Effect of Global Longitudinal Strain at Discharge Period on Predicting Cardiac Defibrillator Implantation in STEMİ Patients with Impaired Left Ventricle Systolic Functions
Abstract
1. Introduction
2. Method
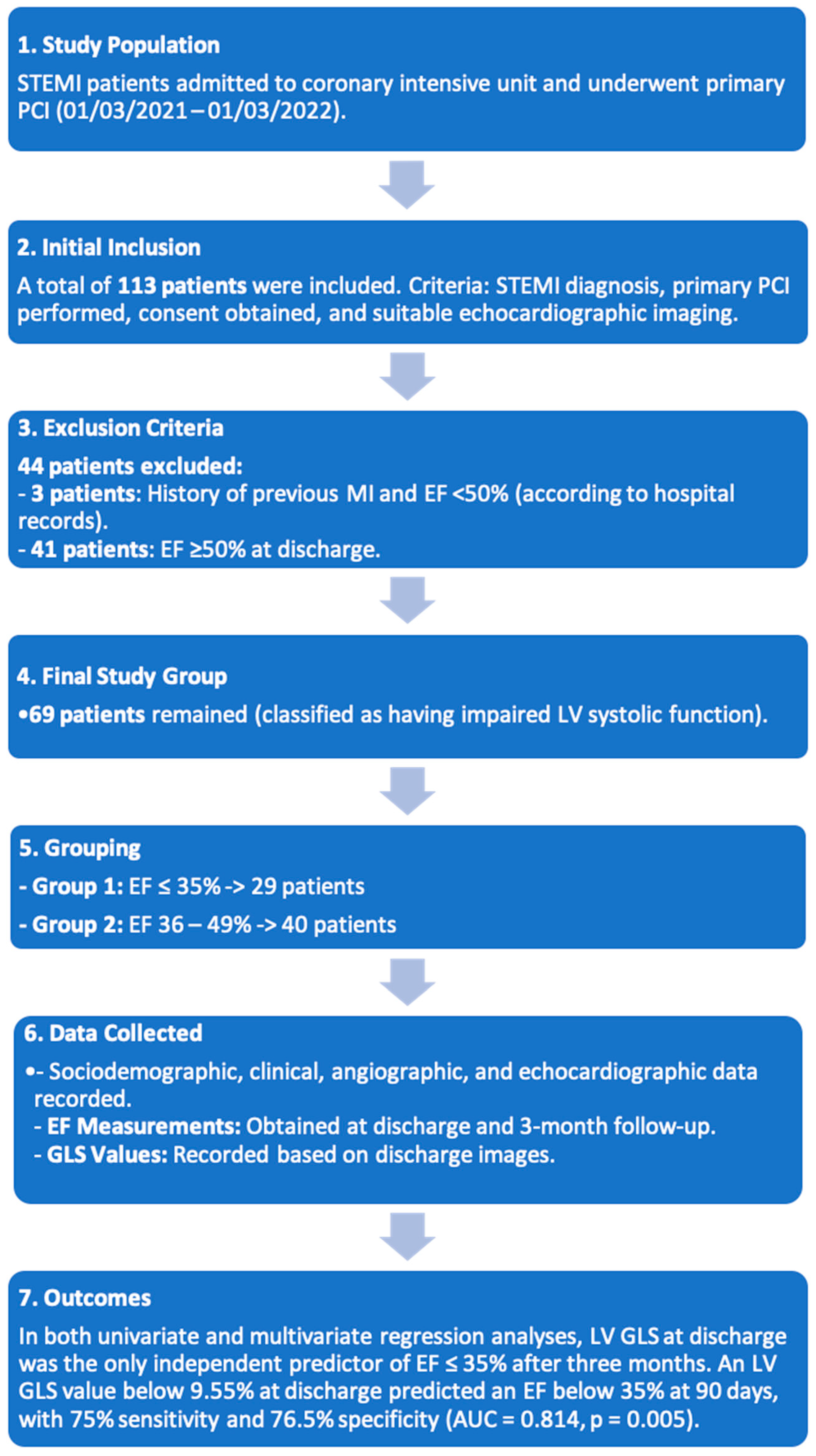
2.1. Study Population
2.2. Study Flowcharts
2.3. Echocardiographic Evaluation
2.3.1. Conventional Echocardiographic Measurements
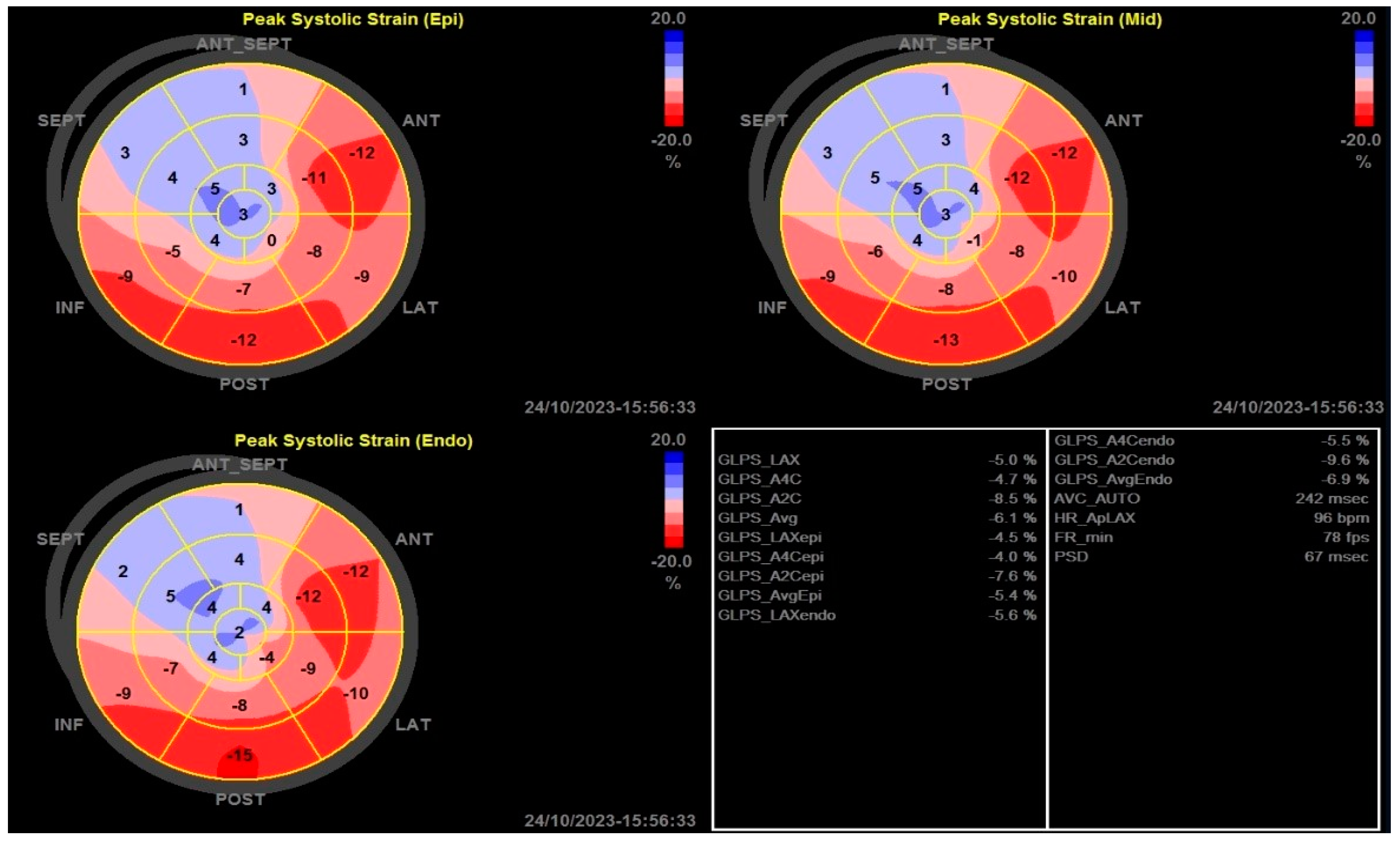
2.3.2. Two-Dimensional Strain Echocardiographic Analyses
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demidova, M.M.; Carlson, J.; Erlinge, D.; Platonov, P.G. Predictors of ventricular fibrillation at reperfusion in patients with acute ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Am. J. Cardiol. 2015, 115, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Epelde, F. Impact of Exercise on Physiological, Biochemical, and Analytical Parameters in Patients with Heart Failure with Reduced Ejection Fraction. Medicina 2024, 60, 2017. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgärtner, F.; et al. Defibrillator implantation early after myocardial infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J. Prophylactic use of an implantable cardioverter–defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef]
- Gronda, E.; Sacchi, S.; Benincasa, G.; Vanoli, E.; Napoli, C. Unresolved issues in left ventricular postischemic remodeling and progression to heart failure. J. Cardiovasc. Med. 2019, 20, 640–649. [Google Scholar] [CrossRef]
- Paul, S. Ventricular remodeling. Crit. Care Nurs. Clin. N. Am. 2003, 15, 407–411. [Google Scholar] [CrossRef]
- Dagres, N.; Hindricks, G. Risk stratification after myocardial infarction: Is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur. Heart J. 2013, 34, 1964–1971. [Google Scholar] [CrossRef]
- Sjøli, B.; Grenne, B.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. The advantage of global strain compared to left ventricular ejection fraction to predict outcome after acute myocardial infarction. Echocardiography 2011, 28, 556–563. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [PubMed]
- Collier, P.; Phelan, D.; Klein, A. A test in context: Myocardial strain measured by speckle-tracking echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [PubMed]
- Bansal, M.; Kasliwal, R.R. How do I do it? Speckle-tracking echocardiography. Indian Heart J. 2013, 65, 117. [Google Scholar] [CrossRef]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic assessment of left ventricular systolic function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar]
- Luis, S.A.; Chan, J.; Pellikka, P.A. Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–138. [Google Scholar]
- Emond, M.; Mock, M.B.; Davis, K.B.; Fisher, L.D.; Holmes, D.R., Jr.; Chaitman, B.R.; Kaiser, G.C.; Alderman, E.; Killip, T., 3rd. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994, 90, 2645–2657. [Google Scholar]
- Curtis, J.P.; Sokol, S.I.; Wang, Y.; Rathore, S.S.; Ko, D.T.; Jadbabaie, F.; Portnay, E.L.; Marshalko, S.J.; Radford, M.J.; Krumholz, H.M. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 2003, 42, 736–742. [Google Scholar]
- Stanton, T.; Leano, R.; Marwick, T.H. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ. Cardiovasc. Imaging 2009, 2, 356–364. [Google Scholar]
- Potter, E.; Marwick, T.H. Assessment of left ventricular function by echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar]
- Nikoo, M.H.; Naeemi, R.; Moaref, A.; Attar, A. Global longitudinal strain for prediction of ventricular arrhythmia in patients with heart failure. ESC Heart Fail. 2020, 7, 2956–2961. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.-B.; Park, J.-H.; Cho, G.-Y. Global longitudinal strain to predict mortality in patients with acute heart failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [PubMed]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar]
- Mignot, A.; Donal, E.; Zaroui, A.; Reant, P.; Salem, A.; Hamon, C.; Monzy, S.; Roudaut, R.; Habib, G.; Lafitte, S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: A multicenter study. J. Am. Soc. Echocardiogr. 2010, 23, 1019–1024. [Google Scholar]
- Cheung, C.C.; Olgin, J.E.; Lee, B.K. Wearable cardioverter-defibrillators: A review of evidence and indications. Trends Cardiovasc. Med. 2021, 31, 196–201. [Google Scholar] [CrossRef]
- Zoppo, F.; Mugnai, G.; Lupo, A.; Zerbo, F. Bridge to avoid ICD implantation with wearable cardioverter defibrillators. Adv. Interv. Cardiol./Postępy Kardiol. Interwencyjnej 2020, 16, 227–228. [Google Scholar]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients with Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef]
- Reindl, M.; Tiller, C.; Holzknecht, M.; Lechner, I.; Beck, A.; Plappert, D.; Gorzala, M.; Pamminger, M.; Mayr, A.; Klug, G.; et al. Prognostic Implications of Global Longitudinal Strain by Feature-Tracking Cardiac Magnetic Resonance in ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2019, 12, e009404. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar]
- Hallén, J. Troponin for the estimation of infarct size: What have we learned? Cardiology 2012, 121, 204–212. [Google Scholar] [CrossRef]
- Khullar, N.; Buckley, A.J.; O’connor, C.; Ibrahim, A.; Ibrahim, A.; Ahern, C.; Cahill, C.; Arnous, S.; Kiernan, T.J. Peak troponin T in STEMI: A predictor of all-cause mortality and left ventricular function. Open Heart 2022, 9, e001863. [Google Scholar] [PubMed]
| Variable | Group 1 | Group 2 | p-Value | |
|---|---|---|---|---|
| Gender n (%) | Female | 3 (%20.6) | 30 (%63.6) | 0.22 |
| Male | 26 (%78.4) | 10 (%36.4) | ||
| Age (Mean ± SD) | 60.2 ± 9.5 | 54.7 ± 11 | 0.03 | |
| Height (cm) | 171 (155–188) | 170 (152–180) | 0.99 | |
| Weight (kg) | 78 (58–120) | 80 (51–116) | 0.53 | |
| Smoking Status n (%) | Never smoked | 8 (%27.6) | 9 (%22.5) | 0.32 |
| Former smoker | 7 (%24.1) | 5 (%12.5) | ||
| Current smoker | 14 (%48.3) | 26 (%65) | ||
| Hypertension n (%) | 10 (34.5%) | 15 (37.5%) | 0.99 | |
| Hyperlipidemia n (%) | 6 (20.7%) | 1 (2.5%) | 0.01 | |
| Diabetes Mellitus n (%) | 8 (27.6%) | 10 (25%) | 0.8 | |
| History of MI n (%) | 8 (27.6%) | 5 (12.5%) | 0.2 | |
| History of PCI (Balloon-Stent) n (%) | 9 (31%) | 5 (12.5%) | 0.1 | |
| History of CABG n (%) | 0 (0%) | 2 (5%) | 0.2 | |
| Peripheral Artery Disease n (%) | 2 (6.9%) | 1 (2.5%) | 0.4 | |
| History of Stroke n (%) | 1 (3.4%) | 2 (5%) | 0.75 | |
| Systolic BP (mmHg, Mean ± SD) | 129.9 ± 22 | 135.2 ± 21.3 | 0.32 | |
| Diastolic BP (mmHg, Median + min–max) | 80 (60–105) | 80 (50–120) | 0.74 | |
| Heart Rate (bpm, Mean + min–max) | 85 (65–101) | 79 (39–105) | 0.11 | |
| Killip Classification n (%) | Class 1 | 23 (79.3%) | 37 (92.5%) | 0.18 |
| Class 2 | 5 (17.2%) | 2 (5%) | ||
| Class 3 | 0 (0%) | 1 (2.5%) | ||
| Class 4 | 1 (3.4%) | 0 (0%) | ||
| Time from Onset of Pain (hours) | 5 (1–28) | 4.5 (1–36) | 0.19 | |
| Hemoglobin (g/dL) | 14.4 (10.5–16.3) | 14.6 (8.3–17.3) | 0.54 | |
| CRP (mg/dL) | 30 (1–286) | 23.5 (2–163) | 0.68 | |
| Glucose (mg/dL) | 115 (81–336) | 109.5 (81–285) | 0.28 | |
| GFR (mL/min) | 92 (57–120) | 101.5 (37–120) | 0.03 | |
| Creatinine (mg/dL) | 0.8 (0.5–1.2) | 0.8 (0.4–1.8) | 0.08 | |
| LDL (mg/dL) | 109 (29–168) | 122.5 (35–290) | 0.31 | |
| Admission Troponin (ng/L) | 887 (1.5–50,000) | 142 (1.4–50,000) | 0.24 | |
| Peak Troponin (ng/L) | 50,000 (389–50,000) | 33,205.5 (642–50,000) | 0.05 | |
| Variable | Group 1 | Group 2 | p-Value | |
|---|---|---|---|---|
| ECG Diagnosis n (%) | Anterior MI n (%) | 26 (89.7%) | 26 (65%) | 0.55 |
| Inferior MI n (%) | 3 (10.3%) | 12 (30%) | ||
| Lateral MI n (%) | 0 (0%) | 2 (5%) | ||
| Number of Affected Vessels | 2 (1–3) | 1 (1–3) | 0.001 | |
| Door-to-Balloon Time (minutes) | 31 (10–72) | 29.5 (15–95) | 0.75 | |
| Target Vessel for Intervention n (%) | LAD n (%) | 26 (89.7%) | 26 (65%) | 0.06 |
| RCA n (%) | 2 (6.9%) | 11 (27.5%) | ||
| CX n (%) | 1 (3.4%) | 3 (7.5%) | ||
| Procedure Success n (%) | Unsuccessful n (%) | 0 (0%) | 1 (2.5%) | 0.4 |
| Successful n (%) | 29 (100%) | 39 (97.5%) | ||
| Guideline-Directed Medical Therapy at Discharge | ASA n (%) | 29 (100%) | 40 (100%) | - |
| P2Y12 Inhibitor n (%) | 29 (100%) | 40 (100%) | - | |
| Beta Blocker n (%) | 29 (100%) | 35 (87.5%) | 0.05 | |
| CCB n (%) | 2 (6.9%) | 11 (27.5%) | 0.032 | |
| ACEi/ARB n (%) | 25 (86.2%) | 34 (85%) | 0.9 | |
| ARNi n (%) | 4 (13.8%) | 2 (5%) | 0.2 | |
| MRA n (%) | 22 (75.9%) | 11 (22%) | <0.001 | |
| SGLT-2 Inhibitor n (%) | 8 (27.6%) | 8 (20%) | 0.65 | |
| Diuretic n (%) | 8 (27.6%) | 4 (10%) | 0.06 | |
| Statin n (%) | 29 (100%) | 40 (100%) | - | |
| 3-Month Follow-up Outcome Data | Recurrent hospital admission | 4 (13.8%) | 3 (7.5%) | 0.4 |
| Recurrent MI | 2 (6.9%) | 0 (0%) | 0.1 | |
| Recurrent intervention | 13 (44.8%) | 4 (10%) | 0.001 | |
| Heart-related death | 1 (3.4%) | 0 (0%) | 0.42 | |
| Variable | Group 1 (Mean + Min–Max) | Group 2 (Mean + Min–Max) | p-Value |
|---|---|---|---|
| EF at Discharge (%) | 32 (20–35) | 44 (37–49) | <0.001 |
| LV GLS | −8.9 (4.3–13.7) | −15.1 (11.4–20.3) | <0.001 |
| EF on Day 90 (%) | 35 (20–46) | 53 (40–60) | <0.001 |
| LVEDD (mm) | 53 (46–61) | 47 (42–54) | <0.001 |
| LVESD (mm) | 41 (32–54) | 32 (30–44) | <0.001 |
| IVS Thickness (mm) | 10 (7–12) | 11 (8–13) | 0.02 |
| PW Thickness (mm) | 10 (6–11) | 10 (8–12) | 0.12 |
| LA Diameter (mm) | 41 (35–52) | 37 (32–43) | <0.001 |
| E/e′ Ratio | 13.6 (7.3–21.7) | 11.4 (5.7–17.7) | <0.001 |
| RA Diameter (mm) | 39 (30–48) | 36 (31–42) | 0.10 |
| RV Diameter (mm) | 33 (27–45) | 31.5 (25–37) | 0.11 |
| Variable | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 0.979 (0.903–1.061) | 0.60 | ||
| Gender | 0 (0–0) | 1.00 | ||
| Smoking | 1.121 (0.4–3.14) | 0.83 | ||
| Hypertension | 0.476 (0.094–2.418) | 0.37 | ||
| Hyperlipidemia | 0.65 (0.098–4.29) | 0.65 | ||
| Diabetes Mellitus | 0.367 (0.06–2.252) | 0.28 | ||
| Heart Failure | 0 (0–0) | 1.00 | ||
| History of MI | 0.367 (0.06–2.252) | 0.28 | ||
| MI Location | 0 (0–0) | 1.00 | ||
| Total Ischemic Time | 0.984 (0.888–1.091) | 0.76 | ||
| Door-to-Balloon Time | 0.972 (0.914–1.033) | 0.35 | ||
| Number of Diseased Vessels | 0.922 (0.355–2.394) | 0.87 | ||
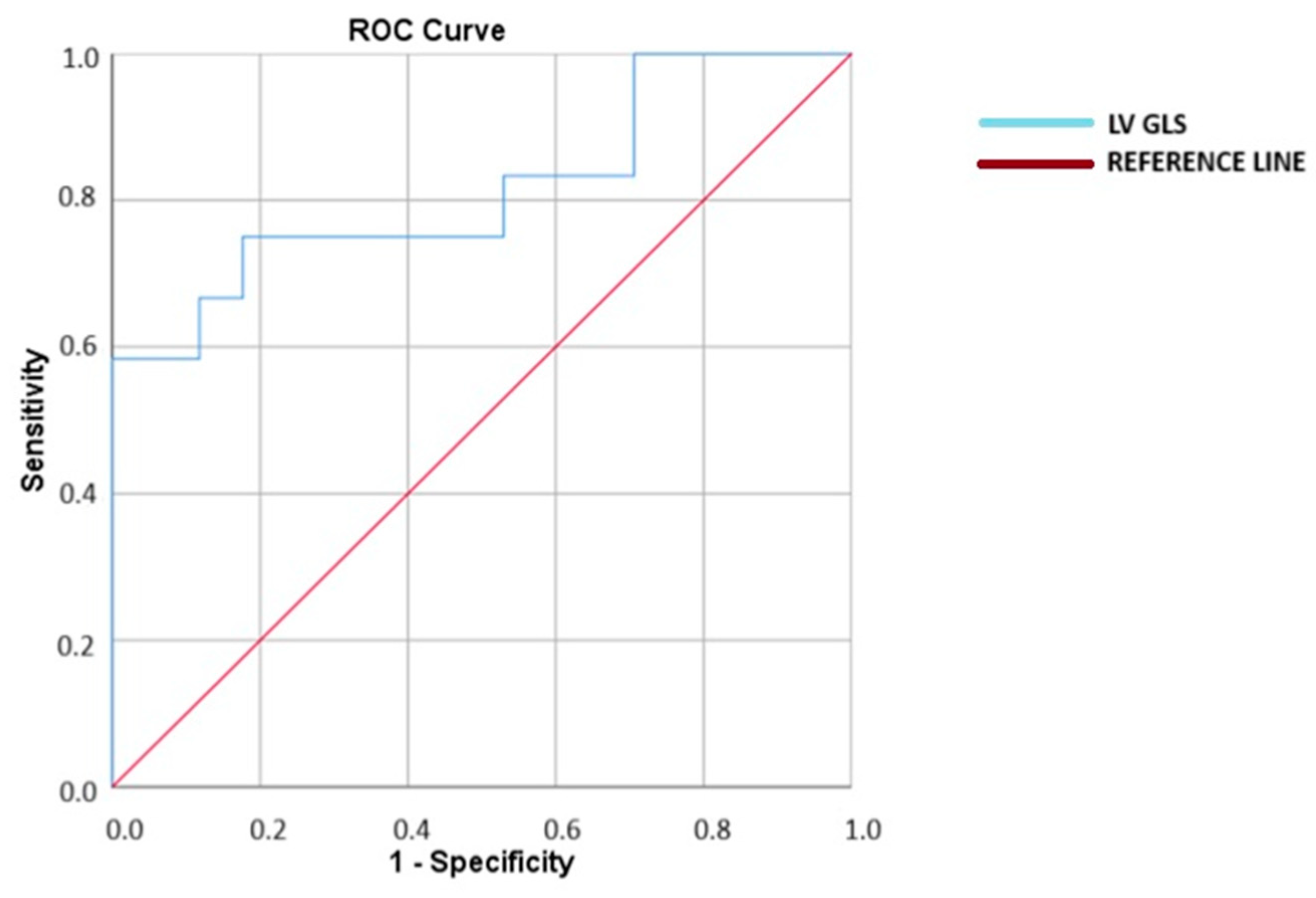
| LV GLS | 1.651 (1.134–2.405) | 0.009 | 1.926 (1.024–2.625) | 0.042 |
| Peak Troponin Level | 1.002 (0.985–1.050) | 0.03 | 1.003 (0.990–1.069) | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkuş, Ö.F.; Gürdoğan, M. Effect of Global Longitudinal Strain at Discharge Period on Predicting Cardiac Defibrillator Implantation in STEMİ Patients with Impaired Left Ventricle Systolic Functions. Medicina 2025, 61, 545. https://doi.org/10.3390/medicina61030545
Akkuş ÖF, Gürdoğan M. Effect of Global Longitudinal Strain at Discharge Period on Predicting Cardiac Defibrillator Implantation in STEMİ Patients with Impaired Left Ventricle Systolic Functions. Medicina. 2025; 61(3):545. https://doi.org/10.3390/medicina61030545
Chicago/Turabian StyleAkkuş, Ömer Ferudun, and Muhammet Gürdoğan. 2025. "Effect of Global Longitudinal Strain at Discharge Period on Predicting Cardiac Defibrillator Implantation in STEMİ Patients with Impaired Left Ventricle Systolic Functions" Medicina 61, no. 3: 545. https://doi.org/10.3390/medicina61030545
APA StyleAkkuş, Ö. F., & Gürdoğan, M. (2025). Effect of Global Longitudinal Strain at Discharge Period on Predicting Cardiac Defibrillator Implantation in STEMİ Patients with Impaired Left Ventricle Systolic Functions. Medicina, 61(3), 545. https://doi.org/10.3390/medicina61030545






