Augmented Reality in Scoliosis Correction Surgery: Efficiency and Accuracy in Pedicle Screw Instrumentation
Abstract
:1. Introduction
2. Methods
2.1. Patient Collection
2.2. Registration Procedures
2.3. Surgical Techniques
2.4. Radiological Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, B.P.; Zhang, Y.P.; Qiao, M.; Qiu, Y.; Mao, S.H. Accuracy of freehand pedicle screw placement in surgical correction of thoracolumbar kyphosis secondary to ankylosing spondylitis: A computed tomography investigation of 2314 consecutive screws. World Neurosurg. 2018, 116, e850–e855. [Google Scholar] [CrossRef] [PubMed]
- Abul-Kasim, K.; Ohlin, A. The rate of screw misplacement in segmental pedicle screw fixation in adolescent idiopathic scoliosis. Acta Orthop. 2011, 82, 50–55. [Google Scholar] [CrossRef]
- Hersh, A.; Mahapatra, S.; Weber-Levine, C.; Awosika, T.; Theodore, J.N.; Zakaria, H.M.; Liu, A.; Witham, T.F.; Theodore, N. Augmented reality in spine surgery: A narrative review. HSS J. 2021, 17, 351–358. [Google Scholar] [CrossRef]
- Jin, M.; Liu, Z.; Qiu, Y.; Yan, H.; Han, X.; Zhu, Z. Incidence and risk factors for the misplacement of pedicle screws in scoliosis surgery assisted by O-arm navigation-analysis of a large series of one thousand, one hundred and forty five screws. Int. Orthop. 2017, 41, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, Y.P.; Taylor, W.; Oygar, A.; Kim, W.K. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008, 8, 584–590. [Google Scholar] [CrossRef]
- Ruatti, S.; Dubois, C.; Chipon, E.; Kerschbaumer, G.; Milaire, M.; Moreau-Gaudry, A.; Tonetti, J.; Merloz, P. Interest of intra-operative 3D imaging in spine surgery: A prospective randomized study. Eur. Spine J. 2016, 25, 1738–1744. [Google Scholar] [CrossRef]
- Tajsic, T.; Patel, K.; Farmer, R.; Mannion, R.J.; Trivedi, R.A. Spinal navigation for minimally invasive thoracic and lumbosacral spine fixation: Implications for radiation exposure, operative time, and accuracy of pedicle screw placement. Eur. Spine J. 2018, 27, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Venier, A.; Croci, D.; Robert, T.; Distefano, D.; Presilla, S.; Scarone, P. Use of intraoperative computed tomography improves outcome of minimally invasive transforaminal lumbar interbody fusion: A single-center retrospective cohort study. World Neurosurg. 2021, 148, e572–e580. [Google Scholar] [CrossRef]
- Gertzbein, S.D.; Robbins, S.E. Accuracy of pedicular screw placement in vivo. Spine 1990, 15, 11–14. [Google Scholar] [CrossRef]
- Safaee, M.; Oh, T.; Pekmezci, M.; Clark, A.J. Cone beam intraoperative computed tomography-based image guidance for minimally invasive transforaminal interbody fusion. J. Vis. Exp. 2019, 150, e57830. [Google Scholar] [CrossRef]
- Lian, X.; Navarro-Ramirez, R.; Berlin, C.; Jada, A.; Moriguchi, Y.; Zhang, Q.; Härtl, R. Total 3D Airo® navigation for minimally invasive transforaminal lumbar interbody fusion. BioMed Res. Int. 2016, 2016, 5027340. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, J.; Riese, F.; Allam, Y.; Reichert, T.; Koeppert, H.; Gutberlet, M. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: Comparison between freehand and O-arm based navigation techniques. Eur. Spine J. 2011, 20, 875–881. [Google Scholar] [CrossRef]
- Mehbodniya, A.H.; Moghavvemi, M.; Narayanan, V.; Waran, V. Frequency and causes of line of sight issues during neurosurgical procedures using optical image-guided systems. World Neurosurg. 2019, 122, e449–e454. [Google Scholar] [CrossRef]
- Molina, C.A.; Sciubba, D.M.; Greenberg, J.K.; Khan, M.; Witham, T. Clinical accuracy, technical precision, and workflow of the first in human use of an augmented-reality head-mounted display stereotactic navigation system for spine surgery. Oper. Neurosurg. 2021, 20, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Yahanda, A.T.; Moore, E.; Ray, W.Z.; Pennicooke, B.; Jennings, J.W.; Molina, C.A. First in-human report of the clinical accuracy of thoracolumbar percutaneous pedicle screw placement using augmented reality guidance. Neurosurg. Focus 2021, 51, E10. [Google Scholar] [CrossRef] [PubMed]
- Elmi-Terander, A.; Nachabe, R.; Skulason, H.; Pedersen, K.; Söderman, M.; Racadio, J.; Babic, D.; Gerdhem, P.; Edström, E. Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine 2018, 43, 1018–1023. [Google Scholar] [CrossRef]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the Microsoft HoloLens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Tang, Y.; Li, H.; Wang, W.; Li, C.; Zhou, Y. Percutaneous placement of lumbar pedicle screws via intraoperative CT image-based augmented reality-guided technology. J. Neurosurg. Spine 2020, 32, 542–547. [Google Scholar] [CrossRef]
- Müller, F.; Roner, S.; Liebmann, F.; Spirig, J.M.; Fürnstahl, P.; Farshad, M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020, 20, 621–628. [Google Scholar] [CrossRef]
- Dennler, C.; Jaberg, L.; Spirig, J.; Agten, C.; Götschi, T.; Fürnstahl, P.; Farshad, M. Augmented reality-based navigation increases precision of pedicle screw insertion. J. Orthop. Surg. Res. 2020, 15, 174. [Google Scholar] [CrossRef]
- Yanni, D.S.; Ozgur, B.M.; Louis, R.G.; Shekhtman, Y.; Iyer, R.R.; Boddapati, V.; Iyer, A.; Patel, P.D.; Jani, R.; Cummock, M.; et al. Real-time navigation guidance with intraoperative CT imaging for pedicle screw placement using an augmented reality head-mounted display: A proof-of-concept study. Neurosurg. Focus 2021, 51, E11. [Google Scholar] [CrossRef] [PubMed]
- Spirig, J.M.; Roner, S.; Liebmann, F.; Fürnstahl, P.; Farshad, M. Augmented reality-navigated pedicle screw placement: A cadaveric pilot study. Eur. Spine J. 2021, 30, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Frisk, H.; Lindqvist, E.; Persson, O.; Weinzierl, J.; Bruetzel, L.K.; Cewe, P.; Burström, G.; Edström, E.; Elmi-Terander, A. Feasibility and accuracy of thoracolumbar pedicle screw placement using an augmented reality head-mounted device. Sensors 2022, 22, 522. [Google Scholar] [CrossRef]
- Felix, B.; Kalatar, S.B.; Moatz, B.; Hofstetter, C.; Karsy, M.; Parr, R.; Gibby, W. Augmented reality spine surgery navigation: Increasing pedicle screw insertion accuracy for both open and minimally invasive spine surgeries. Spine 2022, 47, 865–872. [Google Scholar] [CrossRef]
- Cao, B.; Yuan, B.; Xu, G.; Zhao, Y.; Sun, Y.; Wang, Z.; Zhou, S.; Xu, Z.; Wang, Y.; Chen, X. A pilot human cadaveric study on accuracy of the augmented reality surgical navigation system for thoracolumbar pedicle screw insertion using a new intraoperative rapid registration method. J. Digit. Imaging 2023, 36, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Edström, E.; Burström, G.; Persson, O.; Charalampidis, A.; Nachabe, R.; Gerdhem, P.; Elmi-Terander, A. Does augmented reality navigation increase pedicle screw density compared to freehand technique in deformity surgery? single surgeon case series of 44 patients. Spine 2020, 45, E1085–E1090. [Google Scholar] [CrossRef]
- Peng, Y.N.; Tsai, L.C.; Hsu, H.C.; Kao, C.H. Accuracy of robot-assisted versus conventional freehand pedicle screw placement in spine surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Transl. Med. 2020, 8, 824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakai, D.; Schol, J.; Kawachi, A.; Sako, K.; Hiyama, A.; Katoh, H.; Sato, M.; Watanabe, M. Adolescent idiopathic scoliotic deformity correction surgery assisted by smart glasses can enhance correction outcomes and accuracy and also improve surgeon fatigue. World Neurosurg. 2023, 178, e96–e103. [Google Scholar] [CrossRef]
- Bäcker, H.C.; Freibott, C.E.; Perka, C.; Weidenbaum, M. Surgeons’ learning curve of renaissance robotic surgical system. Int. J. Spine Surg. 2020, 14, 818–823. [Google Scholar] [CrossRef]
- Butler, A.J.; Colman, M.W.; Lynch, J.; Phillips, F.M. Augmented reality in minimally invasive spine surgery: Early efficiency and complications of percutaneous pedicle screw instrumentation. Spine J. 2023, 23, 27–33. [Google Scholar] [CrossRef]
- Burström, G.; Nachabe, R.; Homan, R.; Hoppenbrouwers, J.; Holthuizen, R.; Persson, O.; Edström, E.; Elmi-Terander, A. Frameless patient tracking with adhesive optical skin markers for augmented reality surgical navigation in spine surgery. Spine 2020, 45, 1598–1604. [Google Scholar] [CrossRef]
- Fatima, N.; Massaad, E.; Hadzipasic, M.; Shankar, G.M.; Shin, J.H. Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional freehand technique: A systematic review and meta-analysis. Spine J. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef]
- Farshad, M.; Fürnstahl, P.; Spirig, J.M. First in man in-situ augmented reality pedicle screw navigation. N. Am. Spine Soc. J. 2021, 6, 100065. [Google Scholar] [CrossRef]
- Charles, Y.P.; Cazzato, R.L.; Nachabe, R.; Chatterjea, A.; Steib, J.P.; Gangi, A. Minimally invasive transforaminal lumbar interbody fusion using augmented reality surgical navigation for percutaneous pedicle screw placement. Clin. Spine Surg. 2021, 34, E415–E424. [Google Scholar] [CrossRef]
- Bhatt, F.R.; Orosz, L.D.; Tewari, A.; Boyd, D.; Roy, R.; Good, C.R.; Schuler, T.C.; Haines, C.M.; Jazini, E. Augmented reality-assisted spine surgery: An early experience demonstrating safety and accuracy with 218 screws. Glob. Spine J. 2023, 13, 2047–2052. [Google Scholar] [CrossRef]
- Harel, R.; Anekstein, Y.; Raichel, M.; Molina, C.A.; Ruiz-Cardozo, M.A.; Orrú, E.; Khan, M.; Mirovsky, Y.; Smorgick, Y. The XVS system during open spinal fixation procedures in patients requiring pedicle screw placement in the lumbosacral spine. World Neurosurg. 2022, 164, e1226–e1232. [Google Scholar] [CrossRef]
- Sommer, F.; Hussain, I.; Kirnaz, S.; Goldberg, J.L.; Navarro-Ramirez, R.; McGrath, L.B., Jr.; Schmidt, F.A.; Medary, B.; Gadjradj, P.S.; Härtl, R. Augmented reality to improve surgical workflow in minimally invasive transforaminal lumbar interbody fusion—A feasibility study with case series. Neurospine 2022, 19, 574–585. [Google Scholar] [CrossRef]
- Rush, A.J., 3rd; Shepard, N.; Nolte, M.; Siemionow, K.; Phillips, F. Augmented reality in spine surgery: Current state of the art. Int. J. Spine Surg. 2022, 16, 22–27. [Google Scholar] [CrossRef]
- Li, C.R.; Shen, C.C.; Yang, M.Y.; Lee, C.H. Intraoperative augmented reality in minimally invasive spine surgery: A case report. Asian J. Surg. 2023, 46, 2825–2826. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Nimsky, C. Microscope-based augmented reality in degenerative spine surgery: Initial experience. World Neurosurg. 2019, 128, e541–e551. [Google Scholar] [CrossRef]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Voellger, B.; Nimsky, C. Spine surgery supported by augmented reality. Glob. Spine J. 2020, 10, S41–S55. [Google Scholar] [CrossRef]
- Onuma, H.; Sakai, K.; Arai, Y.; Torigoe, I.; Tomori, M.; Sakaki, K.; Hirai, T.; Egawa, S.; Kobayashi, Y.; Okawa, A.; et al. Augmented reality support for anterior decompression and fusion using floating method for cervical ossification of the posterior longitudinal ligament. J. Clin. Med. 2023, 12, 2898. [Google Scholar] [CrossRef]
- McClendon, J.; Almekkawi, A.K.; Abi-Aad, K.R.; Maiti, T. Use of Pheno room, augmented reality, and 3-rod technique for 3-dimensional correction of adolescent idiopathic scoliosis. World Neurosurg. 2020, 137, 291. [Google Scholar] [CrossRef]
- Carl, B.; Bopp, M.; Saß, B.; Voellger, B.; Nimsky, C. Implementation of augmented reality support in spine surgery. Eur. Spine J. 2019, 28, 1697–1711. [Google Scholar] [CrossRef]
- Sommer, F.; Hussain, I.; Kirnaz, S.; Goldberg, J.; McGrath, L.; Navarro-Ramirez, R.; Waterkeyn, F.; Schmidt, F.; Gadjradj, P.S.; Härtl, R. Safety and feasibility of augmented reality assistance in minimally invasive and open resection of benign intradural extramedullary tumors. Neurospine 2022, 19, 501–512. [Google Scholar] [CrossRef]
- Tigchelaar, S.S.; Medress, Z.A.; Quon, J.; Dang, P.; Barbery, D.; Bobrow, A.; Kin, C.; Louis, R.; Desai, A. Augmented reality neuronavigation for en bloc resection of spinal column lesions. World Neurosurg. 2022, 167, 102–110. [Google Scholar] [CrossRef]
- Lin, M.S.; Huang, C.W.; Tsou, H.K.; Tzeng, C.Y.; Kao, T.H.; Lin, R.H.; Chen, T.Y.; Li, C.R.; Lee, C.Y. Advances in surgical treatment for atlantoaxial instability focusing on rheumatoid arthritis: Analysis of a series of 67 patients. Int. J. Rheum. Dis. 2023, 26, 1996–2006. [Google Scholar] [CrossRef]
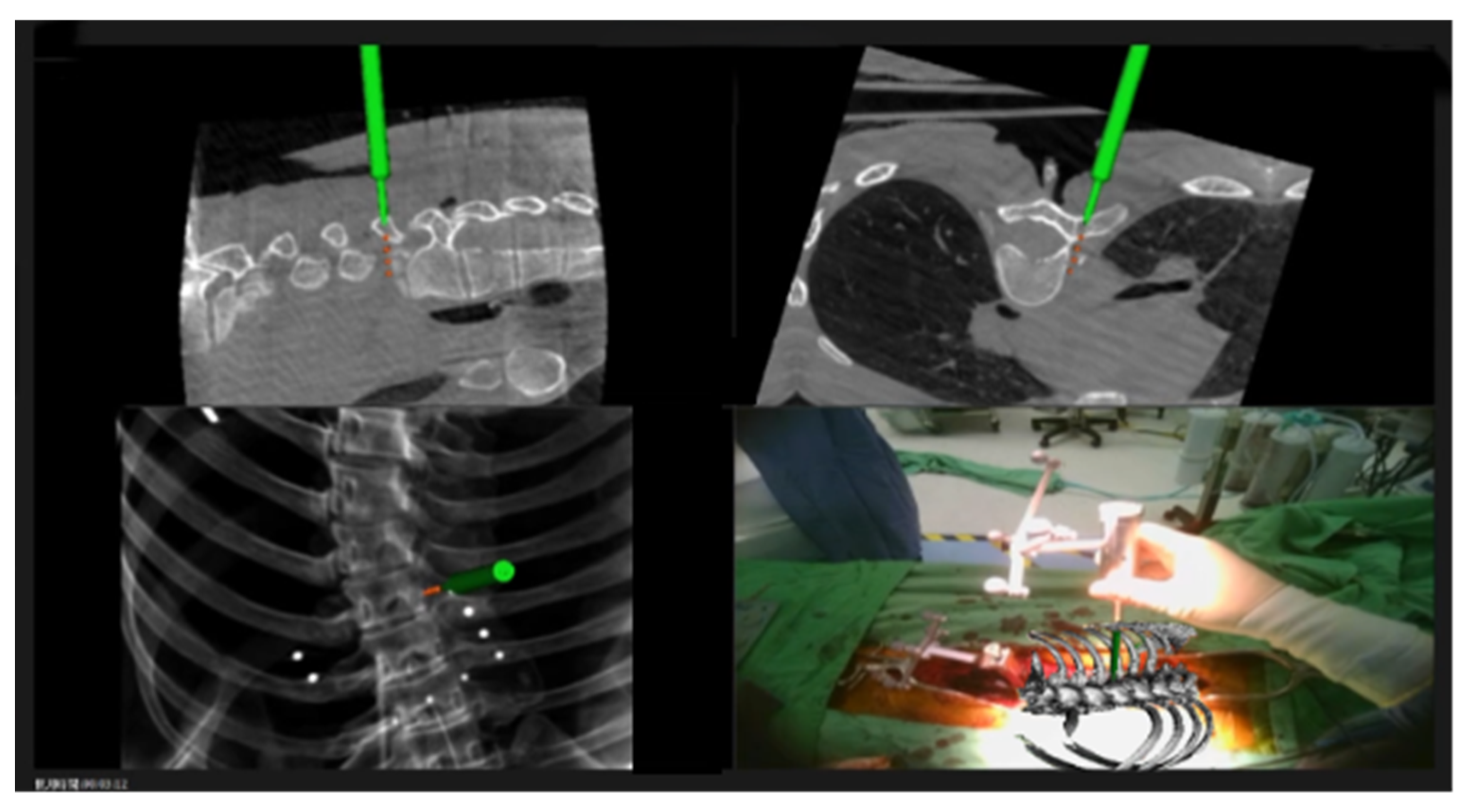
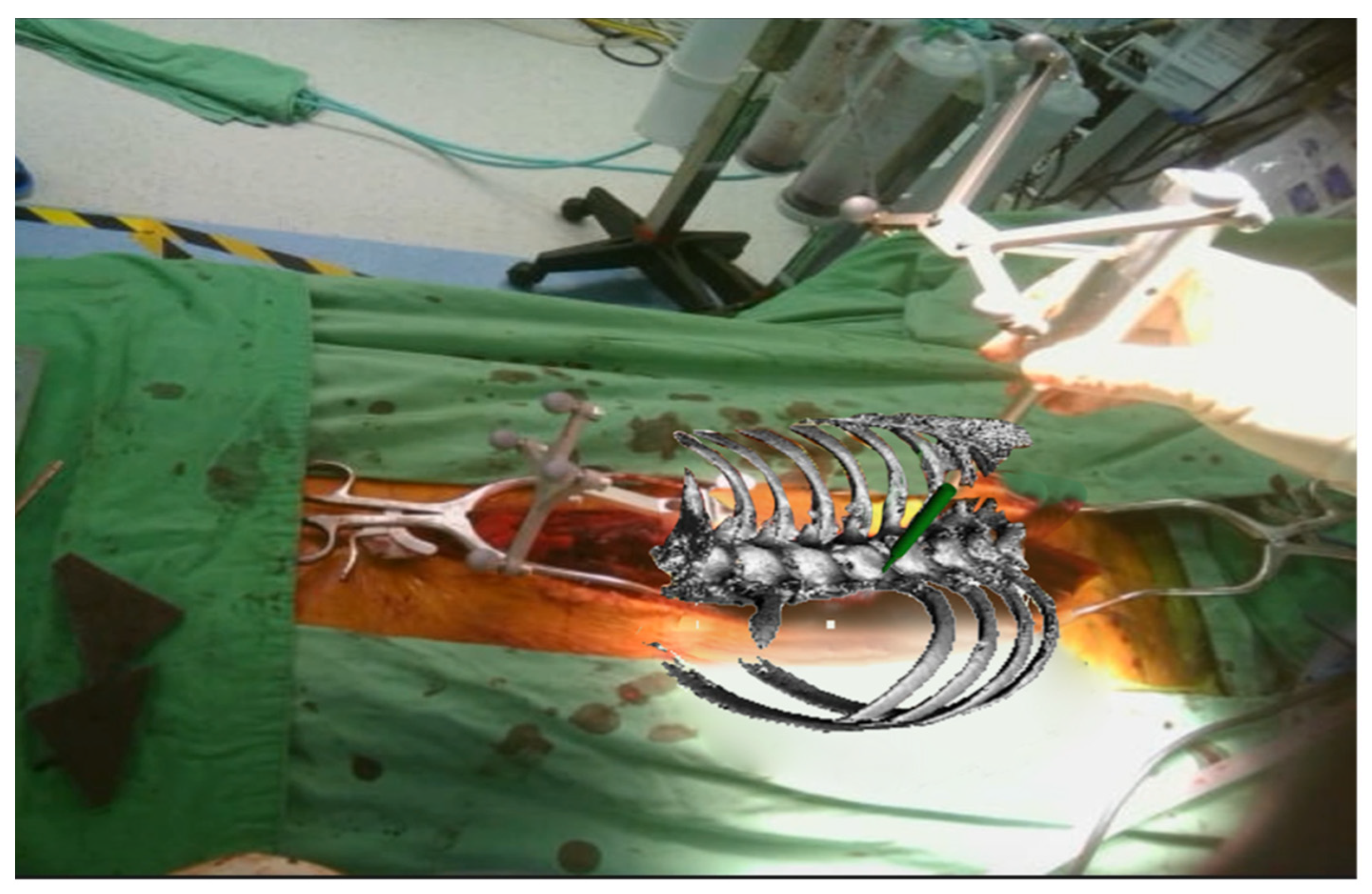
| Demographic Factor | |
|---|---|
| Average age (years) | 22.0 |
| Average body mass index (kg/m2) | 20.17 |
| Sex (Male/Female) | 1 (10%)/9 (90%) |
| Case No. | Instrumented Segments | Largest Preoperative Cobb Angle (°) | Largest Postoperative Cobb Angle (°) |
|---|---|---|---|
| 1 | Left T2 and bilateral T3 to L2 | 72 | 9 |
| 2 | Bilateral T2 to L4 | 98 | 6 |
| 3 | Bilateral T2 to L2 | 45 | 5 |
| 4 | Bilateral T5 to L5 | 60 | 9 |
| 5 | Bilateral T2 to L2 | 85 | 13 |
| 6 | Bilateral T3 to L2 | 78 | 14 |
| 7 | Bilateral T2 to L3 | 72 | 6 |
| 8 | Bilateral T3 to L3 | 52 | 6 |
| 9 | Bilateral T1 to L3 | 75 | 15 |
| 10 | Bilateral T2 to L5 | 124 | 16 |
| Total | GRS A | GRS B | GRS C | Accuracy | |
|---|---|---|---|---|---|
| T-spine | 197 | 132 | 60 | 5 | 97.4% |
| L-spine | 60 | 41 | 19 | 0 | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-N.; Li, C.-R.; Liao, S.-S.; Shen, C.-C.; Chen, K.-Y.; Lee, C.-H.; Yang, M.-Y. Augmented Reality in Scoliosis Correction Surgery: Efficiency and Accuracy in Pedicle Screw Instrumentation. Medicina 2025, 61, 576. https://doi.org/10.3390/medicina61040576
Chang C-N, Li C-R, Liao S-S, Shen C-C, Chen K-Y, Lee C-H, Yang M-Y. Augmented Reality in Scoliosis Correction Surgery: Efficiency and Accuracy in Pedicle Screw Instrumentation. Medicina. 2025; 61(4):576. https://doi.org/10.3390/medicina61040576
Chicago/Turabian StyleChang, Chia-Ning, Chi-Ruei Li, Sian-Siang Liao, Chiung-Chyi Shen, Kai-Yuan Chen, Chung-Hsin Lee, and Meng-Yin Yang. 2025. "Augmented Reality in Scoliosis Correction Surgery: Efficiency and Accuracy in Pedicle Screw Instrumentation" Medicina 61, no. 4: 576. https://doi.org/10.3390/medicina61040576
APA StyleChang, C.-N., Li, C.-R., Liao, S.-S., Shen, C.-C., Chen, K.-Y., Lee, C.-H., & Yang, M.-Y. (2025). Augmented Reality in Scoliosis Correction Surgery: Efficiency and Accuracy in Pedicle Screw Instrumentation. Medicina, 61(4), 576. https://doi.org/10.3390/medicina61040576






