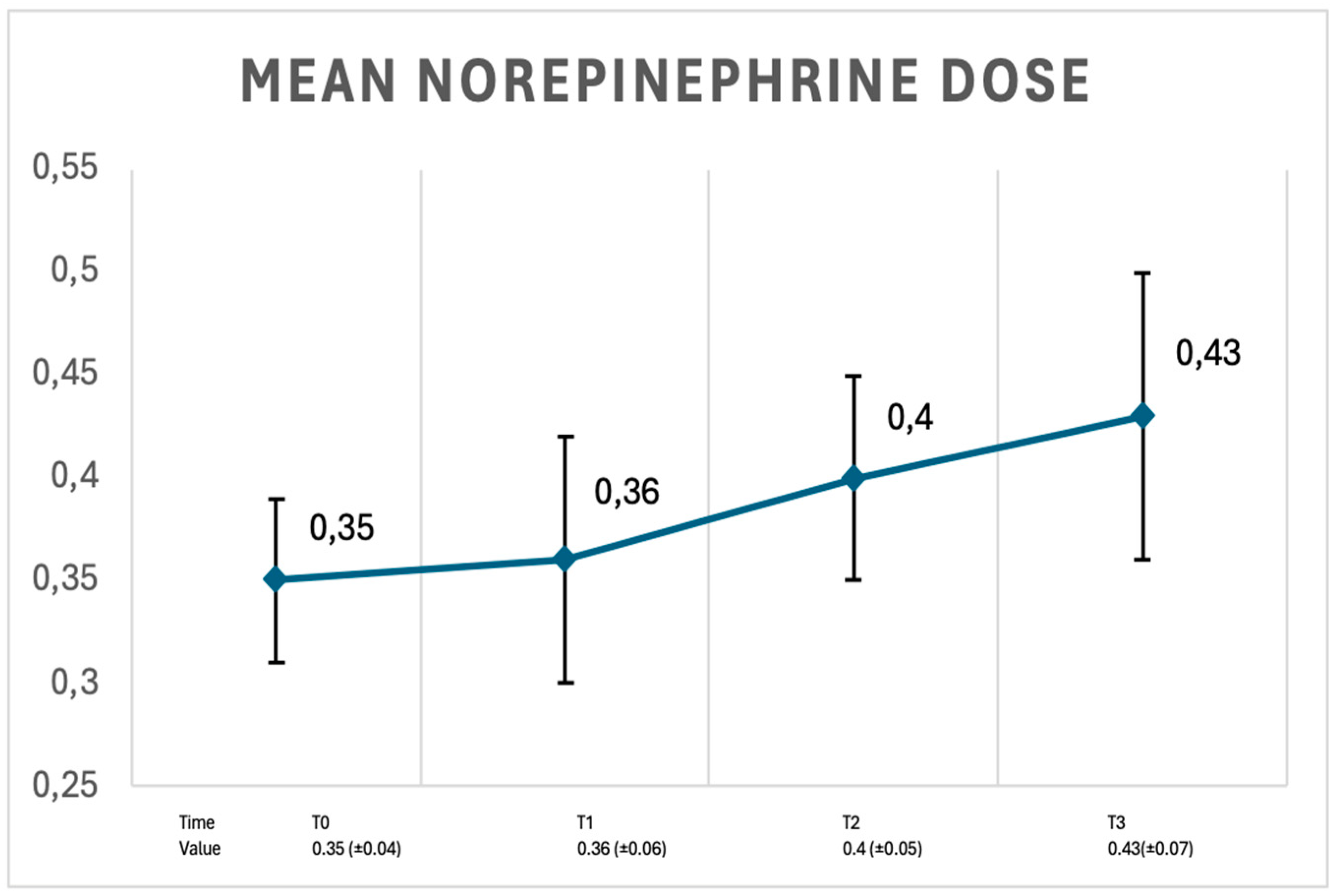
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| CO | cardiac output |
| CI | cardiac index |
| ICU | intensive care unit |
| SV | stroke volume |
| SVI | indexed stroke volume |
| SVV | stroke volume variation |
| SVR | systemic vascular resistance |
| SVRI | indexed systemic vascular resistance |
| MAP | mean arterial pressure |
| PAC | pulmonary artery catheterization |
References
- Giuseppetti, G.M.; Argalia, G.; Abbattista, T. Liver cirrhosis: Evaluation of haemodynamic changes using an ultrasound contrast agent. Eur. J. Radiol. 2004, 51, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.M.; Busk, T.M.; Møller, S. Validation of non-invasive haemodynamic methods in patients with liver disease: The Finometer and the Task Force Monitor. Clin. Physiol. Funct. Imaging 2018, 38, 384–389. [Google Scholar] [CrossRef]
- Han, S.; Park, J.; Hong, S.H.; Park, C.S.; Choi, J.; Chae, M.S. Cardiovascular manifestation of end-stage liver disease and perioperative echocardiography for liver transplantation: Anesthesiologist’s view. Anesth. Pain. Med. 2022, 17, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lee, S.S. Cirrhotic cardiomyopathy: Getting to the heart of the matter. Hepatology 1996, 24, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Groszmann, R.J. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology 2006, 43, S121–S131. [Google Scholar] [CrossRef]
- Frank, P.; Logemann, F.; Gras, C.; Palmaers, T. Noninvasive continuous arterial pressure monitoring during anesthesia induction in patients undergoing cardiac surgery. Ann. Card. Anaesth. 2021, 24, 281–287. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, H.J.; Hwang, I.E.; Lee, H.C.; Kim, W.H.; Yang, S.M. Comparison of invasive and non-invasive measurements of cardiac index and systemic vascular resistance in living-donor liver transplantation: A prospective, observational study. BMC Anesthesiol. 2023, 23, 359. [Google Scholar] [CrossRef]
- Truijen, J.; van Lieshout, J.J.; Wesselink, W.A.; Westerhof, B.E. Noninvasive continuous hemodynamic monitoring. J. Clin. Monit. Comput. 2012, 26, 267–278. [Google Scholar] [CrossRef]
- Saugel, B.; Hoppe, P.; Nicklas, J.Y.; Kouz, K.; Körner, A.; Hempel, J.C.; Vos, J.J.; Schön, G.; Scheeren, T.W.L. Continuous noninvasive pulse wave analysis using finger cuff technologies for arterial blood pressure and cardiac output monitoring in perioperative and intensive care medicine: A systematic review and meta-analysis. Br. J. Anaesth. 2020, 125, 25–37. [Google Scholar] [CrossRef]
- Saugel, B.; Cecconi, M.; Wagner, J.Y.; Reuter, D.A. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br. J. Anaesth. 2015, 114, 562–575. [Google Scholar] [CrossRef]
- Saugel, B.; Reuter, D.A. Are we ready for the age of non-invasive haemodynamic monitoring? Br. J. Anaesth. 2014, 113, 340–343. [Google Scholar] [CrossRef]
- Salzwedel, C.; Puig, J.; Carstens, A.; Bein, B.; Molnar, Z.; Kiss, K.; Hussain, A.; Belda, J.; Kirov, M.Y.; Sakka, S.G.; et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: A multi-center, prospective, randomized study. Crit. Care 2013, 17, R191. [Google Scholar] [CrossRef]
- Møller, S.; Henriksen, J.H.; Bendtsen, F. Pathogenetic background for treatment of ascites and hepatorenal syndrome. Hepatol. Int. 2008, 2, 416–428. [Google Scholar] [CrossRef]
- Tan, B.G.; Tang, Z.; Ou, J.; Zhou, H.Y.; Li, R.; Chen, T.W.; Zhang, X.M.; Li, H.J. An improved model based on quantitative features of right liver lobe, maximum varices, and portal vein system measured on magnetic resonance imaging to predict oesophagogastric variceal haemorrhage secondary to hepatitis B-related cirrhosis. Quant. Imaging Med. Surg. 2023, 13, 7741–7752. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Henriksen, J.H. Cardiovascular complications of cirrhosis. Postgrad. Med. J. 2009, 85, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Imai, T.; Takeda, C.; Mizota, T.; Kawamoto, S. Agreement between continuous cardiac output measured by the fourth generation FloTrac/Vigileo system and a pulmonary artery catheter in adult liver transplantation. Sci. Rep. 2022, 12, 11198. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamamoto, T.; Fuse, M.; Kobayashi, N.; Takeda, S.; Aoyagi, T. Pulse dye densitometry using indigo carmine is useful for cardiac output measurement, but not for circulating blood volume measurement. Eur. J. Anaesthesiol. 2004, 21, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Ogura, Y.; Onishi, Y.; Kamei, H.; Kurata, N.; Kainuma, M.; Takahashi, H.; Suzuki, S.; Ichikawa, T.; Mizuno, S.; et al. Systemic hemodynamics in advanced cirrhosis: Concerns during perioperative period of liver transplantation. World J. Hepatol. 2016, 8, 1047–1060. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Maeda, T.; Miyazaki, E.; Hotta, N.; Sato, H.; Hamaguchi, E.; Kanazawa, H.; Ohnishi, Y.; Kamei, M. Accuracy of the ClearSight™ system in patients undergoing abdominal aortic aneurysm surgery. J. Anesth. 2019, 33, 364–371. [Google Scholar] [CrossRef]
- Maeda, T.; Hattori, K.; Sumiyoshi, M.; Kanazawa, H.; Ohnishi, Y. Accuracy and trending ability of the fourth generation FloTrac/Vigileo System™ in patients undergoing abdominal aortic aneurysm surgery. J. Anesth. 2018, 32, 387–393. [Google Scholar] [CrossRef]
- Kobe, J.; Mishra, N.; Arya, V.K.; Al-Moustadi, W.; Nates, W.; Kumar, B. Cardiac output monitoring: Technology and choice. Ann. Card. Anaesth. 2019, 22, 6–17. [Google Scholar] [PubMed]
- Hofer, C.K.; Cannesson, M. Monitoring fluid responsiveness. Acta Anaesthesiol. Taiwan. 2011, 49, 59–65. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.; Kim, H.; Kim, Y.J.; Kim, M.; Choi, J.H. Accuracy of noninvasive continuous arterial pressure monitoring using ClearSight during one-lung ventilation. Medicine 2021, 100, e25152. [Google Scholar] [CrossRef]
- Alhenc-Gelas, F. A new channel for the control of renin secretion in juxtaglomerular cells. Kidney Int. 2020, 98, 543–545. [Google Scholar] [CrossRef]
- Kamath, S.A.; Drazner, M.H.; Tasissa, G.; Rogers, J.G.; Stevenson, L.W.; Yancy, C.W. Correlation of impedance cardiography with invasive hemodynamic measurements in patients with advanced heart failure: The BioImpedance CardioGraphy (BIG) substudy of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial. Am. Heart J. 2009, 158, 217–223. [Google Scholar] [PubMed]
- Wang, Y.; Huang, W.; Han, J.; Tian, Y.; Wang, C.; Li, L. A comparison of ClearSight noninvasive cardiac output and pulmonary artery bolus thermodilution cardiac output in cardiac surgery patients. Perioper. Med. 2022, 11, 24. [Google Scholar] [CrossRef]
- Teboul, J.L.; Saugel, B.; Cecconi, M.; De Backer, D.; Hofer, C.K.; Monnet, X.; Perel, A.; Pinsky, M.R.; Reuter, D.A.; Rhodes, A.; et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016, 42, 1350–1359. [Google Scholar] [CrossRef]
- Monnet, X.; Lai, C. Which haemodynamic monitoring should we chose for critically ill patients with acute circulatory failure? Curr. Opin. Crit. Care. 2023, 29, 275–280. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Cecconi, M.; Chew, M.S.; Hajjar, L.; Monnet, X.; Ospina-Tascón, G.A.; Ostermann, M.; Pinsky, M.R.; Vincent, J.L. A plea for personalization of the hemodynamic management of septic shock. Crit. Care 2022, 26, 372. [Google Scholar] [CrossRef]
- Yamada, T.; Vacas, S.; Gricourt, Y.; Cannesson, M. Improving Perioperative Outcomes Through Minimally Invasive and Non-invasive Hemodynamic Monitoring Techniques. Front. Med. 2018, 5, 144. [Google Scholar] [CrossRef]
- Pour-Ghaz, I.; Manolukas, T.; Foray, N.; Raja, J.; Rawal, A.; Ibebuogu, U.N.; Khouzam, R.N. Accuracy of non-invasive and minimally invasive hemodynamic monitoring: Where do we stand? Ann. Transl. Med. 2019, 7, 421. [Google Scholar] [CrossRef] [PubMed]

| Age (years) | 58.8 (±11.77) |
| ASA II/III (n, %) | 22 (43.13)/29(56.86) |
| Gender F/M (n, %) | 15 (29.4)/36(70.6) |
| Etiology of cirrhosisEthanolic/viral/autoimmune (n, %) | 30 (58.8)/18(35.2)/3(5.8) |
| Total bilirubin (mg/dL) | 10.86 (±12.07) |
| GOT/GPT (U/L) | 850 (±1760)/427 (±764) |
| Cholinesterase (U/L) | 2292 (±1033) |
| Fibrinogen (mg/dL) | 202 (±111) |
| Albumin (g/dL) | 2.96 (±0.39) |
| International normalized ratio (INR) | 2.29 (±1.10) |
| Platelets (μL) | 111,666 (±75,133) |
| SOFA score | 13 (±5) |
| Apache II score | 25 (±14) |
| MELD-Na score | 22 (±11) |
| Child–Pugh A/B/C (n, %) | 4 (7.84)/18 (35.28)/29 (56.84) |
| Acute kidney injury stage I/II/III (AKI) (n, %) | 21 (41.16)/29 (56.84)/10 (19.6) |
| CLIF-C ACLF grade 1/2/3 (Acute-on-Chronic Liver Failure) (n, %) | 7 (13.72)/13 (25.48)/31 (60.76) |
| Measurement | Parameter | FloTrac | ClearSight | p |
|---|---|---|---|---|
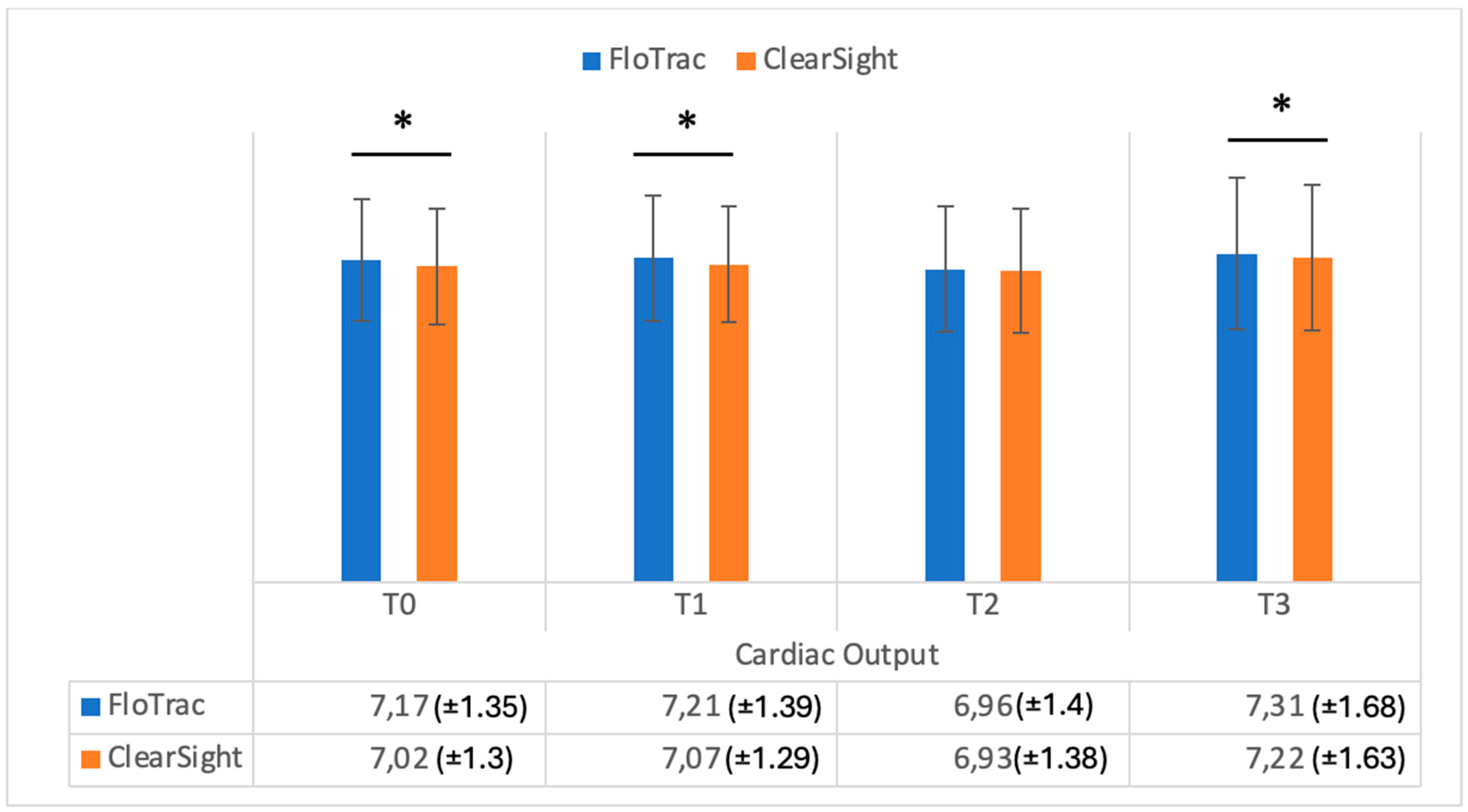
| T0 | CO | 7.17 (±1.35) | 7.02 (±1.30) | 0.001 |
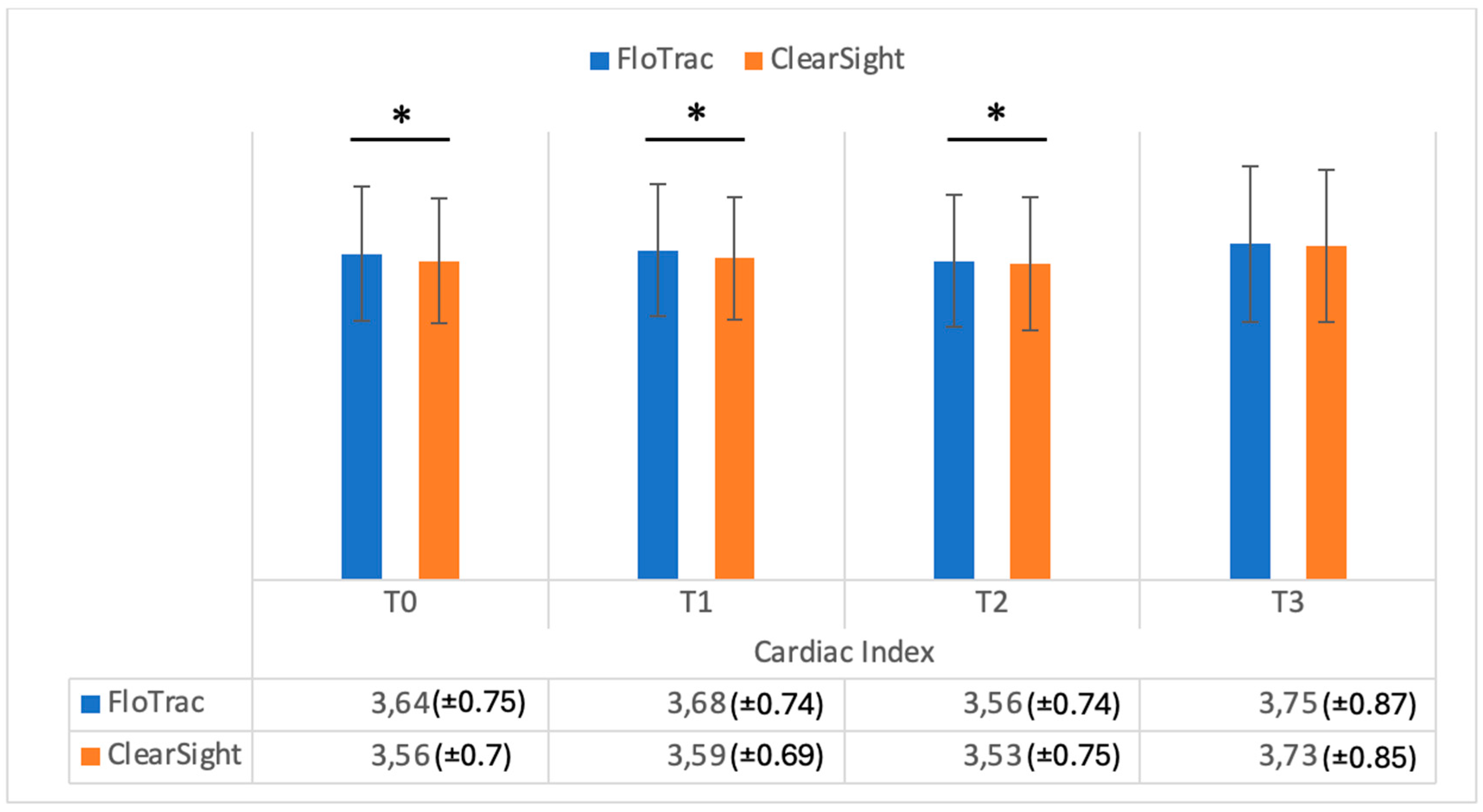
| CI | 3.64 (±0.75) | 3.56 (±0.70) | 0.001 | |
| SV | 55.76 (±13.63) | 54.47 (±13.71) | 0.001 | |
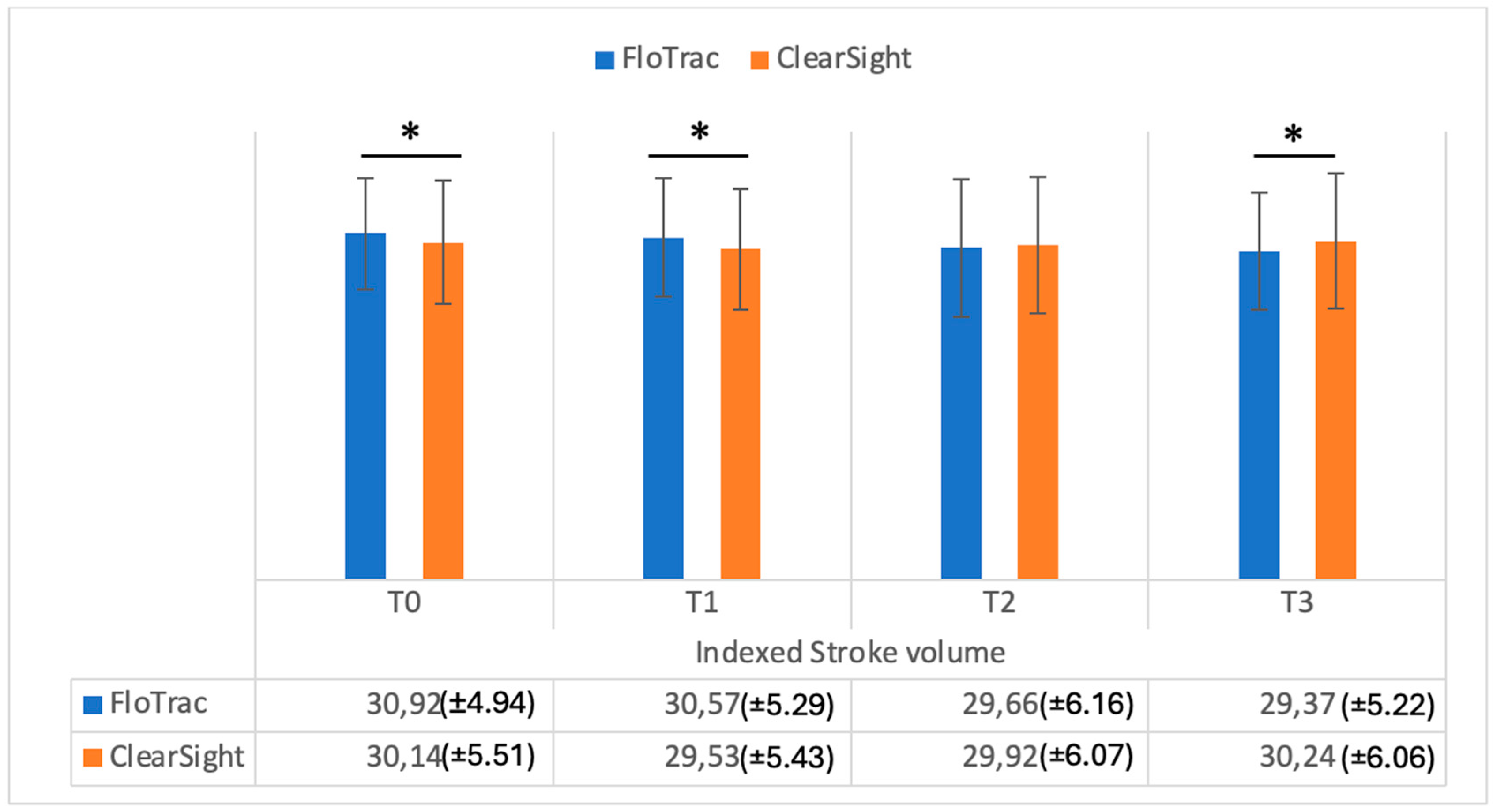
| SVI | 30.92 (±4.94) | 30.14 (±5.51) | 0.001 | |
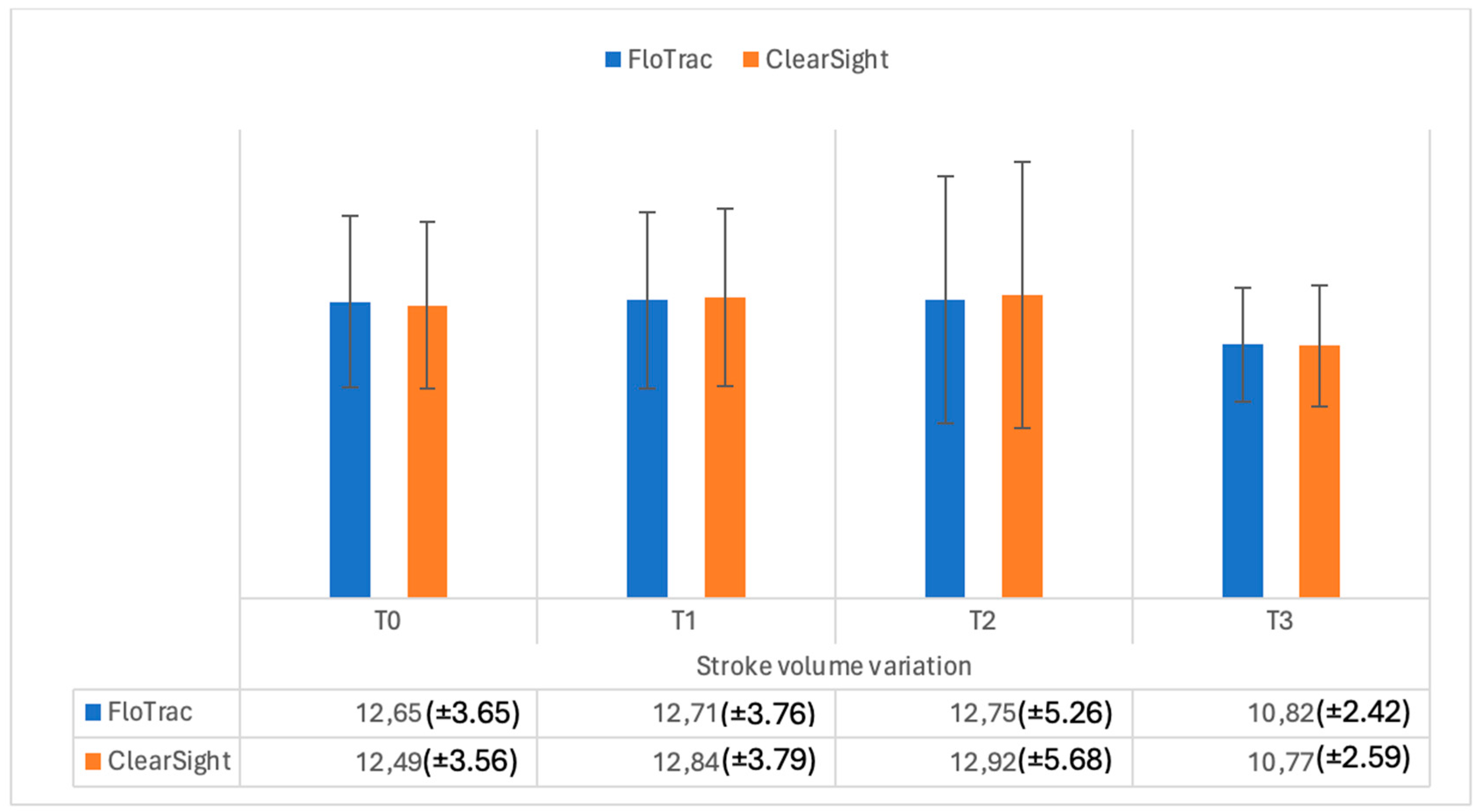
| SVV | 12.65 (±3.65) | 12.49 (±3.56) | 0.29 | |
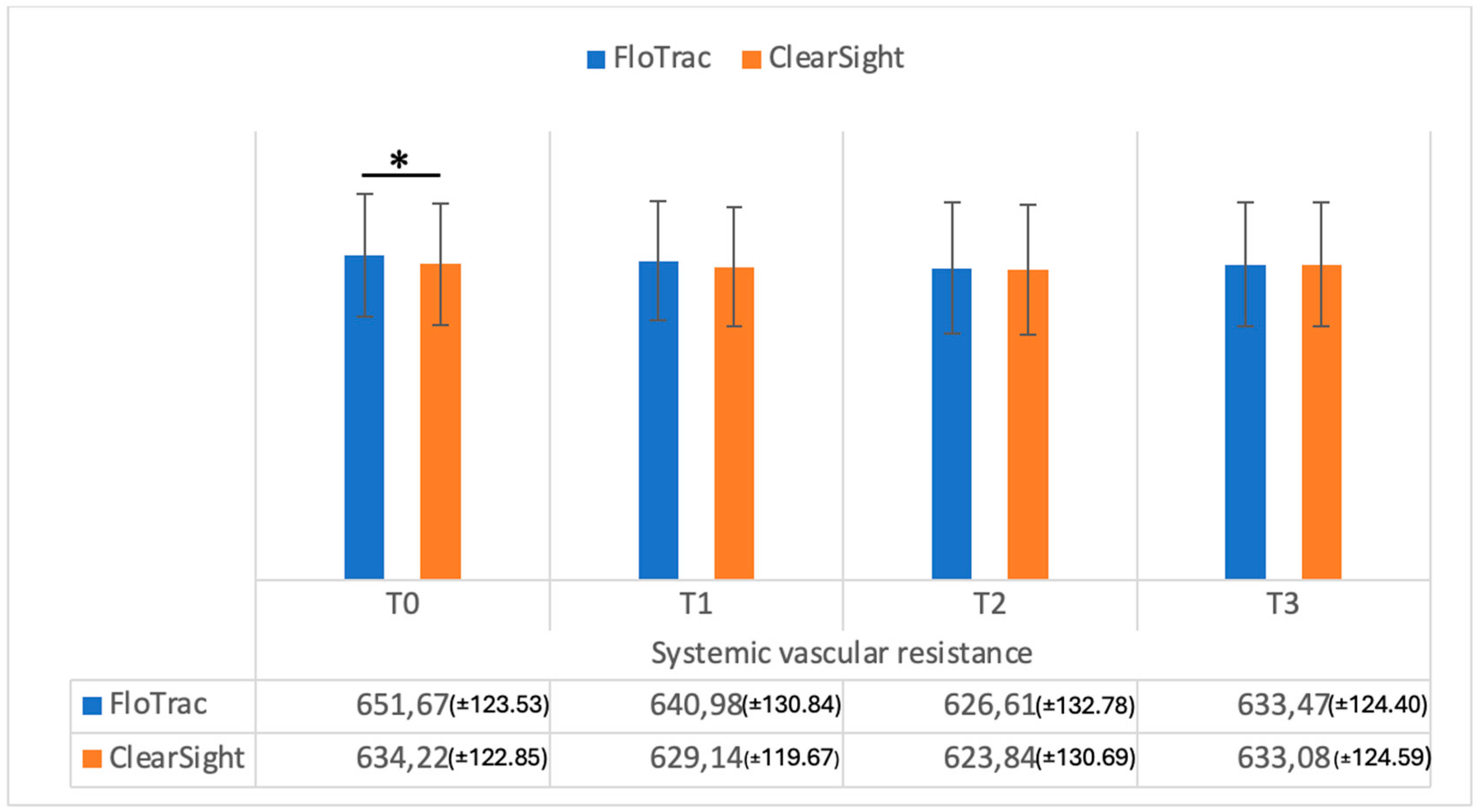
| SVR | 651.67 (±123.53) | 634.22 (±122.85) | 0.001 | |
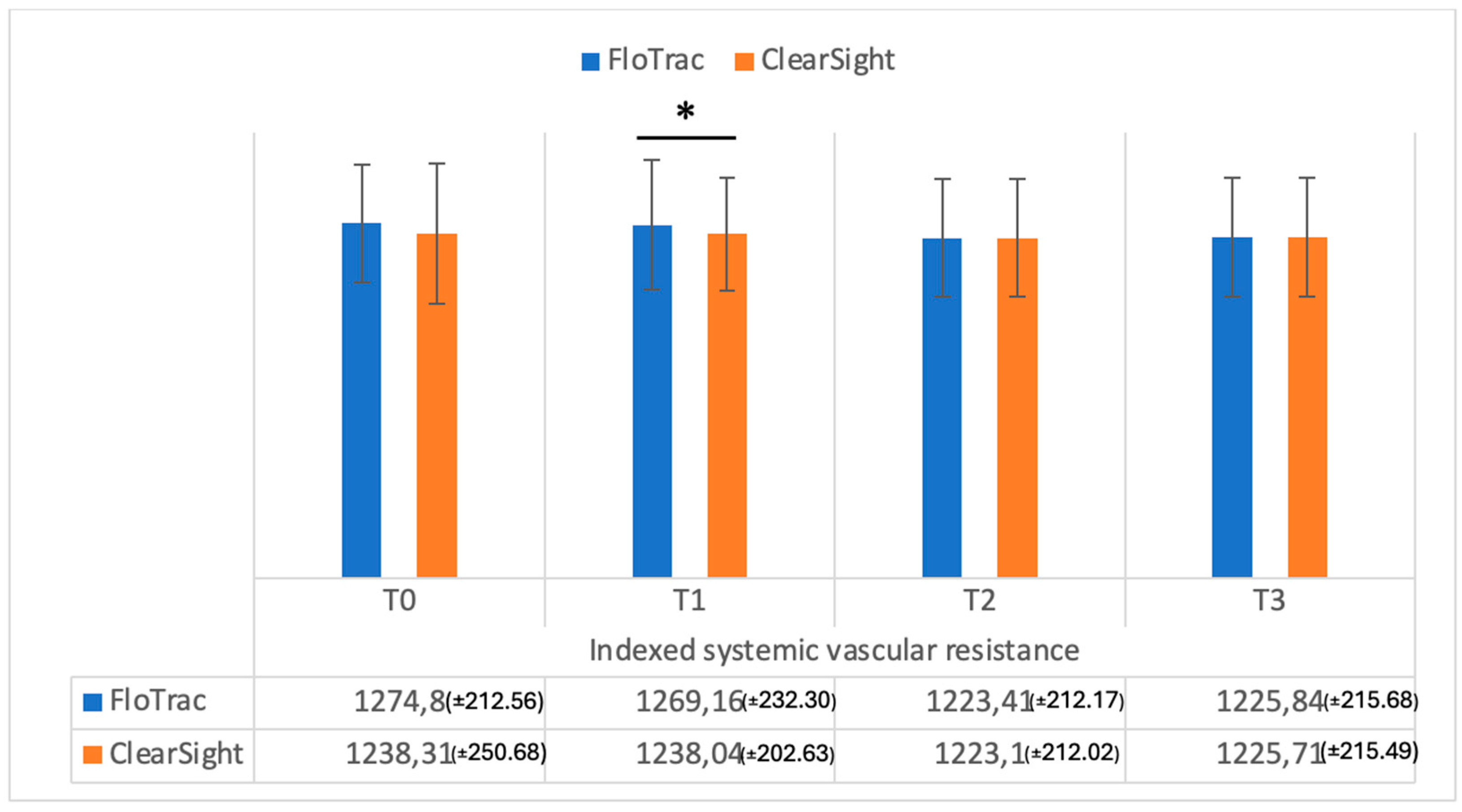
| SVRI | 1274.8 (±212.5) | 1238.31 (±250.68) | 0.09 | |
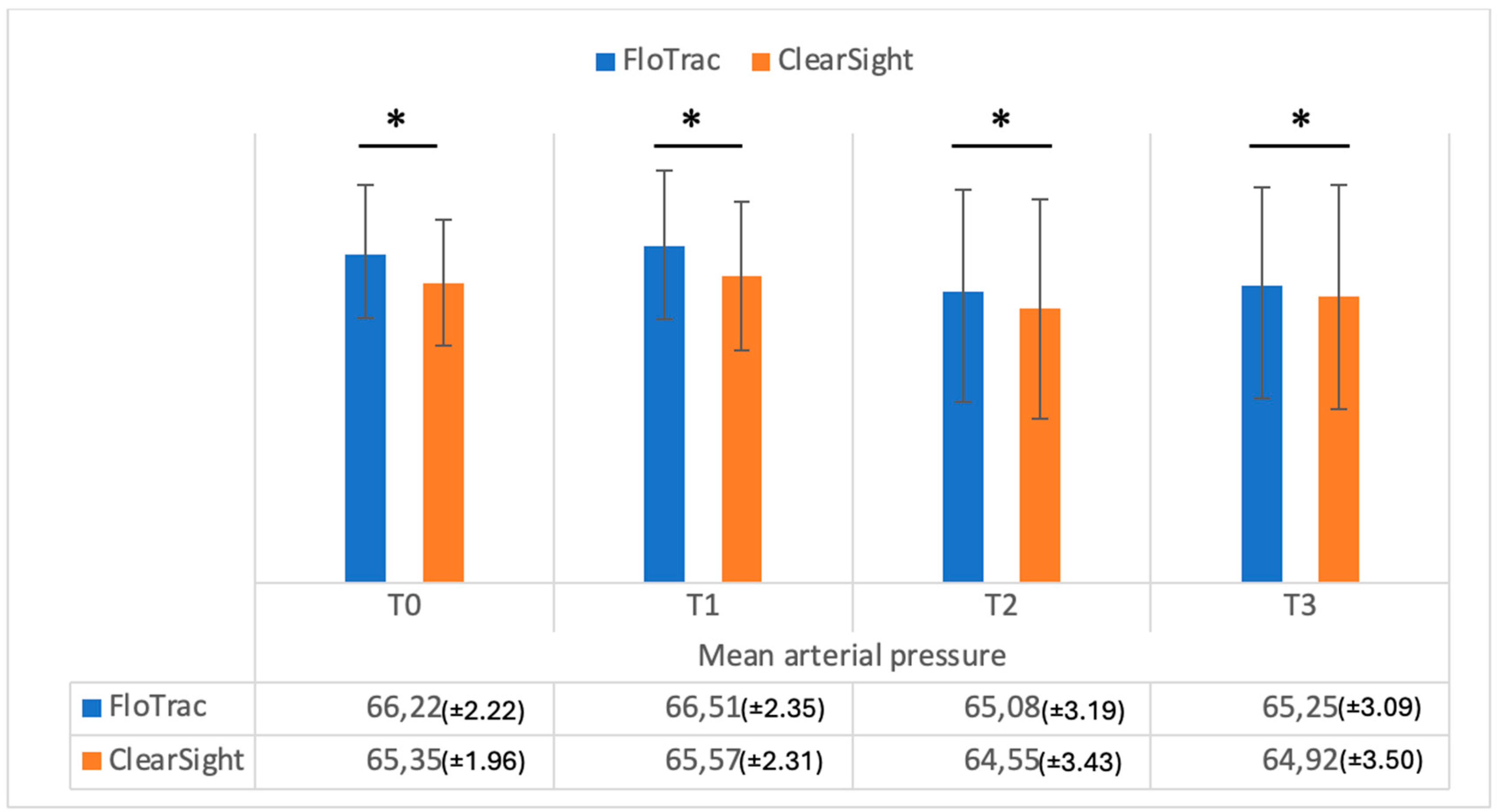
| MAP | 66.22 (±2.20) | 65.35 (±1.96) | 0.001 | |
| T1 | CO | 7.21 (±1.39) | 7.07 (±1.29) | 0.002 |
| CI | 3.68 (±0.74) | 3.59 (±0.69) | 0.001 | |
| SV | 56.27 (±14.26) | 55.29 (±13.52) | 0.002 | |
| SVI | 30.57 (±5.29) | 29.53 (±5.43) | 0.001 | |
| SVV | 12.71 (±3.76) | 12.84 (±3.79) | 0.41 | |
| SVR | 640.98 (±130.84) | 629.14 (±119.67) | 0.07 | |
| SVRI | 1269.16 (±232.30) | 1238.04 (±202.63) | 0.01 | |
| MAP | 66.51 (±2.35) | 65.57 (±2.31) | 0.001 | |
| T2 | CO | 6.96 (±1.40) | 6.93 (±1.38) | 0.1 |
| CI | 3.56 (±0.74) | 3.53 (±0.75) | 0.03 | |
| SV | 54.92 (±13.96) | 54.16 (±14.52) | 0.13 | |
| SVI | 29.66 (±6.16) | 29.92 (±6.07) | 0.13 | |
| SVV | 12.75 (±5.26) | 12.92 (±5.68) | 0.36 | |
| SVR | 626.61 (±132.78) | 623.84 (±130. 69) | 0.26 | |
| SVRI | 1223.41 (±212.17) | 1223.10 (±212.02) | 0.37 | |
| MAP | 65.08 (±3.19) | 64.55 (±3.43) | 0.001 | |
| T3 | CO | 7.31 (±1.68) | 7.22 (±1.63) | 0.002 |
| CI | 3.75 (±0.87) | 3.73 (±0.85) | 0.24 | |
| SV | 58.88 (±16.18) | 58.78 (±16.48) | 0.49 | |
| SVI | 29.37 (±5.22) | 30.24 (±6.06) | 0.02 | |
| SVV | 10.82 (±2.42) | 10.77 (±2.59) | 0.69 | |
| SVR | 633.47 (±124.40) | 633.08 (±124.59) | 0.25 | |
| SVRI | 1225.84 (±215.68) | 1225.71 (±215.49) | 0.55 | |
| MAP | 65.25 (±3.09) | 64.92 (±3.50) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crihan, M.; Alexa, A.L.; Valean, D.; Ionescu, D. Continuous Non-Invasive Hemodynamic Monitoring in Cirrhotic Patients—Friend or Foe? Medicina 2025, 61, 536. https://doi.org/10.3390/medicina61030536
Crihan M, Alexa AL, Valean D, Ionescu D. Continuous Non-Invasive Hemodynamic Monitoring in Cirrhotic Patients—Friend or Foe? Medicina. 2025; 61(3):536. https://doi.org/10.3390/medicina61030536
Chicago/Turabian StyleCrihan, Mirela, Alexandru Leonard Alexa, Dan Valean, and Daniela Ionescu. 2025. "Continuous Non-Invasive Hemodynamic Monitoring in Cirrhotic Patients—Friend or Foe?" Medicina 61, no. 3: 536. https://doi.org/10.3390/medicina61030536
APA StyleCrihan, M., Alexa, A. L., Valean, D., & Ionescu, D. (2025). Continuous Non-Invasive Hemodynamic Monitoring in Cirrhotic Patients—Friend or Foe? Medicina, 61(3), 536. https://doi.org/10.3390/medicina61030536





