Cardiac Telerehabilitation After Heart Attack Using E-Learning Platforms and Monitoring Cardiovascular Risk Factors: A Narrative Review of the Literature
Abstract
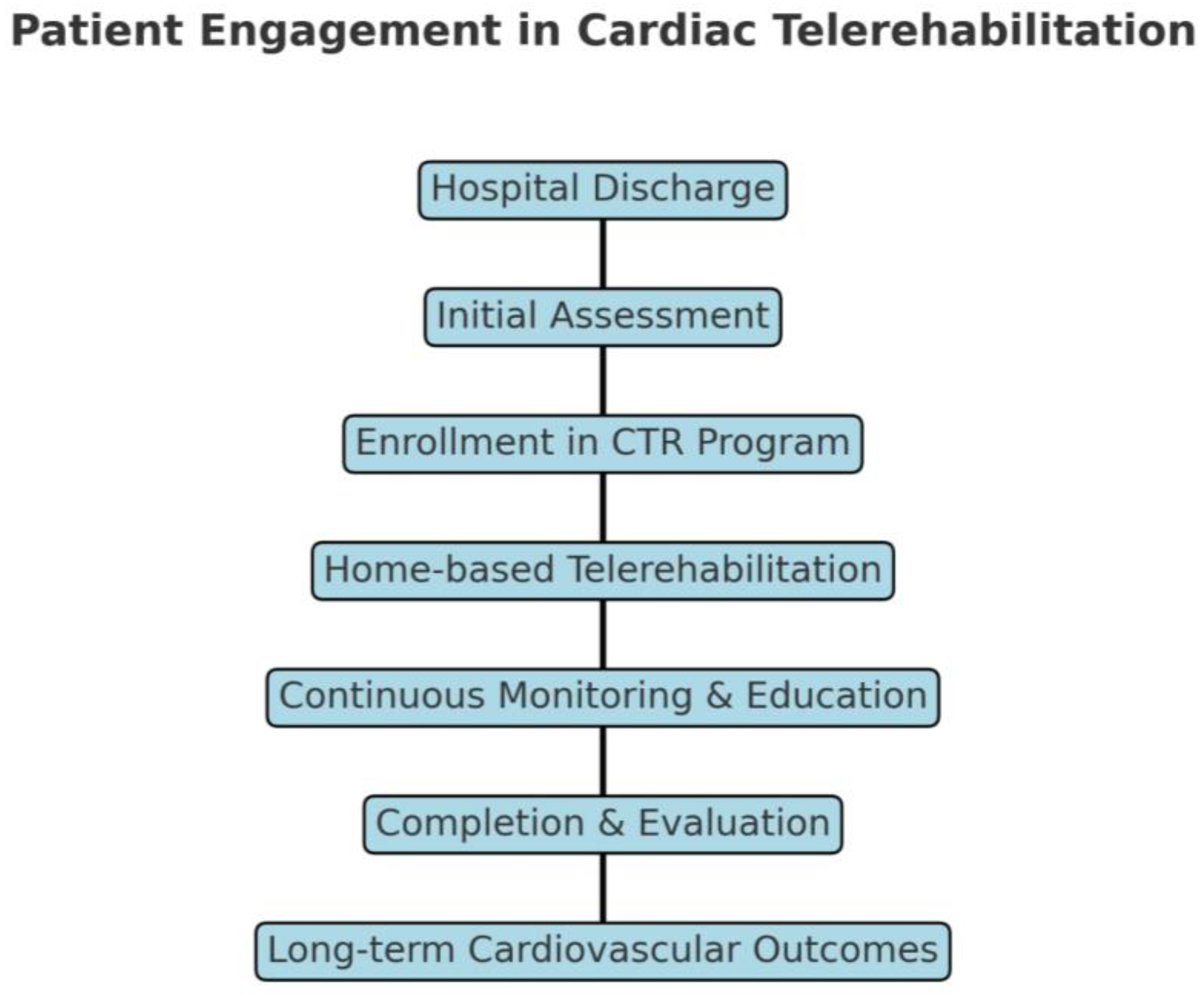
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Methodological Variability
3.2. Long-Term Outcomes and Follow-Up Limitations
3.3. Equity and Accessibility in CTR
3.4. Technological Challenges and Wearable Devices Standardization in CTR
3.5. Non-Exercise Components in CTR
3.6. Cost-Effectiveness of CTR
3.7. Limitations
3.8. Future Research Directions
- Advancing personalization in CTR
- 2.
- Addressing accessibility and health disparities
- 3.
- Evaluating long-term outcomes
- 4.
- Integrating emerging technologies in CTR
- 5.
- Redefining the future of CR through CTR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AMI | Acute myocardial infarction |
| AHA/ACC | American Heart Association/American College of Cardiology |
| BMI | Body mass index |
| CABG | Coronary artery bypass graft |
| CR | Cardiac rehabilitation |
| CTR | Cardiac telerehabilitation |
| CVD | Cardiovascular disease |
| CVRF | Cardiovascular risk factor |
| DHI | Digital health interventions |
| eHealth | Electronic health |
| EHRs | Electronic health records |
| ESC | European Society of Cardiology |
| GDPR | General Data Protection Regulation |
| HDL-C | High-density lipoprotein cholesterol |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| iCBT | Internet-based cognitive behavioral therapy |
| ICER | Incremental Cost-Effectiveness Ratio |
| IMDRF | International Medical Device Regulators Forum |
| IoT | Internet of Things |
| MACE | Major adverse cardiovascular events |
| METs | Metabolic equivalents |
| mHealth | Mobile health |
| ML | Machine Learning |
| PCI | Percutaneous coronary intervention |
| QALY | Quality-Adjusted Life Years |
| RCT | Randomized controlled trial |
| ROI | Return on investment |
| SaMD | Software as a medical device |
| SBP | Systolic blood pressure |
| STEMI | ST-elevation myocardial infarction |
| TAVI | Transcatheter aortic valve implantation |
| VO2 max | Maximum rate of oxygen consumption |
| VR | Virtual reality |
| VV | Virtual visit |
References
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Chindhy, S.; Taub, P.R.; Lavie, C.J.; Shen, J. Current Challenges in Cardiac Rehabilitation: Strategies to Overcome Social Factors and Attendance Barriers. Expert Rev. Cardiovasc. Ther. 2020, 18, 777. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; Dimaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease: The Task Force on Sports Cardiology and Exercise in Patients with Cardiovascular Disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Bauersachs, J.; Dendale, P.; Edvardsen, T.; Gale, C.P.; Jobs, A.; Lambrinou, E.; Mehilli, J.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Bracewell, N.J.; Plasschaert, J.; Conti, C.R.; Keeley, E.C.; Conti, J.B. Cardiac Rehabilitation: Effective yet Underutilized in Patients with Cardiovascular Disease. Clin. Cardiol. 2022, 45, 1128. [Google Scholar] [CrossRef]
- Rodrigo, S.F.; Van Exel, H.J.; Van Keulen, N.; Van Winden, L.; Beeres, S.L.M.A.; Schalij, M.J. Referral and Participation in Cardiac Rehabilitation of Patients Following Acute Coronary Syndrome; Lessons Learned. Int. J. Cardiol. Heart Vasc. 2021, 36, 100858. [Google Scholar] [CrossRef]
- Najem, M.; Duggan, M.; Gambatese, R.; Hill, R.; Yang, S.J.; Batiste, C.; Funahashi, T.; Nkonde-Price, C. Technology Enabled Home-Based Cardiac Rehabilitation among Women with Cardiovascular Disease: A Longitudinal Cohort Study. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200226. [Google Scholar] [CrossRef]
- Heindl, B.; Ramirez, L.; Joseph, L.; Clarkson, S.; Thomas, R.; Bittner, V. Hybrid Cardiac Rehabilitation—The State of the Science and the Way Forward. Prog. Cardiovasc. Dis. 2022, 70, 175–182. [Google Scholar] [CrossRef]
- Seron, P.; Oliveros, M.J.; Marzuca-Nassr, G.N.; Morales, G.; Román, C.; Muñoz, S.R.; Gálvez, M.; Latin, G.; Marileo, T.; Molina, J.P.; et al. Hybrid Cardiac Rehabilitation Program in a Low-Resource Setting: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2350301. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.İ. Telemedicine: Current Concepts and Future Perceptions. Anatol. J. Cardiol. 2019, 22, 21. [Google Scholar] [CrossRef]
- Hayıroğlu, M.İ.; Çınar, T.; Cilli Hayıroğlu, S.; Şaylık, F.; Uzun, M.; Tekkeşin, A.İ. The Role of Smart Devices and Mobile Application on the Change in Peak VO2 in Patients with High Cardiovascular Risk: A Sub-Study of the LIGHT Randomised Clinical Trial. Acta Cardiol. 2023, 78, 1000–1005. [Google Scholar] [CrossRef]
- Milewski, K.; Balsam, P.; Kachel, M.; Sitek, B.; Kolarczyk-Haczyk, A.; Skoczyński, S.; Hirnle, P.; Gawałko, M.; Kołtowski, Ł.; Główczyńska, R.; et al. Actual Status and Future Directions of Cardiac Telerehabilitation. Cardiol. J. 2023, 30, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, R.; Cheng, H.; Xu, L.; Wang, L.; He, C.; Wei, Q. Longer-Term Effects of Cardiac Telerehabilitation on Patients With Coronary Artery Disease: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2023, 11, e46359. [Google Scholar] [CrossRef]
- Ansari, S.; Nadar, B.G.; Estêvão, M.D.; Aguiar, D.R.; Ejeh, J.; Khan, Z. Comparing the Outcomes of Digital and Traditional Cardiac Rehabilitation Practices: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e77757. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.; O’Carroll, V. The Effectiveness of Cardiac Telerehabilitation in Comparison to Centre-Based Cardiac Rehabilitation Programmes: A Literature Review. J. Telemed. Telecare 2022, 30, 631. [Google Scholar] [CrossRef]
- Maddison, R.; Rawstorn, J.C.; Stewart, R.A.H.; Benatar, J.; Whittaker, R.; Rolleston, A.; Jiang, Y.; Gao, L.; Moodie, M.; Warren, I.; et al. Effects and Costs of Real-Time Cardiac Telerehabilitation: Randomised Controlled Non-Inferiority Trial. Heart 2019, 105, 122–129. [Google Scholar] [CrossRef]
- Tedeschi, A.; Palazzini, M.; Trimarchi, G.; Conti, N.; Di Spigno, F.; Gentile, P.; D’Angelo, L.; Garascia, A.; Ammirati, E.; Morici, N.; et al. Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. J. Clin. Med. 2024, 13, 2592. [Google Scholar] [CrossRef]
- Liu, J.-C.; Cheng, C.-Y.; Cheng, T.-H.; Liu, C.-N.; Chen, J.-J.; Hao, W.-R.; Liu, J.-C.; Cheng, C.-Y.; Cheng, T.-H.; Liu, C.-N.; et al. Unveiling the Potential: Remote Monitoring and Telemedicine in Shaping the Future of Heart Failure Management. Life 2024, 14, 936. [Google Scholar] [CrossRef]
- Mariani, M.V.; Pierucci, N.; Forleo, G.B.; Schiavone, M.; Bernardini, A.; Gasperetti, A.; Mitacchione, G.; Mei, M.; Giunta, G.; Piro, A.; et al. The Feasibility, Effectiveness and Acceptance of Virtual Visits as Compared to In-Person Visits among Clinical Electrophysiology Patients during the COVID-19 Pandemic. J. Clin. Med. 2023, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Xu, T.; Shi, G.; Li, J.; Shuai, W.; Yang, Y.; Yang, Y.; Tian, W.; Zhou, Y. Cardiac Telerehabilitation under 5G Internet of Things Monitoring: A Randomized Pilot Study. Sci. Rep. 2023, 13, 18886. [Google Scholar] [CrossRef] [PubMed]
- Calvo-López, M.; Tolós, R.A.; Expósito, J.M.; Gruosso, D.; Andrea, R.; Roque, M.; Falces, C.; Yago, G.; Araguas, J.S.; Pastor, N.; et al. Cardio4Health Study, a Cardiac Telerehabilitation Pilot Program Aimed at Patients After an Ischemic Event: Cross-Sectional Study. JMIR Cardio 2023, 7, e44179. [Google Scholar] [CrossRef] [PubMed]
- Jameie, S.; Haybar, H.; Aslani, A.; Saadat, M. Development and Usability Evaluation of Web-Based Telerehabilitation Platform for Patients After Myocardial Infarction. Stud. Health Technol. Inform. 2019, 261, 68–74. [Google Scholar] [CrossRef]
- Orzechowski, P.; Kowalik, I.; Piotrowicz, E. Feasibility of Hybrid Telerehabilitation as a Component of the Managed Care after Acute Myocardial Infarction (MC-AMI) Program in a 12-Month Follow-up: Experience from a Single Center. Pol. Arch. Intern. Med. 2023, 133, 16456. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Anifanti, M.; Koukouvou, G.; Ntovoli, A.; Alexandris, K.; Kouidi, E. Exploring the Effects of Real-Time Online Cardiac Telerehabilitation Using Wearable Devices Compared to Gym-Based Cardiac Exercise in People with a Recent Myocardial Infarction: A Randomised Controlled Trial. Front. Cardiovasc. Med. 2024, 11, 1410616. [Google Scholar] [CrossRef]
- Ślązak, A.; Przybylska, I.; Paprocka-Borowicz, M. Evaluation of Change in Body Composition, Including Phase Angle, in Post-Myocardial Infarction Patients Rehabilitated under the KOS-Zawał (MC-AMI) Programme. J. Clin. Med. 2024, 13, 2784. [Google Scholar] [CrossRef]
- Milewski, K.; Małecki, A.; Orszulik-Baron, D.; Kachel, M.; Hirnle, P.; Orczyk, M.; Dunal, R.; Mikołajowski, G.; Janas, A.; Nowak, Z.; et al. The Use of Modern Telemedicine Technologies in an Innovative Optimal Cardiac Rehabilitation Program for Patients after Myocardial Revascularization: Concept and Design of RESTORE, a Randomized Clinical Trial. Cardiol. J. 2019, 26, 594–603. [Google Scholar] [CrossRef]
- Krzowski, B.; Boszko, M.; Peller, M.; Hoffman, P.; Żurawska, N.; Skoczylas, K.; Osak, G.; Kołtowski, Ł.; Grabowski, M.; Opolski, G.; et al. Mobile App and Digital System for Patients after Myocardial Infarction (AfterAMI): Results from a Randomized Trial. J. Clin. Med. 2023, 12, 2886. [Google Scholar] [CrossRef]
- Salarvand, S.; Farzanpour, F.; Gharaei, H.A. The Effect of Personalized Mobile Health (MHealth) in Cardiac Rehabilitation for Discharged Elderly Patients after Acute Myocardial Infarction on Their Inner Strength and Resilience. BMC Cardiovasc. Disord. 2024, 24, 116. [Google Scholar] [CrossRef]
- Treskes, R.W.; van Winden, L.A.M.; van Keulen, N.; van der Velde, E.T.; Beeres, S.L.M.A.; Atsma, D.E.; Schalij, M.J. Effect of Smartphone-Enabled Health Monitoring Devices vs. Regular Follow-up on Blood Pressure Control Among Patients After Myocardial Infarction: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e202165. [Google Scholar] [CrossRef] [PubMed]
- Zullig, L.L.; Peterson, E.D.; Shah, B.R.; Grambow, S.C.; Oddone, E.Z.; McCant, F.; Lindquist, J.H.; Bosworth, H.B. Secondary Prevention Risk Interventions via Telemedicine and Tailored Patient Education (SPRITE): A Randomized Trial to Improve Post Myocardial Infarction Management. Patient Educ. Couns. 2022, 105, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Krackhardt, F.; Maier, L.S.; Appel, K.F.; Köhler, T.; Ghanem, A.; Tschoepe, C.; vom Dahl, J.; Degenhardt, R.; Niklasson, A.; Ahlqvist, M.; et al. Design and Rationale for the “Me & My Heart” (EMocial) Study: A Randomized Evaluation of a New Smartphone-Based Support Tool to Increase Therapy Adherence of Patients with Acute Coronary Syndrome. Clin. Cardiol. 2019, 42, 1054–1062. [Google Scholar] [CrossRef]
- Manzoor, S.; Hisam, A.; Aziz, S.; Mashhadi, S.F.; Ul Haq, Z. Effectiveness of Mobile Health Augmented Cardiac Rehabilitation on Behavioural Outcomes among Post-Acute Coronary Syndrome Patients: A Randomised Controlled Trial. J. Coll. Physicians Surg. Pak. 2021, 31, 1148–1153. [Google Scholar] [CrossRef]
- Ding, E.Y.; Erskine, N.; Stut, W.; McManus, D.D.; Peterson, A.; Wang, Z.; Valle, J.E.; Albuquerque, D.; Alonso, A.; Botkin, N.F.; et al. MI-PACE Home-Based Cardiac Telerehabilitation Program for Heart Attack Survivors: Usability Study. JMIR Hum. Factors 2021, 8, e18130. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; Houben, V.J.G.; Kraal, J.J.; Spee, R.F.; Kemps, H.M.C. Predictors of Cardiac Rehabilitation Referral, Enrolment and Completion after Acute Myocardial Infarction: An Exploratory Study. Neth. Heart J. 2021, 29, 151–157. [Google Scholar] [CrossRef]
- Diby, K.F.; Gnaba, A.; Ouattara, P.; Ayegnon, G.; Coulibaly, A.; Tro, G.; Dakoi, S.A.; Sall, F.; Adoubi, A.; N’guessan, K.E.; et al. Tele-ECG Improves Diagnosis of Acute Coronary Syndrome and ST-Elevation Myocardial Infarction in Côte d’Ivoire. Digit. Health 2024, 10, 20552076241262276. [Google Scholar] [CrossRef]
- Teixeira, A.B.; Zancaner, L.F.; Ribeiro, F.F.d.F.; Pintyá, J.P.; Schmidt, A.; Maciel, B.C.; Marin-Neto, J.A.; Miranda, C.H. Reperfusion Therapy Optimization in Acute Myocardial Infarction with ST-Segment Elevation Using WhatsApp®-Based Telemedicine. Arq. Bras. Cardiol. 2022, 118, 556–564. [Google Scholar] [CrossRef]
- Mehta, S.; Grines, C.L.; Botelho, R.; Fernandez, F.; Cade, J.; Dusilek, C.; Prudente, M.; Cavalcanti, R.; Campos, C.; Alcocer Gamba, M. STEMI Telemedicine for 100 Million Lives. Catheter. Cardiovasc. Interv. 2021, 98, 1066–1071. [Google Scholar] [CrossRef]
- Kim, J.; Aryee, L.M.D.; Bang, H.; Prajogo, S.; Choi, Y.K.; Hoch, J.S.; Prado, E.L. Effectiveness of Digital Mental Health Tools to Reduce Depressive and Anxiety Symptoms in Low- and Middle-Income Countries: Systematic Review and Meta-Analysis. JMIR Ment. Health 2023, 10, e43066. [Google Scholar] [CrossRef]
- Humphries, S.M.; Wallert, J.; Norlund, F.; Wallin, E.; Burell, G.; Von Essen, L.; Held, C.; Olsson, E.M.G. Internet-Based Cognitive Behavioral Therapy for Patients Reporting Symptoms of Anxiety and Depression After Myocardial Infarction: U-CARE Heart Randomized Controlled Trial Twelve-Month Follow-Up. J. Med. Internet Res. 2021, 23, e25465. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, T.; Scherrenberg, M.; Intan-Goey, V.; Falter, M.; Kindermans, H.; Frederix, I.; Dendale, P. Efficacy of Digital Health Interventions on Depression and Anxiety in Patients with Cardiac Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. Digit. Health 2022, 3, 445. [Google Scholar] [CrossRef]
- Forbes, A.; Keleher, M.R.; Venditto, M.; DiBiasi, F. Assessing Patient Adherence to and Engagement With Digital Interventions for Depression in Clinical Trials: Systematic Literature Review. J. Med. Internet Res. 2023, 25, e43727. [Google Scholar] [CrossRef]
- Şaylık, F.; Çınar, T.; Hayıroğlu, M.İ.; Tekkeşin, A.İ. Digital Health Interventions in Patient Management Following Acute Coronary Syndrome: A Meta-Analysis of the Literature. Anatol. J. Cardiol. 2023, 27, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Falter, M.; Scherrenberg, M.; Kindermans, H.; Kizilkilic, S.; Kaihara, T.; Dendale, P. Willingness to Participate in Cardiac Telerehabilitation: Results from Semi-Structured Interviews. Eur. Heart J. Digit. Health 2021, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Urbančíková, N.; Manakova, N.; Bielcheva, G. Socio-Economic and Regional Factors of Digital Literacy Related to Prosperity. Qual. Innov. Prosper. 2017, 21, 124–141. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; Brini, A.; Kuijpers, R.W.F.H.; Kraal, J.J.; Kemps, H.M.C. Predictors of Non-Participation in a Cardiac Telerehabilitation Programme: A Prospective Analysis. Eur. Heart J. Digit. Health 2022, 3, 81–89. [Google Scholar] [CrossRef]
- Samayoa, L.; Grace, S.L.; Gravely, S.; Scott, L.B.; Marzolini, S.; Colella, T.J.F. Sex Differences in Cardiac Rehabilitation Enrollment: A Meta-Analysis. Can. J. Cardiol. 2014, 30, 793–800. [Google Scholar] [CrossRef]
- Sawan, M.A.; Calhoun, A.E.; Fatade, Y.A.; Wenger, N.K. Cardiac Rehabilitation in Women, Challenges and Opportunities. Prog. Cardiovasc. Dis. 2022, 70, 111–118. [Google Scholar] [CrossRef]
- Supervía, M.; Medina-Inojosa, J.R.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.W.; Pérez-Terzic, C.M.; Brewer, L.P.C.; Leth, S.E.; Thomas, R.J. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin. Proc. 2017, 92, S0025-6196(17)30026-5. [Google Scholar] [CrossRef]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and Facilitators to the Use of E-Health by Older Adults: A Scoping Review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef]
- Fitzpatrick, P.J. Improving Health Literacy Using the Power of Digital Communications to Achieve Better Health Outcomes for Patients and Practitioners. Front. Digit. Health 2023, 5, 1264780. [Google Scholar] [CrossRef]
- Lu, J.K.; Wang, W.; Goh, J.; Maier, A.B. A Practical Guide for Selecting Continuous Monitoring Wearable Devices for Community-Dwelling Adults. Heliyon 2024, 10, e33488. [Google Scholar] [CrossRef] [PubMed]
- Digital Health Technologies for Remote Data Acquisition in Clinical Investigations|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations (accessed on 19 March 2025).
- Ema Guideline on Computerised Systems and Electronic Data in Clinical Trials; European Medicines Agency: Amsterdam, The Netherlands, 2021.
- General Data Protection Regulation (GDPR) Compliance Guidelines. Available online: https://gdpr.eu/ (accessed on 19 March 2025).
- International Medical Device Regulators Forum (IMDRF)|International Medical Device Regulators Forum. Available online: https://www.imdrf.org/ (accessed on 19 March 2025).
- ISO 27001:2013; A Guide to Information Security Management. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 13485:2016; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59752.html (accessed on 19 March 2025).
- ISO 82304-1:2016; Health Software—Part 1: General Requirements for Product safety. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59543.html (accessed on 19 March 2025).
- ISO—International Organization for Standardization. Available online: https://www.iso.org/home.html (accessed on 19 March 2025).
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef]
- Frederix, I.; Vanhees, L.; Dendale, P.; Goetschalckx, K. A Review of Telerehabilitation for Cardiac Patients. J. Telemed. Telecare 2015, 21, 45–53. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; Van Der Poort, E.K.J.; Kemps, H.M.C.; Van Den Akker-Van Marle, M.E.; Kraal, J.J. Cost-Effectiveness of Cardiac Telerehabilitation With Relapse Prevention for the Treatment of Patients With Coronary Artery Disease in the Netherlands. JAMA Netw. Open 2021, 4, e2136652. [Google Scholar] [CrossRef]
- Batalik, L.; Filakova, K.; Sladeckova, M.; Dosbaba, F.; Su, J.; Pepera, G. The Cost-Effectiveness of Exercise-Based Cardiac Telerehabilitation Intervention: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2023, 59, 248. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Mojdehdehbaher, S. AI-Driven Telerehabilitation: Benefits and Challenges of a Transformative Healthcare Approach. AI 2025, 6, 62. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Y.; Ke, Q.Q.; Su, J.K.; Yang, Q.H. Mobilizing Artificial Intelligence to Cardiac Telerehabilitation. Rev. Cardiovasc. Med. 2022, 23, 45. [Google Scholar] [CrossRef]
| Type | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Type of Article | Randomized Controlled Trials, Prospective/Retrospective Cohort Study, Original Study, Pilot Study, Open Access publications | Narrative Review, Critical Review, Closed Access publications (full-text only available for a fee) |
| Population | Male and female patients following a myocardial infarction (STEMI) or acute coronary syndrome (Non-STEMI, unstable angina) | Cardiac patients without a history of myocardial infarction or acute coronary syndrome, including those with heart failure with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF), hypertension, post-transcatheter aortic valve implantation (TAVI), arrhythmias, chronic coronary syndrome, bypass grafting (CABG), significant neurological and psychological disorders, mobility impairments, and the very elderly |
| Interventions | Telemedicine or cardiac telerehabilitation involving home-based patients, smart wearable devices, exercise programs, and dietary interventions | Cardiac rehabilitation programs conducted in hospitals without a home-based component |
| Outcomes | Patient compliance with treatments, level of improvement in heart function after telerehabilitation, percentage of patients who experienced or did not experience a new heart attack, accessibility to smart devices, efficiency of online communication, risk/benefit analysis, and the percentage of patients from rural and urban environments | Articles lacking a significant impact on the role of post-infarction cardiac telerehabilitation or those that did not monitor cardiovascular risk factors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trache, D.; Șerbănoiu, L.I.; Bistriceanu, M.I.A.; Olteanu, G.; Andronic, O.; Călin, L.; Busnatu, Ș.-S. Cardiac Telerehabilitation After Heart Attack Using E-Learning Platforms and Monitoring Cardiovascular Risk Factors: A Narrative Review of the Literature. Medicina 2025, 61, 635. https://doi.org/10.3390/medicina61040635
Trache D, Șerbănoiu LI, Bistriceanu MIA, Olteanu G, Andronic O, Călin L, Busnatu Ș-S. Cardiac Telerehabilitation After Heart Attack Using E-Learning Platforms and Monitoring Cardiovascular Risk Factors: A Narrative Review of the Literature. Medicina. 2025; 61(4):635. https://doi.org/10.3390/medicina61040635
Chicago/Turabian StyleTrache, Dragoș, Liviu Ionuț Șerbănoiu, Mircea Ioan Alexandru Bistriceanu, Gabriel Olteanu, Octavian Andronic, Liviu Călin, and Ștefan-Sebastian Busnatu. 2025. "Cardiac Telerehabilitation After Heart Attack Using E-Learning Platforms and Monitoring Cardiovascular Risk Factors: A Narrative Review of the Literature" Medicina 61, no. 4: 635. https://doi.org/10.3390/medicina61040635
APA StyleTrache, D., Șerbănoiu, L. I., Bistriceanu, M. I. A., Olteanu, G., Andronic, O., Călin, L., & Busnatu, Ș.-S. (2025). Cardiac Telerehabilitation After Heart Attack Using E-Learning Platforms and Monitoring Cardiovascular Risk Factors: A Narrative Review of the Literature. Medicina, 61(4), 635. https://doi.org/10.3390/medicina61040635








