Breaking Barriers—The Promise and Challenges of Limb Osseointegration Surgery
Abstract
1. Introduction
2. Heterogeneity in the Limb Amputation Population
3. Varied Implant Technologies and Clinician Access
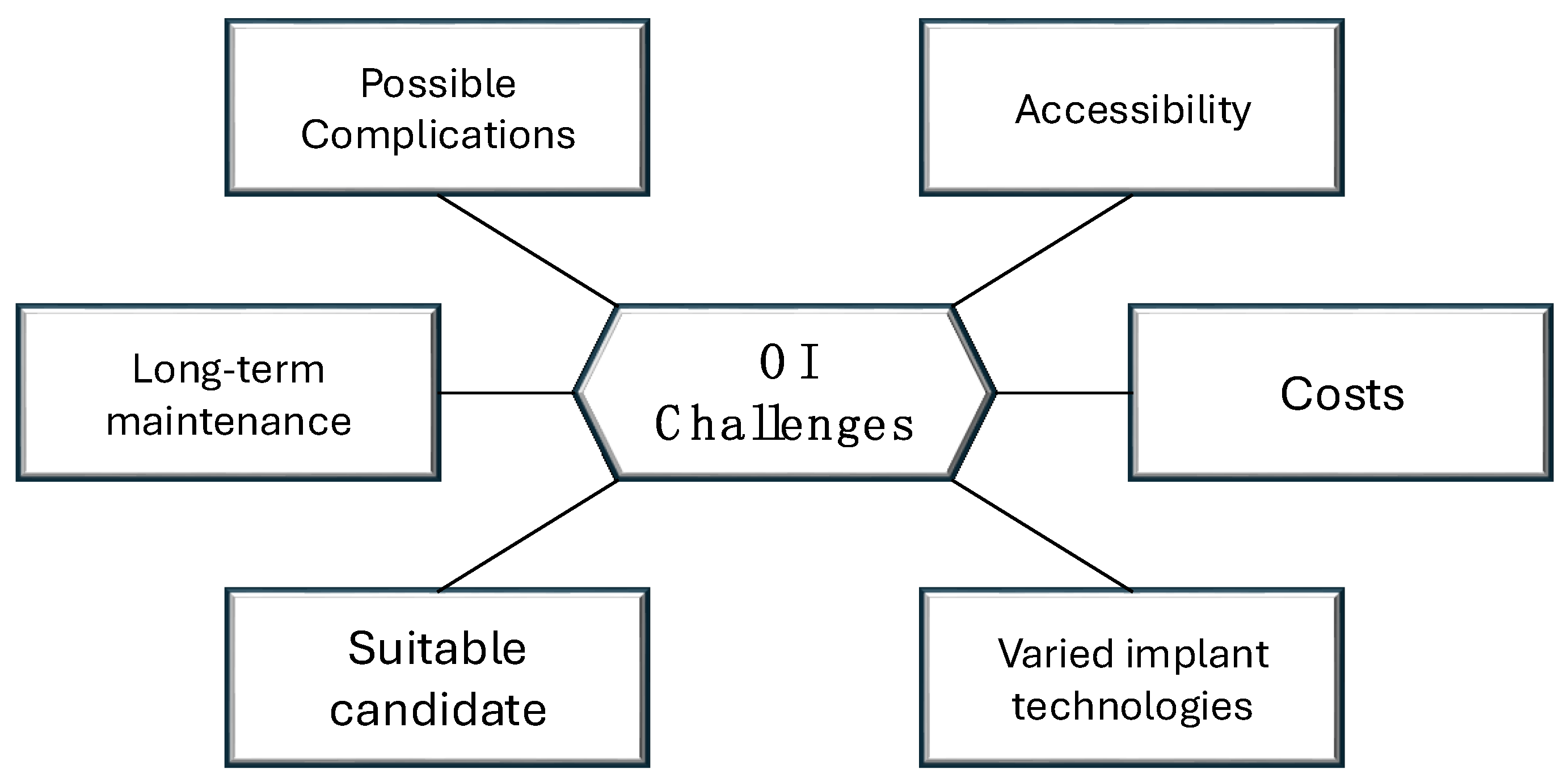
4. Potential Risks and Complications
5. Patient Selection for Limb Osseointegration: Key Considerations
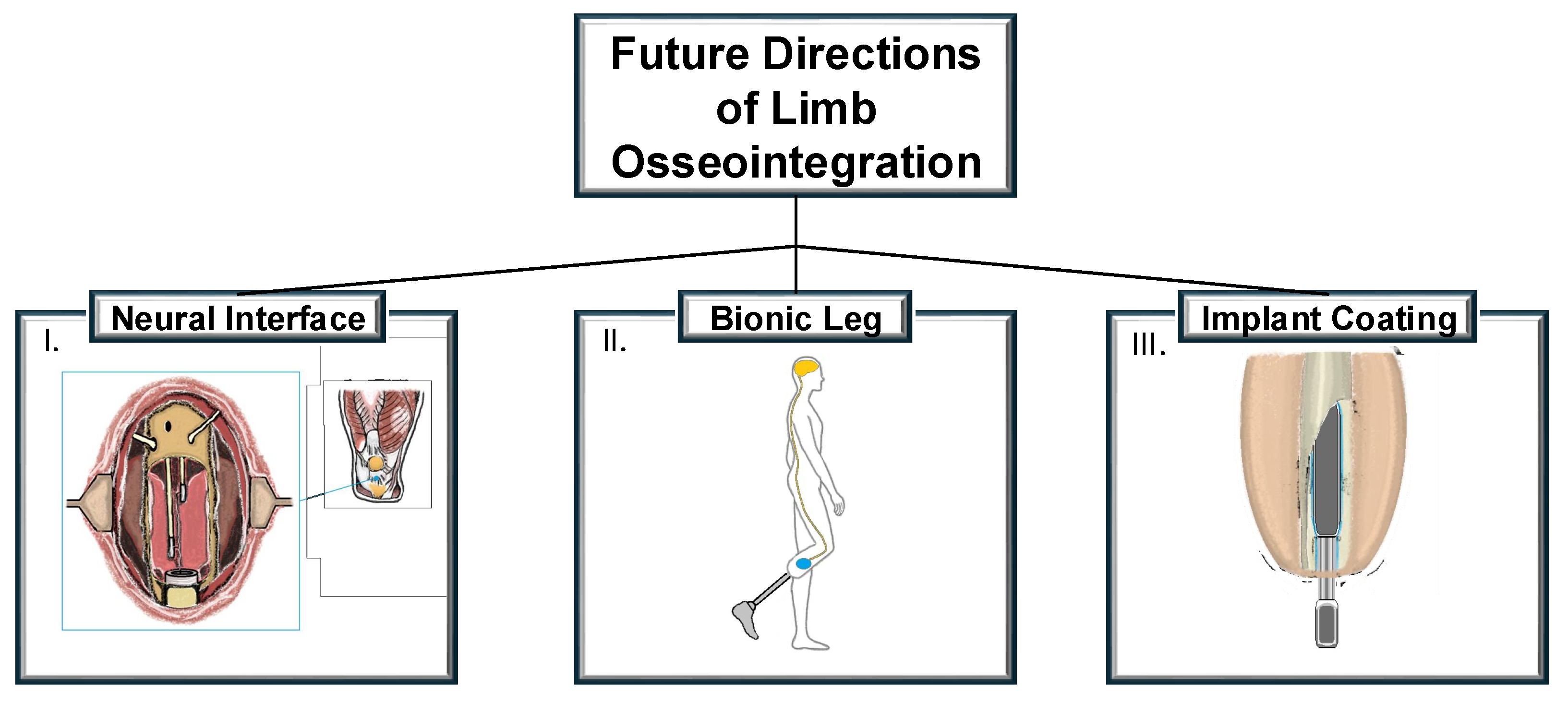
6. Innovations and Future Directions
7. Conclusions
Future Perspectives
- -
- To build a strong evidence base, it is essential to fund and conduct large-scale, multi-center trials that track the long-term outcomes of osseointegration.
- -
- Unified, evidence-based rehabilitation protocols should be developed to ensure consistency in post-surgical care and improve patient recovery outcomes. These guidelines should encompass both physical rehabilitation and psychological support, as adapting to the new prosthetic system can be a challenging process.
- -
- Governments and healthcare systems need to recognize the long-term benefits of osseointegration and explore funding options to make this technology more accessible to patients. Public–private partnerships could be key in making osseointegration more affordable while also advancing research into lower-cost, equally effective implant options.
- -
- The future of amputee rehabilitation will require the seamless integration of osseointegration with other technologies such as targeted muscle reinnervation, myoelectric sensors, and sensory feedback systems. A coordinated approach that combines these innovations could significantly improve the quality of life for an amputee, offering them greater independence and functionality.
Author Contributions
Funding
Conflicts of Interest
References
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, D.; Gu, S.; Xiao, S.; Song, F. The global burden of traumatic amputation in 204 countries and territories. Front. Public Health 2023, 11, 1258853. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Belsi, A.; McGregor, A.H. Issues faced by people with amputation(s) during lower limb prosthetic rehabilitation: A thematic analysis. Prosthet. Orthot. Int. 2022, 46, 61–67. [Google Scholar] [CrossRef]
- Pezzin, L.E.; Dillingham, T.R.; Mackenzie, E.J.; Ephraim, P.; Rossbach, P. Use and satisfaction with prosthetic limb devices and related services. Arch. Phys. Med. Rehabil. 2004, 85, 723–729. [Google Scholar] [CrossRef] [PubMed]
- DadeMatthews, O.O.; Roper, J.A.; Vazquez, A.; Shannon, D.M.; Sefton, J.M. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet. Orthot. Int. 2024, 48, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brånemark, R. Osseointegrated prostheses for rehabilitation following amputation: The pioneering Swedish model. Unfallchirurg 2017, 120, 285–292. [Google Scholar] [CrossRef]
- Baumann, M.; Price, C.; Brousseau, L.; Loftsgaarden, M.; Powell, J.; Sanders, J.; Childers, W.L. The relationship between residual limb health, motion within the socket, and prosthetic suspension. PM R 2023, 15, 510–521. [Google Scholar] [CrossRef]
- Pospiech, P.T.; Wendlandt, R.; Aschoff, H.H.; Ziegert, S.; Schulz, A.P. Quality of life of persons with transfemoral amputation: Comparison of socket prostheses and osseointegrated prostheses. Prosthet. Orthot. Int. 2021, 45, 20–25. [Google Scholar] [CrossRef]
- Eskridge, S.L.; Dougherty, A.L.; Watrous, J.R.; McCabe, C.T.; Cancio, J.M.; Mazzone, B.N.; Galarneau, M.R. Prosthesis satisfaction and quality of life in US service members with combat-related major lower-limb amputation. Prosthet. Orthot. Int. 2022, 46, 68–74. [Google Scholar] [CrossRef]
- Saleib, R.M.; Pekbay, B.; Verhofstad, M.H.J.; Paping, M.A.; Van Vledder, M.G.; Van Waes, O.J.F. Analyzing research trends and developments in osseointegration in patients with extremity amputations: Systematic bibliometric analysis and research recommendations. Prosthet. Orthot. Int. 2024. [Google Scholar] [CrossRef]
- Hughes, W.; Goodall, R.; Salciccioli, J.D.; Marshall, D.C.; Davies, A.H.; Shalhoub, J. Editor’s Choice—Trends in Lower Extremity Amputation Incidence in European Union 15+ Countries 1990–2017. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Mealy, A.; Tierney, S.; Sorensen, J. Lower extremity amputations in Ireland: A registry-based study. Ir. J. Med. Sci. 2022, 191, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.; Coman, H.; Coșoreanu, A.; Militaru, A.M.; Popescu-Vâlceanu, H.C.; Teodoru, I.; Mihai, D.A.; Elian, V.; Gavan, N.A.; Radulian, G. Incidence of Lower Extremity Amputation in Romania: A Nationwide 5-Year Cohort Study, 2015–2019. Medicina 2023, 59, 1199. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Thomas, G.N.; Gill, P.; Chan, C.; Torella, F. Lower limb amputation in England: Prevalence, regional variation and relationship with revascularisation, deprivation and risk factors. A retrospective review of hospital data. J. R. Soc. Med. 2014, 107, 483–489. [Google Scholar] [CrossRef]
- Evans, D.R.; Lazarides, A.L.; Visgauss, J.D.; Somarelli, J.A.; Blazer, D.G., 3rd; Brigman, B.E.; Eward, W.C. Limb salvage versus amputation in patients with osteosarcoma of the extremities: An update in the modern era using the National Cancer Database. BMC Cancer 2020, 20, 995. [Google Scholar] [CrossRef]
- Sume, B.W.; Geneti, S.A. Determinant Causes of Limb Amputation in Ethiopia: A Systematic Review and Meta-Analysis. Ethiop. J. Health Sci. 2023, 33, 891–902. [Google Scholar] [CrossRef]
- Xu, J.; Haider, A.; Sheikh, A.; González-Fernández, M. Epidemiology and Impact of Limb Loss in the United States and Globally. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 679–690. [Google Scholar] [CrossRef]
- Thanni, L.O.; Tade, A.O. Extremity amputation in Nigeria—A review of indications and mortality. Surgeon 2007, 5, 213–217. [Google Scholar] [CrossRef]
- Al-Ajlouni, Y.A.; Abouzid, M.; Tanashat, M.; Basheer, A.A.; Al Ta’ani, O.; Bilgin-Badur, N.; Islam, M. Temporal trends in lower extremity amputation in Middle East and North Africa (MENA) region: Analysis of the GBD dataset 1990–2019. Int. J. Equity Health 2024, 23, 178. [Google Scholar] [CrossRef]
- Riandini, T.; Pang, D.; Toh, M.P.H.S.; Tan, C.S.; Choong, A.M.T.L.; Lo, Z.J.; Chandrasekar, S.; Tai, E.S.; Tan, K.B.; Venkataraman, K. National Rates of Lower Extremity Amputation in People With and Without Diabetes in a Multi-Ethnic Asian Population: A Ten Year Study in Singapore. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 147–155. [Google Scholar] [CrossRef]
- Hassan Al Imam, M.; Alamgir, H.; Jahan Akhtar, N.; Hossain, Z.; Islam, R.; Sohrab Hossain, M. Characterisation of persons with lower limb amputation who attended a tertiary rehabilitation centre in Bangladesh. Disabil. Rehabil. 2020, 42, 1995–2001. [Google Scholar] [CrossRef]
- Unnikrishnan, E.P.; Rollands, R.; Parambil, S.M. Epidemiology of major limb amputations: A cross sectional study from a South Indian tertiary care hospital. Int. Surg. J. 2017, 4, 1642–1646. [Google Scholar]
- Chung, H.J.; Chun, D.I.; Kang, E.M.; Kim, K.; Lee, J.; Jeon, Y.J.; Cho, J.; Won, S.; Yi, Y. Trend and Seasonality of Diabetic Foot Amputation in South Korea: A Population-Based Nationwide Study. Int. J. Environ. Res. Public Health 2022, 19, 4111. [Google Scholar] [CrossRef]
- Rivera, J.A.; Churovich, K.; Anderson, A.B.; Potter, B.K. Estimating Recent US Limb Loss Prevalence and Updating Future Projections. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100376. [Google Scholar] [CrossRef] [PubMed]
- Portela, F.S.O.; Louzada, A.C.S.; da Silva, M.F.A.; Teivelis, M.P.; Kuzniec, S.; Wolosker, N. Editor’s Choice—Analysis of Lower Limb Amputations in Brazil’s Public Health System over 13 Years. Eur. J. Vasc. Endovasc. Surg. 2024, 68, 91–98. [Google Scholar] [CrossRef]
- Dillon, M.P.; Fortington, L.V.; Akram, M.; Erbas, B.; Kohler, F. Geographic Variation of the Incidence Rate of Lower Limb Amputation in Australia from 2007-12. PLoS ONE 2017, 12, e0170705. [Google Scholar] [CrossRef]
- Juhnke, D.L.; Aschoff, H.H. Endo-Exo-Prothesen nach Gliedmaßenamputation [Endo-exo prostheses following limb-amputation]. Orthopade 2015, 44, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hoellwarth, J.S.; Al-Jawazneh, S.; Oomatia, A.; Tetsworth, K.; Al Muderis, M. Transfemoral Osseointegration for Amputees with Well-Managed Diabetes Mellitus. JB JS Open Access 2024, 9, e23.00168. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Gillatt, D.; Blom, A.W. Systematic review of the safety and efficacy of osseointegration prosthesis after limb amputation. Br. J. Surg. 2018, 105, 1731–1741. [Google Scholar] [CrossRef]
- Thesleff, A.; Brånemark, R.; Håkansson, B.; Ortiz-Catalan, M. Biomechanical characterisation of bone-anchored implant systems for amputation limb prostheses: A systematic review. Ann. Biomed. Eng. 2018, 46, 377–391. [Google Scholar] [CrossRef]
- Muderis, M.A.; Tan, Y.C.; Lu, W.; Tetsworth, K.; Axelrod, D.; Haque, R.; Akhtar, M.A.; Roberts, C.; Doshi, K.; Al-Jawazneh, S.; et al. Transtibial osseointegration following unilateral traumatic amputation: An observational study of patients with at least two years follow-up. Injury 2024, 55, 111568. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Haidary, A.; Tetsworth, K.; Oomatia, A.; Al Muderis, M. Transfemoral Osseointegration in Association With Total Hip Replacement: Observational Cohort Study of Patients With Follow-Up Exceeding 2 Years. Arthroplast. Today 2024, 28, 101463. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Felländer-Tsai, L. The bone anchored prostheses for amputees—Historical development, current status, and future aspects. Biomaterials 2021, 273, 120836. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar] [PubMed]
- Hagberg, K.; Ghassemi Jahani, S.A.; Kulbacka-Ortiz, K.; Thomsen, P.; Malchau, H.; Reinholdt, C. A 15-year follow-up of transfemoral amputees with bone-anchored transcutaneous prostheses. Bone Joint J. 2020, 102, 55–63. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Akhtar, M.A.; Al Muderis, M. The Clinical History and Basic Science Origins of Transcutaneous Osseointegration for Amputees. Adv. Orthop. 2022, 2022, 7960559. [Google Scholar] [CrossRef]
- Aschoff, H.H.; Örgel, M.; Sass, M.; Fischer, D.C.; Mittlmeier, T. Transcutaneous Osseointegrated Prosthesis Systems (TOPS) for Rehabilitation After Lower Limb Loss: Surgical Pearls. JBJS Essent Surg Tech. 2024, 14, e23.00010. [Google Scholar] [CrossRef]
- Al Muderis, M.; Khemka, A.; Lord, S.J.; Van de Meent, H.; Frölke, J.P. Safety of Osseointegrated Implants for Transfemoral Amputees: A Two-Center Prospective Cohort Study. J. Bone Joint Surg. Am. 2016, 98, 900–909. [Google Scholar] [CrossRef]
- Haque, R.; Al-Jawazneh, S.; Hoellwarth, J.; Akhtar, M.A.; Doshi, K.; Tan, Y.C.; Lu, W.Y.; Roberts, C.; Al Muderis, M. Osseointegrated reconstruction and rehabilitation of transtibial amputees: The Osseointegration Group of Australia surgical technique and protocol for a prospective cohort study. BMJ Open 2020, 10, e038346. [Google Scholar] [CrossRef]
- McGough, R.L.; Goodman, M.A.; Randall, R.L.; Forsberg, J.A.; Potter, B.K.; Lindsey, B. The Compress® transcutaneous implant for rehabilitation following limb amputation. Unfallchirurg 2017, 120, 300–305. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Humanitarian Device Exemption (HDE). OPRA. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H080004 (accessed on 15 January 2025).
- Evans, A.R.; Tetsworth, K.; Quinnan, S.; Wixted, J.J. Transcutaneous osseointegration for amputees. OTA Int. 2024, 7 (Suppl. S2), e326. [Google Scholar] [CrossRef] [PubMed]
- Frossard, L.A.; Merlo, G.; Burkett, B.; Quincey, T.; Berg, D. Cost-effectiveness of bone-anchored prostheses using osseointegrated fixation: Myth or reality? Prosthet. Orthot. Int. 2018, 42, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.S.; Rehani, M.; Stiegelmar, R. Osseointegration for Lower-Limb Amputation: A Systematic Review of Clinical Outcomes. JBJS Rev. 2017, 5, e10. [Google Scholar] [CrossRef] [PubMed]
- Black, G.G.; Vaeth, A.M.; Kochheiser, M.; Chen, Y.; Truong, A.Y.; Reif, T.; Rozbruch, S.R.; Henry, M.W.; Otterburn, D.M. Infection After Lower-Limb Osseointegration: A Single-Center Retrospective Evaluation of Pathogens, Management, and Outcomes. Ann. Plast. Surg. 2024, 92 (Suppl. S4), S96–S100. [Google Scholar] [CrossRef]
- Sabaté Brescó, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic Mechanisms and Host Interactions in Staphylococcus epidermidis Device-Related Infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Oomatia, A.; Akhtar, M.A.; Xu, H.; Al Muderis, M. Association Between Osseointegration of Lower Extremity Amputation and Mortality Among Adults. JAMA Netw. Open. 2022, 5, e2235074. [Google Scholar] [CrossRef]
- Alam, S.H.; Hoellwarth, J.S.; Tetsworth, K.; Oomatia, A.; Taylor, T.N.; Al Muderis, M. Development of an evidence-based diagnostic algorithm for infection in patients with transcutaneous osseointegration following amputation. J. Bone Jt. Infect. 2024, 9, 49–57. [Google Scholar] [CrossRef]
- Monument, M.J.; Bernthal, N.M.; Bowles, A.J.; Jones, K.B.; Randall, R.L. What are the 5-year survivorship outcomes of compressive endoprosthetic osseointegration fixation of the femur? Clin. Orthop. Relat. Res. 2015, 473, 883–890. [Google Scholar] [CrossRef]
- Nehler, M.R.; Coll, J.R.; Hiatt, W.R.; Regensteiner, J.G.; Schnickel, G.T.; Klenke, W.A.; Strecker, P.K.; Anderson, M.W.; Jones, D.N.; Whitehill, T.A.; et al. Functional outcome in a contemporary series of major lower extremity amputations. J. Vasc. Surg. 2003, 38, 7–14. [Google Scholar] [CrossRef]
- Groundland, J.; Brown, J.M.; Monument, M.; Bernthal, N.; Jones, K.B.; Randall, R.L. What Are the Long-term Surgical Outcomes of Compressive Endoprosthetic Osseointegration of the Femur with a Minimum 10-year Follow-up Period? Clin. Orthop. Relat. Res. 2022, 480, 539–548. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Kendrew, J.; Kang, N.V.; van Waes, O.; Al-Maawi, Q.; Roberts, C.; Al Muderis, M. Periprosthetic osseointegration fractures are infrequent and management is familiar. Bone Joint J. 2020, 102, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Reetz, D.; van de Meent, H.; Schreuder, H.; Frölke, J.P.; Leijendekkers, R. What Are the Risk Factors for Mechanical Failure and Loosening of a Transfemoral Osseointegrated Implant System in Patients with a Lower-limb Amputation? Clin. Orthop. Relat. Res. 2022, 480, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Wanivenhaus, F.; Mauler, F.; Stelzer, T.; Tschopp, A.; Böni, T.; Berli, M.C. Revision Rate and Risk Factors After Lower Extremity Amputation in Diabetic or Dysvascular Patients. Orthopedics 2016, 39, e149–e154. [Google Scholar] [CrossRef] [PubMed]
- Rennie, C.; Rodriguez, M.; Futch, K.N.; Krasney, L.C. Complications Following Osseointegrated Transfemoral and Transtibial Implants: A Systematic Review. Cureus 2024, 16, e57045. [Google Scholar] [CrossRef]
- Overmann, A.L.; Forsberg, J.A. The state of the art of osseointegration for limb prosthesis. Biomed. Eng. Lett. 2019, 10, 5–16. [Google Scholar] [CrossRef]
- McMenemy, L.; Ramasamy, A.; Sherman, K.; Mistlin, A.; Phillip, R.; Evriviades, D.; Kendrew, J. Direct Skeletal Fixation in bilateral above knee amputees following blast: 2 year follow up results from the initial cohort of UK service personnel. Injury 2020, 51, 735–743. [Google Scholar] [CrossRef]
- Wnuk-Scardaccione, A.; Zawojska, K.; Barłowska-Trybulec, M.; Mazur-Biały, A.I. Exercise Therapy in Nonspecific Low Back Pain among Individuals with Lower-Limb Amputation: A Systematic Review. Life 2023, 13, 772. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kenealy, T.; Garrett, M.; Bramley, D.; Drury, P.L.; Elley, C.R. Ethnicity and risk of lower limb amputation in people with Type 2 diabetes: A prospective cohort study. Diabet. Med. 2016, 33, 55–61. [Google Scholar] [CrossRef]
- Lin, C.; Liu, J.; Sun, H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A meta-analysis. PLoS ONE 2020, 15, e0239236. [Google Scholar] [CrossRef]
- Alexander, K.A.; Raggatt, L.J.; Millard, S.; Batoon, L.; Chiu-Ku Wu, A.; Chang, M.K.; Hume, D.A.; Pettit, A.R. Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration. Immunol. Cell Biol. 2017, 95, 7–16. [Google Scholar] [CrossRef]
- Atallah, R.; Li, J.J.; Lu, W.; Leijendekkers, R.; Frölke, J.P.M.; Al Muderis, M. Osseointegrated Transtibial Implants in Patients with Peripheral Vascular Disease: A Multicenter Case Series of 5 Patients with 1-Year Follow-up. J. Bone Joint Surg. Am. 2017, 99, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, M.; Reif, T.J.; Rozbruch, S.R.; Hoellwarth, J.S. Lower Extremity Osseointegration Postoperative Rehabilitation Protocols: A Scoping Review. Phys. Ther. 2024, 105, pzae139. [Google Scholar] [CrossRef] [PubMed]
- Al Muderis, M.; Lu, W.; Tetsworth, K.; Bosley, B.; Li, J.J. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: The Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open 2017, 7, e013508. [Google Scholar] [CrossRef]
- Murphy, E.P.; Sheridan, G.A.; Page, B.J.; Greenstein, M.D.; Hoellwarth, J.S.; Fragomen, A.T.; Rozbruch, S.R. Modern Internet Search Analytics and Osseointegration: What are Patients Asking and Reading Online? Strateg. Trauma Limb Reconstr. 2023, 18, 163–168. [Google Scholar]
- Balk, E.M.; Gazula, A.; Markozannes, G.; Kimmel, H.J.; Saldanha, I.J.; Trikalinos, T.A.; Resnik, L.J. Psychometric Properties of Functional, Ambulatory, and Quality of Life Instruments in Lower Limb Amputees: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 2354–2370. [Google Scholar] [CrossRef]
- Gailey, R.S.; Gaunaurd, I.A.; Kirk-Sanchez, N.J.; Gard, S.A.; Kristal, A. The development and reliability testing of the Functional Lower-Limb Amputee Gait Assessment. Clin. Rehabil. 2023, 37, 1656–1669. [Google Scholar] [CrossRef]
- Crowson, M.G.; Tucci, D.L. Mini Review of the Cost-Effectiveness of Unilateral Osseointegrated Implants in Adults: Possibly Cost-Effective for the Correct Indication. Audiol. Neurootol. 2016, 21, 69–71. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Hu, G.; Wang, L.; Zheng, J.; Zhan, J.; Zhu, Y.; Zhong, C.; Shi, X.; Liu, S.; et al. Immobilization of an antimicrobial peptide on silicon surface with stable activity by click chemistry. J. Mater. Chem. B 2018, 6, 68–74. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhang, Y.; He, H.; Xiong, S.; Chen, P.; Li, C.; Wang, L.; Lu, G.; Xu, Y. A dual-functional PEEK implant coating for anti-bacterial and accelerated osseointegration. Colloids Surf. B Biointerfaces 2023, 224, 113196. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Dingle, A.M.; Ness, J.P.; Novello, J.; Millevolte, A.X.T.; Zeng, W.; Sanchez, R.; Nemke, B.; Lu, Y.; Suminski, A.J.; Markel, M.D.; et al. Experimental Basis for Creating an Osseointegrated Neural Interface for Prosthetic Control: A Pilot Study in Rabbits. Mil. Med. 2020, 185 (Suppl. S1), 462–469. [Google Scholar] [CrossRef]
- Zhou, P.; He, F.; Liu, B.; Wei, S. Nerve electrical stimulation enhances osseointegration of implants in the beagle. Sci. Rep. 2019, 9, 4916. [Google Scholar] [CrossRef]
- Klinder, A.; Möws, F.; Ziebart, J.; Su, Y.; Gabler, C.; Jonitz-Heincke, A.; van Rienen, U.; Ellenrieder, M.; Bader, R. Effects of electrical stimulation with alternating fields on the osseointegration of titanium implants in the rabbit tibia—A pilot study. Front. Bioeng. Biotechnol. 2024, 12, 1395715. [Google Scholar] [CrossRef]
- Sun, R.; Bai, L.; Yang, Y.; Ding, Y.; Zhuang, J.; Cui, J. Nervous System-Driven Osseointegration. Int. J. Mol. Sci. 2022, 23, 8893. [Google Scholar] [CrossRef]
- Mastinu, E.; Doguet, P.; Botquin, Y.; Hakansson, B.; Ortiz-Catalan, M. Embedded System for Prosthetic Control Using Implanted Neuromuscular Interfaces Accessed Via an Osseointegrated Implant. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 867–877. [Google Scholar] [CrossRef]
- Israel, J.S.; Dingle, A.M.; Sanchez, R.J.; Kapur, S.K.; Brodnick, S.; Richner, T.J.; Ness, J.P.; Novello, J.; Williams, J.C.; Poore, S.O. Neuroma Implantation into Long Bones: Clinical Foundation for a Novel Osseointegrated Peripheral Nerve Interface. Plast. Reconstr. Surg. Glob. Open. 2018, 6, e1788. [Google Scholar] [CrossRef]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef]
- Tran, M.; Gabert, L.; Hood, S.; Lenzi, T. A lightweight robotic leg prosthesis replicating the biomechanics of the knee, ankle, and toe joint. Sci. Robot. 2022, 7, eabo3996. [Google Scholar] [CrossRef]
- Troyk, P.R.; DeMichele, G.A.; Kerns, D.A.; Weir, R.F. IMES: An implantable myoelectric sensor. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2007, 2007, 1730–1733. [Google Scholar]
- Stockinger, T.; Wirthl, D.; Mao, G.; Drack, M.; Pruckner, R.; Demchyshyn, S.; Steiner, M.; Egger, F.; Müller, U.; Schwödiauer, R.; et al. iSens: A Fiber-Based, Highly Permeable and Imperceptible Sensor Design. Adv. Mater. 2021, 33, e2102736. [Google Scholar] [CrossRef]
- Farina, D.; Vujaklija, I.; Brånemark, R.; Bull, A.M.J.; Dietl, H.; Graimann, B.; Hargrove, L.J.; Hoffmann, K.P.; Huang, H.H.; Ingvarsson, T.; et al. Toward higher-performance bionic limbs for wider clinical use. Nat. Biomed. Eng. 2023, 7, 473–485. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Hoellwarth, J.S.; Tetsworth, K.; Oomatia, A.; Al Muderis, M. Osseointegration Following Transfemoral Amputation After Infected Total Knee Replacement: A Case Series of 10 Patients With a Mean Follow-up of 5 Years. Arthroplast. Today 2022, 16, 21–30. [Google Scholar] [CrossRef]
| Region | Country | References | Time of Observation | Primary Causes | Secondary Causes |
|---|---|---|---|---|---|
| Europe | Ireland | Maely A./2022 [12] | 2016–2019 | Of the total number of all 3104 amputations, approximately 51.3% (n = 1592) of the minor amputations and 16.9% (n = 525) of the major amputations were performed on patients with diabetes diagnoses | - |
| Europe | Romania | Rusu E/2023 [13] | 2015–2019 | Of the total number of non-traumatic amputations, 51.2% were performed on patients with diabetes (n = 40,499) | Trauma-related, 11.4% (n = 9013) |
| Europe | England | Ahmad n/2014 [14] | 2003–2009 | There were 25,312 major lower limb amputations, and the most common disease risk factors were diabetes (43.7%) and hypertension and coronary heart disease (39%) | Trauma-related, 12% |
| Africa | Ethiopia | Sume BW/2023 [16] | 2000–2022 | The authors reviewed18,900 study participants, and the major cause of limb amputations was trauma (11.05%), with traditional bone setters (24.10%) | Burn, 10.63%; diabetic foot ulcer, 9.93% |
| Africa | Nigeria | Al-Ajlouni YA/2024 [18] | 1991–2005 | There were 1642 amputations, and the most frequent indications for amputation were trauma (34%), complications in traditional bone-setting (TBS) (23%), and malignant tumors (14.5%) | Diabetic gangrene, 12.3%; infections, 5.1%; peripheral artery disease, 2.1%; and burns, 2.1% |
| Asia | Bangladesh | Hassan Al Imam M/2020 [21] | 2014–2016 | Of the 332 participants, road traffic accidents were the leading cause (58.7%) | Peripheral vascular diseases, 7.5%; hit by sharp objects, 7.2% |
| Asia | South Korea | Chung HJ/2022 [23] | 2011–2018 | There were 8156 amputation cases, with the leading causes being peripheral vascular disease (PAD) (63.7%) and diabetes (32.1%) | - |
| North America | USA | Rivera JA/2024 [24] | 2016–2019 | There was a total of 2,118,175 amputations, with PAD and diabetes (83%) being the leading causes | Trauma-related, 9% |
| South America | Brazil | Portela FSO/2024 [25] | 2008–2020 | There were 633 455 amputations, with PAD (62.7%) and diabetes (23%) being the leading causes | Trauma-related, 11% |
| Australia | Australia | Dillon MP/2017 [26] | 2007–2012 | There was a total of 35,306 amputations, with diabetes (44.6%) and PAD (32.2%) being the leading causes | Trauma related (9.5%), Cancer (5.4%) |
| Implant System | Advantages | Disadvantages |
|---|---|---|
| OPRA | Proven long-term success for both upper- and lower-limb amputees | Higher risk of infection due to concentrated stresses around the threads |
| The design features enhance torsional stability and enable implantation in cases with a short residual limb | Possible complications with soft tissue overgrowth and irritation | |
| High patient satisfaction with functional outcomes | Expensive, with a long two-stage surgical process | |
| Compress | Minimally invasive surgical procedure | Limited clinical data compared to OPRA; long-term outcomes still being studied |
| Potential for a lower risk of infection due to internal components | Potential for less stability in certain cases, particularly with bone quality, higher risk of aseptic loosening | |
| Simplicity of performing revision surgery | Stress shielding or the removal of physiologic stress on bone by an implant may lead to osteopenia and reduced cortical thickness | |
| OPL | Designed to minimize the risk of infection as the polished adapter helps prevent fractures in the surrounding bone | Limited long-term clinical evidence |
| One-stage procedure, faster loading of the operated limb | The complexity of internal design could potentially lead to greater difficulty in adjustments, repairs, or revisions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnuk-Scardaccione, A.; Bilski, J. Breaking Barriers—The Promise and Challenges of Limb Osseointegration Surgery. Medicina 2025, 61, 542. https://doi.org/10.3390/medicina61030542
Wnuk-Scardaccione A, Bilski J. Breaking Barriers—The Promise and Challenges of Limb Osseointegration Surgery. Medicina. 2025; 61(3):542. https://doi.org/10.3390/medicina61030542
Chicago/Turabian StyleWnuk-Scardaccione, Agnieszka, and Jan Bilski. 2025. "Breaking Barriers—The Promise and Challenges of Limb Osseointegration Surgery" Medicina 61, no. 3: 542. https://doi.org/10.3390/medicina61030542
APA StyleWnuk-Scardaccione, A., & Bilski, J. (2025). Breaking Barriers—The Promise and Challenges of Limb Osseointegration Surgery. Medicina, 61(3), 542. https://doi.org/10.3390/medicina61030542






