Evaluation of Prefrontal Cortex Activation and Static Balance Mechanisms in Adolescent Idiopathic Scoliosis Using fNIRS
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup and Study Design
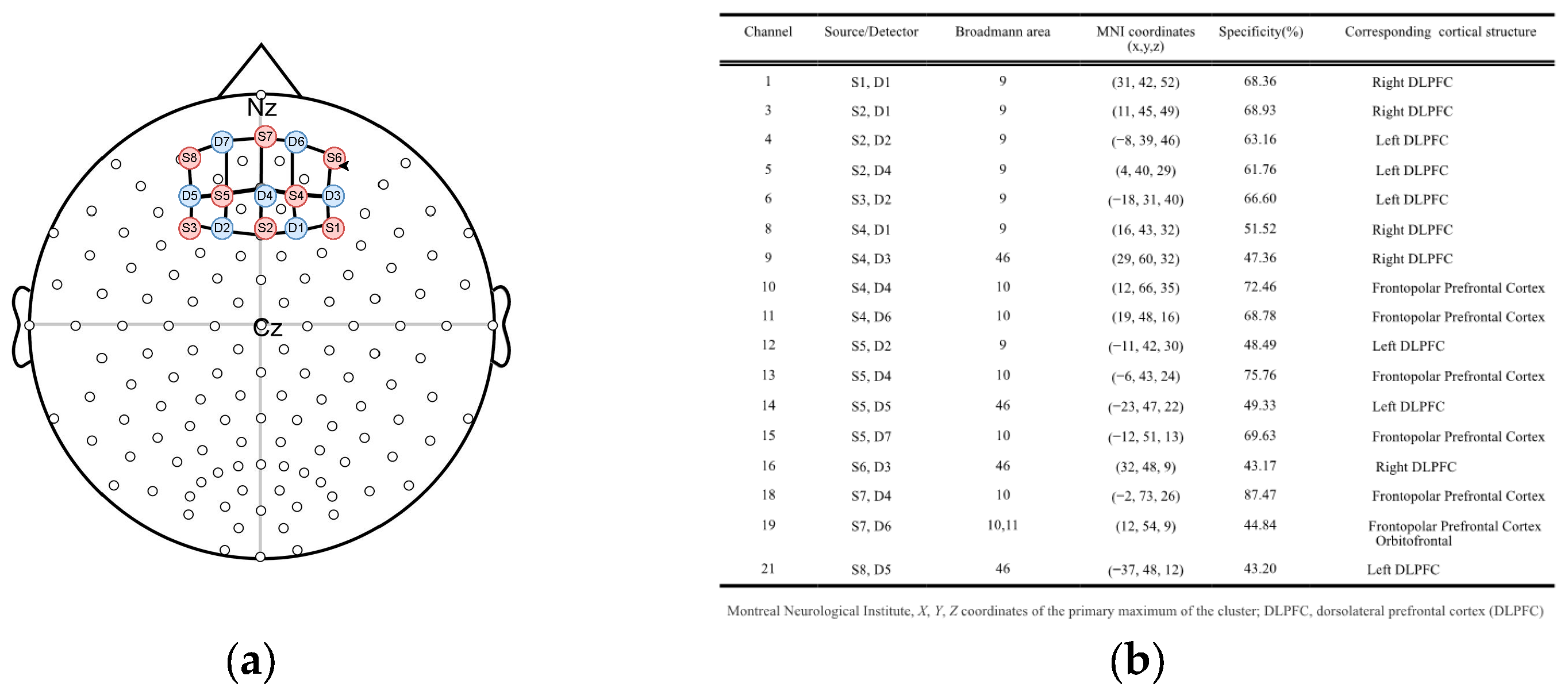
2.3. fNIRS Data Acquisition
2.4. fNIRS Data Processing and Analyses
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Archer, J.E.; Baird, C.; Gardner, A.; Rushton, A.B.; Heneghan, N.R. Evaluating measures of quality of life in adult scoliosis: A systematic review and narrative synthesis. Spine Deform. 2022, 10, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Negrini, F.; Fusco, C.; Zaina, F. Idiopathic scoliosis patients with curves more than 45 Cobb degrees refusing surgery can be effectively treated through bracing with curve improvements. Spine J. 2011, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.; Negrini, S.; Rigo, M.; Kotwicki, T.; Hawes, M.C.; Grivas, T.B.; Maruyama, T.; Landauer, F. Indications for conservative management of scoliosis (SOSORT guidelines). Stud. Health Technol. Inform. 2008, 135, 164. [Google Scholar] [PubMed]
- HwangBo, P.N. The effects of Pilates exercise using the three dimensional Schroth breathing technique on the physical factors of scoliosis patients. J. Korean Phys. Ther. 2018, 30, 229–233. [Google Scholar] [CrossRef]
- Rrecaj-Malaj, S.; Hykolli, A.; Lumi, S.; Murtezani, A. Quality of life in adolescent’s idiopathic scoliosis before and after physical therapy: A preliminary study. Sport Mont 2018, 16, 69–72. [Google Scholar] [CrossRef]
- Borysov, M.; Moramarco, M.; Sy, N.; Lee, S.G. Postural re-education of scoliosis-state of the art (mini-review). Curr. Pediatr. Rev. 2016, 12, 12–16. [Google Scholar] [CrossRef]
- Gouttebarge, V.; Zwerver, J.; Verhagen, E. Preventing musculoskeletal injuries among recreational adult volleyball players: Design of a randomised prospective controlled trial. BMC Musculoskelet. Disord. 2017, 18, 333. [Google Scholar] [CrossRef]
- Haber, C.K.; Sacco, M. Scoliosis: Lower limb asymmetries during the gait cycle. Arch. Physiother. 2015, 5, 4. [Google Scholar] [CrossRef]
- Parent, E.C.; Vaclavik, M.; Bourgoin, C.; Hebert, C.; Bouwmeester, M.; Cheslock, S.; Collins, R.; Potgieter, S.; Coles, M.; Schreiber, S. Inventory of patient-reported outcome measures used in the non-operative care of Scoliosis: A scoping review. Children 2023, 10, 239. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 1910, 40, 28. [Google Scholar] [PubMed]
- Takakusaki, K.; Takahashi, M.; Obara, K.; Chiba, R. Neural substrates involved in the control of posture. Adv. Robot. 2017, 31, 2–23. [Google Scholar] [CrossRef]
- Kuo, A.D. An optimal state estimation model of sensory integration in human postural balance. J. Neural Eng. 2005, 2, S235. [Google Scholar]
- Peterka, R.J. Sensory integration for human balance control. Handb. Clin. Neurol. 2018, 159, 27–42. [Google Scholar]
- Mierau, A.; Pester, B.; Hülsdünker, T.; Schiecke, K.; Strüder, H.K.; Witte, H. Cortical correlates of human balance control. Brain Topogr. 2017, 30, 434–446. [Google Scholar] [CrossRef]
- Gebel, A.; Lehmann, T.; Granacher, U. Balance task difficulty affects postural sway and cortical activity in healthy adolescents. Exp. Brain Res. 2020, 238, 1323–1333. [Google Scholar] [CrossRef]
- Lanthier, J.; Simoneau, M.; Knoth, I.S.; Lippe, S.; Bluteau, C.; Fortin, C. Increased EEG alpha peak frequency in adolescents with idiopathic scoliosis during balance control in normal upright standing. Neurosci. Lett. 2020, 722, 134836. [Google Scholar] [CrossRef]
- Formaggio, E.; Bertuccelli, M.; Rubega, M.; Di Marco, R.; Cantele, F.; Gottardello, F.; De Giuseppe, M.; Masiero, S. Brain oscillatory activity in adolescent idiopathic scoliosis. Sci. Rep. 2022, 12, 17266. [Google Scholar] [CrossRef]
- Fortin, C.; Pialasse, J.-P.; Knoth, I.S.; Lippé, S.; Duclos, C.; Simoneau, M. Cortical dynamics of sensorimotor information processing associated with balance control in adolescents with and without idiopathic scoliosis. Clin. Neurophysiol. 2019, 130, 1752–1761. [Google Scholar]
- Paramento, M.; Passarotto, E.; Maccarone, M.C.; Agostini, M.; Contessa, P.; Rubega, M.; Formaggio, E.; Masiero, S. Neurophysiological, balance and motion evidence in adolescent idiopathic scoliosis: A systematic review. PLoS ONE 2024, 19, e0303086. [Google Scholar]
- Rojas, R.F.; Huang, X.; Hernandez-Juarez, J.; Ou, K.-L. Physiological fluctuations show frequency-specific networks in fNIRS signals during resting state. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 2550–2553. [Google Scholar]
- Perrey, S. Possibilities for examining the neural control of gait in humans with fNIRS. Front. Physiol. 2014, 5, 204. [Google Scholar]
- Piper, S.K.; Krueger, A.; Koch, S.P.; Mehnert, J.; Habermehl, C.; Steinbrink, J.; Obrig, H.; Schmitz, C.H. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage 2014, 85, 64–71. [Google Scholar] [PubMed]
- Koch, S.P.; Koendgen, S.; Bourayou, R.; Steinbrink, J.; Obrig, H. Individual alpha-frequency correlates with amplitude of visual evoked potential and hemodynamic response. Neuroimage 2008, 41, 233–242. [Google Scholar]
- Baradaran, Y.; Rojas, R.F.; Goecke, R.; Ghahramani, M. A Systematic Review on Functional Near-Infrared Spectroscopy Concurrent with Quantitative Balance Assessment. IEEE Access 2023, 11, 66641–66671. [Google Scholar] [CrossRef]
- Cobb, W.; London, G.B.; Gastaut, H.; Hess, R., Jr.; Jung, R.; Magnus, O.; Terzian, H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhäupl, K.; Villringer, A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 2000, 12, 623–639. [Google Scholar]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar]
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649. [Google Scholar] [CrossRef] [PubMed]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Setarehdan, S.K.; Boas, D.A.; Yücel, M.A. Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky–Golay filtering. Neurophotonics 2018, 5, 015003. [Google Scholar] [CrossRef] [PubMed]
- Cope, M.; Delpy, D.T. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med. Biol. Eng. Comput. 1988, 26, 289–294. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Cannestra, A.F.; Wartenburger, I.; Obrig, H.; Villringer, A.; Toga, A.W. Functional assessment of Broca’s area using near infrared spectroscopy in humans. Neuroreport 2003, 14, 1961–1965. [Google Scholar] [CrossRef]
- Hirth, C.; Obrig, H.; Valdueza, J.; Dirnagl, U.; Villringer, A. Simultaneous assessment of cerebral oxygenation and hemodynamics during a motor task: A combined near infrared and transcranial Doppler sonography study. In Oxygen Transport to Tissue XVIII. Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1997; pp. 461–469. [Google Scholar]
- Sato, T.; Ito, M.; Suto, T.; Kameyama, M.; Suda, M.; Yamagishi, Y.; Ohshima, A.; Uehara, T.; Fukuda, M.; Mikuni, M. Time courses of brain activation and their implications for function: A multichannel near-infrared spectroscopy study during finger tapping. Neurosci. Res. 2007, 58, 297–304. [Google Scholar] [CrossRef]
- Maki, A.; Yamashita, Y.; Watanabe, E.; Koizumi, H. Visualizing human motor activity by using non-invasive optical topography. Front. Med. Biol. Eng. Int. J. Jpn. Soc. Med. Electron. Biol. Eng. 1996, 7, 285–297. [Google Scholar]
- Miyai, I.; Tanabe, H.C.; Sase, I.; Eda, H.; Oda, I.; Konishi, I.; Tsunazawa, Y.; Suzuki, T.; Yanagida, T.; Kubota, K. Cortical mapping of gait in humans: A near-infrared spectroscopic topography study. Neuroimage 2001, 14, 1186–1192. [Google Scholar] [CrossRef]
- Watanabe, E.; Yamashita, Y.; Maki, A.; Ito, Y.; Koizumi, H. Non-invasive functional mapping with multi-channel near infra-red spectroscopic topography in humans. Neurosci. Lett. 1996, 205, 41–44. [Google Scholar] [PubMed]
- Leff, D.R.; Orihuela-Espina, F.; Elwell, C.E.; Athanasiou, T.; Delpy, D.T.; Darzi, A.W.; Yang, G.-Z. Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage 2011, 54, 2922–2936. [Google Scholar] [PubMed]
- Strangman, G.; Boas, D.A.; Sutton, J.P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry 2002, 52, 679–693. [Google Scholar] [PubMed]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Petkov, M.P.; Borsook, D.; Dan, I.; Boas, D.A. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics 2015, 2, 020801. [Google Scholar] [CrossRef]
- Collins, D.L.; Zijdenbos, A.P.; Kollokian, V.; Sled, J.G.; Kabani, N.J.; Holmes, C.J.; Evans, A.C. Design and construction of a realistic digital brain phantom. IEEE Trans. Med. Imaging 1998, 17, 463–468. [Google Scholar]
- Gupta, R.; Tranel, D. Memory, neural substrates. In Encyclopedia of Human Behavior; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 593–600. [Google Scholar]
- Wittenberg, E.; Thompson, J.; Nam, C.S.; Franz, J.R. Neuroimaging of human balance control: A systematic review. Front. Hum. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef]
- Luks, T.L.; Simpson, G.V.; Dale, C.L.; Hough, M.G. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage 2007, 35, 949–958. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, Y.-H.; Jang, S.H.; Chang, W.H.; Park, C.; Kim, S.T. Dynamic changes in the cortico-subcortical network during early motor learning. NeuroRehabilitation 2010, 26, 95–103. [Google Scholar]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. Neuroimage 2008, 43, 329–336. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Kubota, K. Cortical control of postural balance in patients with hemiplegic stroke. Neuroreport 2012, 23, 314–319. [Google Scholar] [CrossRef]
- Ue, S.; Nakahama, K.; Hayashi, J.; Ohgomori, T. Cortical activity associated with the maintenance of balance during unstable stances. PeerJ 2024, 12, e17313. [Google Scholar] [PubMed]
- Xiao, C.; Liang, Q.; Yang, Y.; Mo, M.; Li, W.; Chen, H.; Long, Y.; Huang, J. Changes in Cerebral Cortex Activation during Upright Standing Tasks in Individuals with Chronic Neck Pain: An fNIRS Study. Front. Neurol. 2025, 16, 1531314. [Google Scholar]
- Lamm, C.; Windischberger, C.; Leodolter, U.; Moser, E.; Bauer, H. Evidence for premotor cortex activity during dynamic visuospatial imagery from single-trial functional magnetic resonance imaging and event-related slow cortical potentials. Neuroimage 2001, 14, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Marx, E.; Stephan, T.; Nolte, A.; Deutschländer, A.; Seelos, K.C.; Dieterich, M.; Brandt, T. Eye closure in darkness animates sensory systems. Neuroimage 2003, 19, 924–934. [Google Scholar]
- Helmich, I.; Coenen, J.; Henckert, S.; Pardalis, E.; Schupp, S.; Lausberg, H. Reduced frontopolar brain activation characterizes concussed athletes with balance deficits. Neuroimage Clin. 2020, 25, 102164. [Google Scholar]
| Demographic Characteristics | p-Value | |||
|---|---|---|---|---|
| Sex | 0.439 | |||
| Control Group | n | % | ||
| Female | 6 | 37.5% | ||
| Male | 10 | 62.5% | ||
| AIS Group | ||||
| Female | 7 | 46.67% | ||
| Male | 8 | 53.33% | ||
| Age (years) | Mean ± SD | t | 95% CI | 0.08 |
| Control Group | 12.75 ± 2.64 | −1.816 | −3.65–0.217 | |
| AIS Group | 14.47 ± 2.615 | |||
| BMI (kg/m2) | Mean ± SD | Median (min–max) | 0.782 | |
| Control Group | 20.39 ± 5.31 | 19.24 (16.82–23.22) | ||
| AIS Group | 20.22 ± 3.60 | 19.14 (17.67–24.03) | ||
| Min | Max | Mean ± SD | ||
| Cobb Thoracic | 5.0 | 27.8 | 13.8 ± 6.765 | |
| Cobb Lumbar | 12.0 | 33.6 | 20.673 ± 7.009 | |
| Channel | Control Group Mean ± SD | AIS Group Mean ± SD | t | Cohen’s d | p-Value |
|---|---|---|---|---|---|
| 1 | 0.158 ± 5.545 | 0.888 ± 4.043 | −0.417 | −0.150 | 0.680 |
| 2 | −1.426 ± 5.257 | 0.286 ± 3.563 | −1.054 | −0.379 | 0.300 |
| 3 | 0.409 ± 4.013 | 0.704 ± 8.179 | −0.129 | −0.046 | 0.333 |
| 4 | 2.086 ± 6.857 | −1.064 ± 10.378 | 1.004 | 0.361 | 0.477 |
| 5 | 0.959 ± 7.093 | 1.471 ± 4.092 | −0.244 | −0.088 | 0.752 |
| 6 | 1.020 ± 6.165 | 1.135 ± 7.875 | −0.134 | −0.048 | 0.895 |
| 7 | −0.769 ± 4.037 | 2.581 ± 6.373 | −1.761 | −0.633 | 0.206 |
| 8 | −0.001 ± 3.417 | 1.938 ± 5.557 | −1.179 | −0.424 | 0.248 |
| 9 | −1.539 ± 4.839 | −0.053 ± 3.629 | −0.962 | −0.346 | 0.344 |
| 10 | −0.554 ± 4.408 | 0.585 ± 4.789 | −0.690 | −0.248 | 0.527 |
| 11 | 1.081 ± 8.393 | 2.413 ± 4.074 | −0.556 | −0.200 | 0.268 |
| 12 | −1.515 ± 3.993 | 2.679 ± 3.992 | −2.920 | −1.049 | 0.007 * |
| 13 | −1.618 ± 4.286 | 1.654 ± 3.345 | −2.268 | −0.848 | 0.030 * |
| 14 | −1.991 ± 3.503 | 0.820 ± 5.906 | −1.625 | −0.569 | 0.115 |
| 15 | −0.696 ± 5.801 | 1.633 ± 4.975 | −1.196 | −0.430 | 0.241 |
| 16 | −0.548 ± 3.533 | −0.395 ± 6.101 | −0.086 | −0.031 | 0.932 |
| 17 | −1.550 ± 5.068 | 2.190 ± 5.676 | −1.938 | −0.697 | 0.062 |
| 18 | −1.702 ± 6.525 | 4.068 ± 7.267 | −2.328 | −0.840 | 0.018 * |
| 19 | −0.265 ± 4.995 | 0.602 ± 7.220 | −0.391 | −0.141 | 0.699 |
| 20 | −0.260 ± 4.881 | 0.411 ± 7.374 | −0.301 | −0.108 | 0.766 |
| 21 | −2.698 ± 12.304 | −0.251 ± 6.874 | −0.677 | −0.243 | 0.664 |
| 22 | −0.945 ± 6.158 | 0.898 ± 5.804 | −0.856 | −0.308 | 0.399 |
| Channel | Control Group Mean ± SD | AIS Group Mean ± SD | t | Cohen’s d | p-Value |
|---|---|---|---|---|---|
| 1 | 2.491 ± 4.063 | −0.103 ± 6.122 | 1.367 | 0.510 | 0.183 |
| 2 | 2.306 ± 6.122 | 0.189 ± 5.211 | 0.976 | 0.365 | 0.338 |
| 3 | 1.841 ± 3.588 | 3.521 ± 8.358 | −0.728 | −0.272 | 0.895 |
| 4 | 3.556 ± 4.860 | 3.364 ± 12.283 | 0.057 | 0.021 | 0.405 |
| 5 | 1.521 ± 3.152 | 2.098 ± 4.4956 | −0.381 | −0.142 | 0.861 |
| 6 | 3.745 ± 5.115 | 2.150 ± 7.125 | 0.701 | 0.262 | 0.489 |
| 7 | 3.189 ± 6.343 | 0.526 ± 6.028 | 1.149 | 0.429 | 0.313 |
| 8 | 1.608 ± 5.133 | 2.459 ± 8.403 | −0.336 | −0.125 | 0.739 |
| 9 | 3.273 ± 6.255 | −0.300 ± 6.772 | 1.475 | 0.551 | 0.152 |
| 10 | 2.548 ± 4.970 | 0.823 ± 5.042 | 0.924 | 0.345 | 0.293 |
| 11 | 3.238 ± 5.443 | 2.791 ± 9.237 | 0.162 | 0.061 | 0.599 |
| 12 | 3.243 ± 4.701 | 0.032 ± 5.646 | 1.672 | 0.624 | 0.106 |
| 13 | 3.343 ± 7.146 | −1.075 ± 5.459 | 1.835 | 0.685 | 0.054 |
| 14 | 3.122 ± 5.738 | −1.279 ± 4.230 | 2.301 | 0.859 | 0.029 * |
| 15 | 5.105 ± 5.854 | −1.483 ± 7.381 | 2.683 | 1.002 | 0.012 * |
| 16 | 4.769 ± 5.232 | −1.938 ± 7.421 | 2.852 | 1.065 | 0.008 * |
| 17 | 2.101 ± 5.152 | −1.693 ± 6.717 | 1.723 | 0.643 | 0.096 |
| 18 | 4.169 ± 5.806 | −0.309 ± 7.666 | 1.791 | 0.669 | 0.048 * |
| 19 | 2.519 ± 5.566 | −1.223 ± 6.223 | 1.709 | 0.638 | 0.099 |
| 20 | 2.711 ± 5.752 | 5.752 ± −3.215 | 2.667 | 0.996 | 0.013 * |
| 21 | 4.536 ± 8.394 | 0.042 ± 7.232 | 1.524 | 0.569 | 0.096 |
| 22 | 2.826 ± 5.971 | −0.705 ± 6.720 | 1.498 | 0.559 | 0.146 |
| Control | AIS | |
|---|---|---|
| EO | 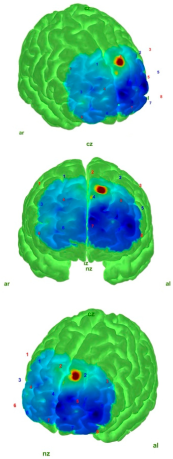 | 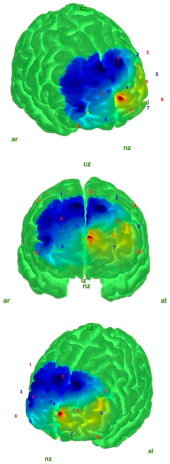 |
| EC | 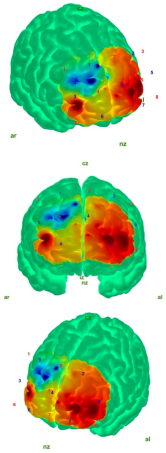 | 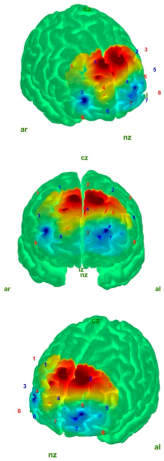 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzen, E.; Tombak, K.; Simsek, B.; Colak, O.H.; Ozen, S. Evaluation of Prefrontal Cortex Activation and Static Balance Mechanisms in Adolescent Idiopathic Scoliosis Using fNIRS. Medicina 2025, 61, 667. https://doi.org/10.3390/medicina61040667
Suzen E, Tombak K, Simsek B, Colak OH, Ozen S. Evaluation of Prefrontal Cortex Activation and Static Balance Mechanisms in Adolescent Idiopathic Scoliosis Using fNIRS. Medicina. 2025; 61(4):667. https://doi.org/10.3390/medicina61040667
Chicago/Turabian StyleSuzen, Esra, Kadriye Tombak, Buket Simsek, Omer Halil Colak, and Sukru Ozen. 2025. "Evaluation of Prefrontal Cortex Activation and Static Balance Mechanisms in Adolescent Idiopathic Scoliosis Using fNIRS" Medicina 61, no. 4: 667. https://doi.org/10.3390/medicina61040667
APA StyleSuzen, E., Tombak, K., Simsek, B., Colak, O. H., & Ozen, S. (2025). Evaluation of Prefrontal Cortex Activation and Static Balance Mechanisms in Adolescent Idiopathic Scoliosis Using fNIRS. Medicina, 61(4), 667. https://doi.org/10.3390/medicina61040667








