A Systematic Review and Meta-Analysis of MIP-1α and MIP-1β Chemokines in Malaria in Relation to Disease Severity
Abstract
1. Introduction
2. Methods
2.1. Protocol of Systematic Review
2.2. Definitions
2.3. The Research Question for This Systematic Review
2.4. Database Searches
2.5. Eligibility Criteria
2.6. Study Selection and Data Extraction
2.7. Risk of Bias Assessment
2.8. Data Synthesis
3. Results
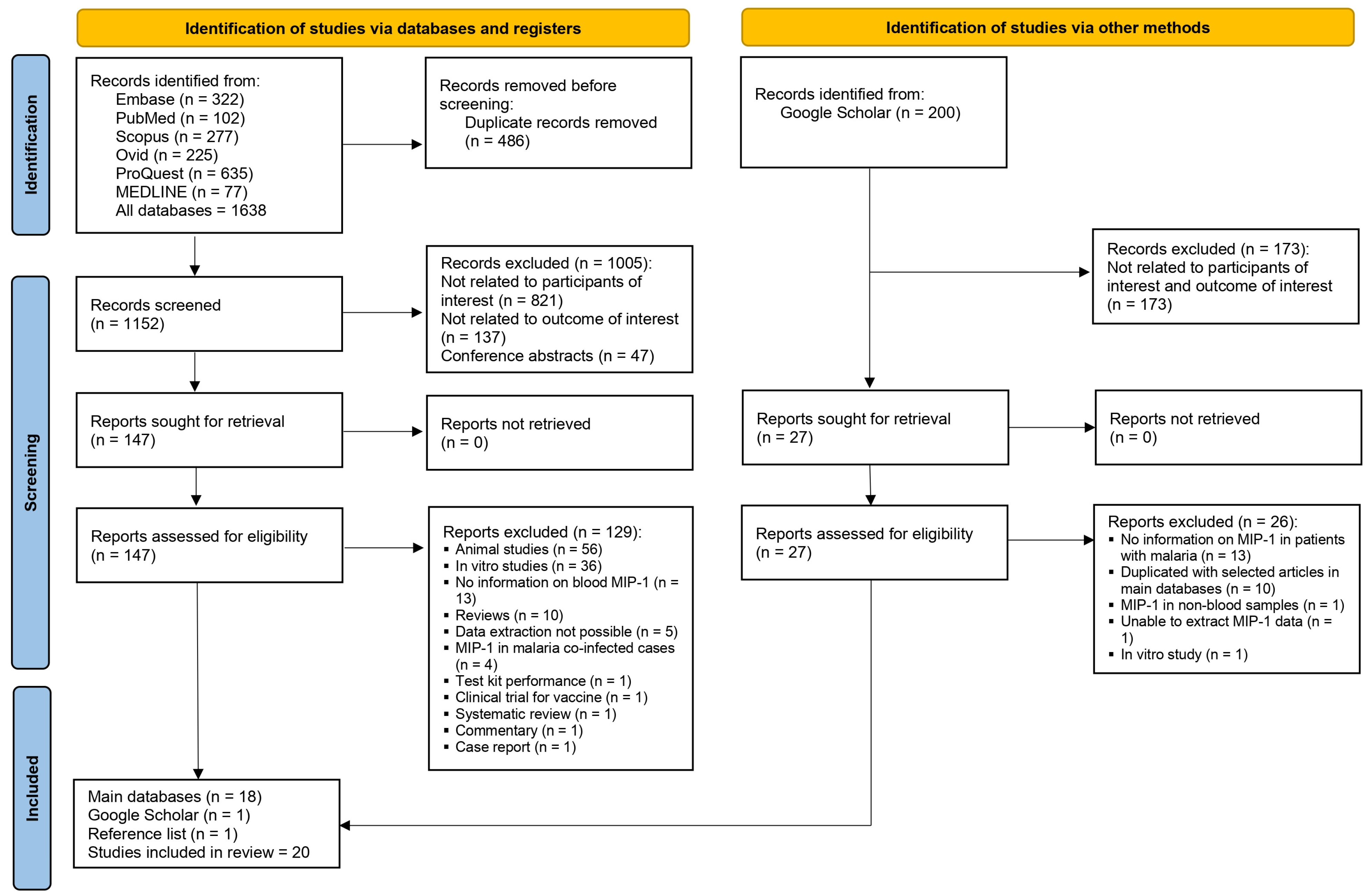
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Risk of Bias
3.4. MIP-1α in Participants with Plasmodium Infections
3.5. MIP-1β in Plasmodium Infections
3.6. MIP-1α/MIP-1β Between Participants with Severe and Uncomplicated Malaria
3.7. MIP-1α/MIP-1β Between Different Severe Malarial Complications, Fatality, and Plasmodium Species
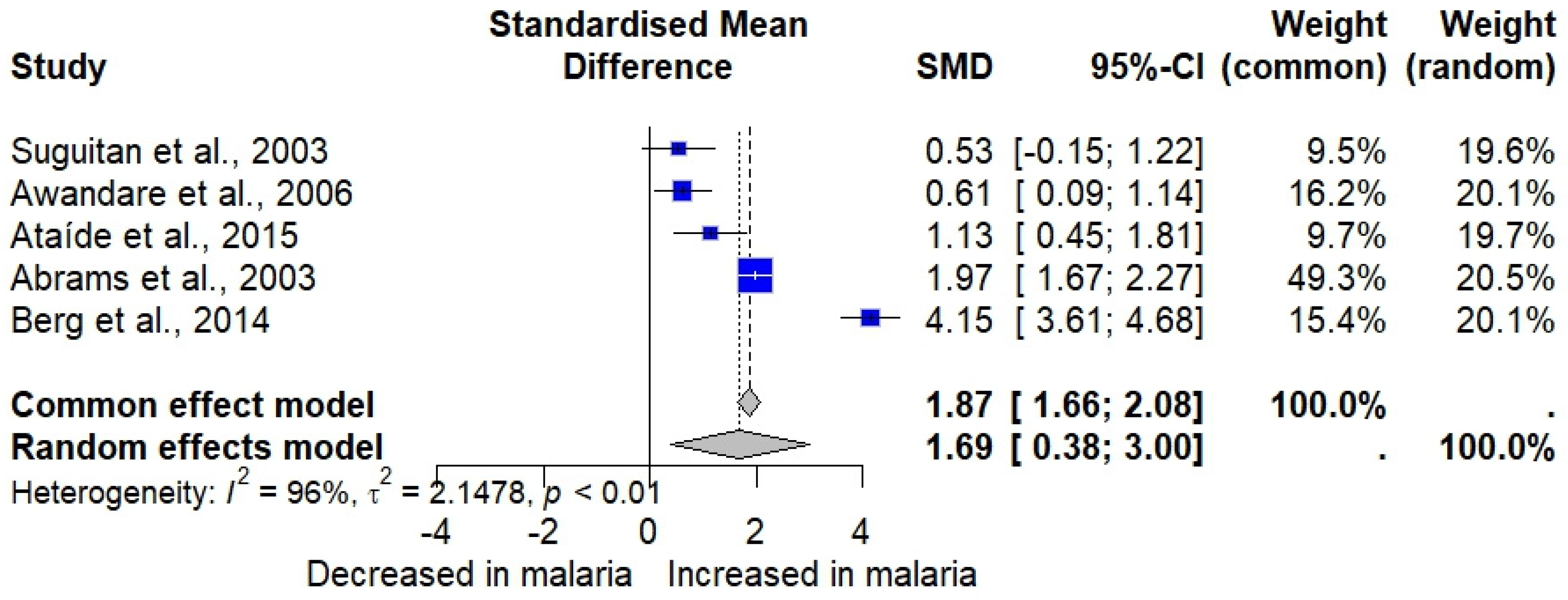
3.8. Meta-Analysis of MIP-1α/MIP-1β in Malaria Cases and Uninfected Individuals
3.9. Meta-Analysis of MIP-1α/MIP-1β in Severe and Uncomplicated Malaria Cases
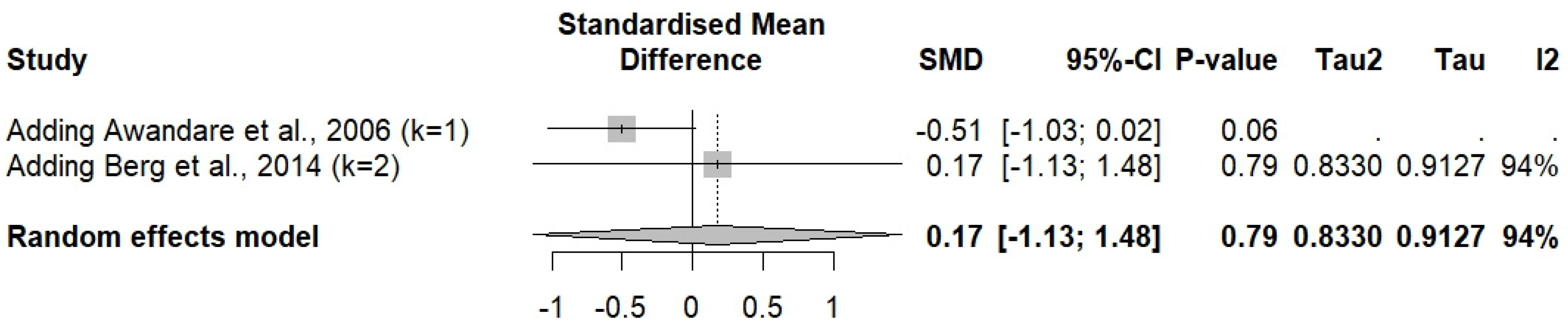
3.10. Sensitivity Analysis
3.11. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 30 September 2024).
- Dayananda, K.K.; Achur, R.N.; Gowda, D.C. Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vector Borne Dis. 2018, 55, 1–8. [Google Scholar]
- Stevenson, M.M.; Riley, E.M. Innate immunity to malaria. Nat. Rev. Immunol. 2004, 4, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Long, C.A.; Zavala, F. Immune Responses in Malaria. Cold Spring Harb. Perspect. Med. 2017, 7, a025577. [Google Scholar]
- Angulo, I.; Fresno, M. Cytokines in the pathogenesis of and protection against malaria. Clin. Vaccine Immunol. 2002, 9, 1145–1152. [Google Scholar]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar]
- Murdoch, C.; Finn, A. Chemokine receptors and their role in inflammation and infectious diseases. Blood J. Am. Soc. Hematol. 2000, 95, 3032–3043. [Google Scholar]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar]
- Ioannidis, L.J.; Nie, C.Q.; Hansen, D.S. The role of chemokines in severe malaria: More than meets the eye. Parasitology 2014, 141, 602–613. [Google Scholar] [CrossRef]
- Dunst, J.; Kamena, F.; Matuschewski, K. Cytokines and chemokines in cerebral malaria pathogenesis. Front. Cell. Infect. Microbiol. 2017, 7, 324. [Google Scholar] [CrossRef]
- Maurer, M.; von Stebut, E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004, 36, 1882–1886. [Google Scholar]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage inflammatory protein-1 alpha (MIP-1 alpha)/CCL3: As a biomarker. In General Methods in Biomarker Research and Their Applications; Preedy, V.R., Patel, V.B., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 223–249. [Google Scholar]
- Sherry, B.A.; Alava, G.; Tracey, K.J.; Martiney, J.; Cerami, A.; Slater, A.F. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 1995, 45, 85–96. [Google Scholar]
- Sarr, D.; Lucchi, N.W.; Owino, S.; Peterson, D.S.; Moore, J.M. The malarial parasite toxin, hemozoin, activates map kinases and promotes a chemotactic and immunostimulatory secretory response in primary human syncytiotrophoblast. Placenta 2010, 31, A116. [Google Scholar]
- Stanisic, D.I.; Cutts, J.; Eriksson, E.; Fowkes, F.J.I.; Rosanas-Urgell, A.; Siba, P.; Laman, M.; Davis, T.M.E.; Manning, L.; Mueller, I.; et al. γδ T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J. Infect. Dis. 2014, 210, 295–305. [Google Scholar] [CrossRef]
- Ochiel, D.O.; Awandare, G.A.; Keller, C.C.; Hittner, J.B.; Kremsner, P.G.; Weinberg, J.B.; Perkins, D.J. Differential regulation of β-chemokines in children with Plasmodium falciparum malaria. Infect. Immun. 2005, 73, 4190–4197. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Opika-Opoka, R.; Byarugaba, J.; Idro, R.; Boivin, M.J. Low levels of RANTES are associated with mortality in children with cerebral malaria. J. Infect. Dis. 2006, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Armah, H.B.; Tongren, J.E.; Ned, R.M.; Wilson, N.O.; Crawford, S.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Singh, N.; et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar. J. 2008, 7, 1–15. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Malaria, 30 November 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121 Pt 1, 1027–1031. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: Adelaide, Australia, 2024. [Google Scholar]
- Kotepui, M.; Kwankaew, P.; Mahittikorn, A.; Kotepui, K.U.; Masangkay, F.R.; Wattanapisit, A.; Wilairatana, P. A systematic review and meta-analysis of blood level of MCP-1/CCL-2 in severe and uncomplicated malaria. Sci. Rep. 2024, 14, 28738. [Google Scholar] [CrossRef]
- Kwankaew, P.; Mahittikorn, A.; Mala, W.; Kotepui, K.U.; Anabire, N.G.; Wilairatana, P.; Kotepui, M. Association between RANTES/CCL5 levels with Plasmodium infections and malaria severity: A systematic review. Malar. J. 2024, 23, 335. [Google Scholar] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar]
- Sutton, A.; Abrams, K.; Jones, D.; Sheldon, T.; Song, F. Methods for Meta-Analysis in Medical Research; Wiley: Chichester, UK, 2000. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 24 November 2024).
- Abrams, E.T.; Brown, H.; Chensue, S.W.; Turner, G.D.H.; Tadesse, E.; Lema, V.M.; Molyneux, M.E.; Rochford, R.; Meshnick, S.R.; Rogerson, S.J. Host response to malaria during pregnancy: Placental monocyte recruitment is associated with elevated beta chemokine expression. J. Immunol. 2003, 170, 2759–2764. [Google Scholar]
- Armah, H.B.; O Wilson, N.; Sarfo, B.Y.; Powell, M.D.; Bond, V.C.; Anderson, W.; A Adjei, A.; Gyasi, R.K.; Tettey, Y.; Wiredu, E.K.; et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar. J. 2007, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Ataíde, R.; Murillo, O.; Dombrowski, J.G.; Souza, R.M.; Lima, F.A.; Lima, G.F.M.C.; Hristov, A.D.; Valle, S.C.N.; Di Santi, S.M.; Epiphanio, S.; et al. Malaria in pregnancy interacts with and alters the angiogenic profiles of the placenta. PLOS Neglected Trop. Dis. 2015, 9, e0003824. [Google Scholar]
- Awandare, G.A.; Goka, B.; Boeuf, P.; Tetteh, J.K.A.; Kurtzhals, J.A.L.; Behr, C.; Akanmori, B.D. Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress. J. Infect. Dis. 2006, 194, 1438–1446. [Google Scholar] [CrossRef][Green Version]
- Berg, A.; Patel, S.; Gonca, M.; David, C.; Otterdal, K.; Ueland, T.; Dalen, I.; Kvaløy, J.T.; Mollnes, T.E.; Aukrust, P.; et al. Cytokine network in adults with falciparum malaria and HIV-1: Increased IL-8 and IP-10 levels are associated with disease severity. PLoS ONE 2014, 9, e114480. [Google Scholar]
- Burgmann, H.; Hollenstein, U.; Wenisch, C.; Thalhammer, F.; Looareesuwan, S.; Graninger, W. Serum concentrations of MIP-1 alpha and interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin. Immunol. Immunopathol. 1995, 76, 32–36. [Google Scholar]
- Chaisavaneeyakorn, S.; Moore, J.M.; Mirel, L.; Othoro, C.; Otieno, J.; Chaiyaroj, S.C.; Shi, Y.P.; Nahlen, B.L.; Lal, A.A.; Udhayakumar, V. Levels of macrophage inflammatory protein 1α-(MIP-1α) and MIP-1β in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin. Vaccine Immunol. 2003, 10, 631–636. [Google Scholar]
- Cox-Singh, J.; Singh, B.; Daneshvar, C.; Planche, T.; Parker-Williams, J.; Krishna, S. Anti-inflammatory cytokines predominate in acute human Plasmodium knowlesi infections. PLoS ONE 2011, 6, e20541. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Mbengue, B.; Dagamajalu, S.; Fall, M.M.; Loke, M.F.; Nguer, C.M.; Thiam, A.; Vadivelu, J.; Dieye, A. Cytokine response during non-cerebral and cerebral malaria: Evidence of a failure to control inflammation as a cause of death in African adults. PeerJ 2016, 4, e1965. [Google Scholar] [CrossRef]
- Dobaño, C.; Bardají, A.; Arévalo-Herrera, M.; Martínez-Espinosa, F.E.; Bôtto-Menezes, C.; Padilla, N.; Menegon, M.; Kochar, S.; Kochar, S.K.; Unger, H.; et al. Cytokine signatures of Plasmodium vivax infection during pregnancy and delivery outcomes. PLoS Neglected Trop. Dis. 2020, 14, e0008155. [Google Scholar]
- Herbert, F.; Tchitchek, N.; Bansal, D.; Jacques, J.; Pathak, S.; Bécavin, C.; Fesel, C.; Dalko, E.; Cazenave, P.-A.; Preda, C.; et al. Evidence of IL-17, IP-10, and IL-10 involvement in multiple-organ dysfunction and IL-17 pathway in acute renal failure associated to Plasmodium falciparum malaria. J. Transl. Med. 2015, 13, 369. [Google Scholar]
- Obeng-Aboagye, E.; Frimpong, A.; Amponsah, J.A.; Danso, S.E.; Owusu, E.D.A.; Ofori, M.F. Inflammatory cytokines as potential biomarkers for early diagnosis of severe malaria in children in Ghana. Malar. J. 2023, 22, 220. [Google Scholar] [CrossRef]
- Ong’echa, J.M.; Davenport, G.C.; Vulule, J.M.; Hittner, J.B.; Perkins, D.J. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect. Immun. 2011, 79, 4674–4680. [Google Scholar] [PubMed]
- Royo, J.; Vianou, B.; Accrombessi, M.; Kinkpé, E.; Ayédadjou, L.; Dossou-Dagba, I.; Ladipo, Y.; Alao, M.J.; Bertin, G.I.; Cot, M.; et al. Elevated plasma interleukin-8 as a risk factor for mortality in children presenting with cerebral malaria. Infect. Dis. Poverty 2023, 12, 1–15. [Google Scholar] [CrossRef]
- Suguitan, J.A.L.; Leke, R.G.F.; Fouda, G.; Zhou, A.; Thuita, L.; Metenou, S.; Fogako, J.; Megnekou, R.; Taylor, D.W. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 2003, 188, 1074–7082. [Google Scholar] [CrossRef]
- Thuma, P.E.; van Dijk, J.; Bucala, R.; Debebe, Z.; Nekhai, S.; Kuddo, T.; Nouraie, M.; Weiss, G.; Gordeuk, V.R. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J. Infect. Dis. 2010, 203, 211–219. [Google Scholar] [CrossRef]
- Frimpong, A.; Owusu, E.D.A.; Amponsah, J.A.; Obeng-Aboagye, E.; van der Puije, W.; Frempong, A.F.; Kusi, K.A.; Ofori, M.F. Cytokines as potential biomarkers for differential diagnosis of sepsis and other non-septic disease conditions. Front. Cell. Infect. Microbiol. 2022, 12, 901433. [Google Scholar] [CrossRef]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Fahey, T.J., 3rd; Tracey, K.J.; Tekamp-Olson, P.; Cousens, L.S.; Jones, W.G.; Shires, G.T.; Cerami, A.; Sherry, B. Macrophage inflammatory protein 1 modulates macrophage function. J. Immunol. 1992, 148, 2764–2769. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mala, W.; Milanez, G.D.J.; Masangkay, F.R.; Kotepui, K.U.; Kotepui, M. Increased interleukin-6 levels associated with malaria infection and disease severity: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 5982. [Google Scholar] [CrossRef] [PubMed]
- Mahittikorn, A.; Mala, W.; Srisuphanunt, M.; Masangkay, F.R.; Kotepui, K.U.; Wilairatana, P.; Kotepui, M. Tumour necrosis factor-alpha as a prognostic biomarker of severe malaria: A systematic review and meta-analysis. J. Travel Med. 2022, 29, taac053. [Google Scholar] [CrossRef]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef]
- Langhorne, J.; Ndungu, F.M.; Sponaas, A.-M.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- McLean, A.R.; Ataide, R.; Simpson, J.A.; Beeson, J.G.; Fowkes, F.J.I. Malaria and immunity during pregnancy and postpartum: A tale of two species. Parasitology 2015, 142, 999–1015. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Scopel, K.K.; Bastos, M.S.; Ferreira, M.U. Cytokine balance in human malaria: Does Plasmodium vivax elicit more inflammatory responses than Plasmodium falciparum? PLoS ONE 2012, 7, e44394. [Google Scholar] [CrossRef]
- Lillard, J.W., Jr.; Singh, U.P.; Boyaka, P.N.; Singh, S.; Taub, D.D.; McGhee, J.R. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood 2003, 101, 807–814. [Google Scholar] [CrossRef]
- Aliberti, J.C.; Machado, F.S.; Souto, J.T.; Campanelli, A.P.; Teixeira, M.M.; Gazzinelli, R.T.; Silva, J.S. Beta-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect. Immun. 1999, 67, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Bliss, S.K.; Marshall, A.J.; Zhang, Y.; Denkers, E.Y. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-Inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J. Immunol. 1999, 162, 7369–7375. [Google Scholar]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jennes, W.; Vereecken, C.; Fransen, K.; de Roo, A.; Kestens, L. Disturbed secretory capacity for macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta in progressive HIV infection. AIDS Res. Hum. Retroviruses 2004, 20, 1087–1091. [Google Scholar]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [PubMed]
- Alonso-Dominguez, J.; Gallego-Rodriguez, M.; Martinez-Barros, I.; Calderon-Cruz, B.; Leiro-Fernandez, V.; Perez-Gonzalez, A.; Poveda, E. High levels of IL-1beta, TNF-alpha and MIP-1alpha one month after the onset of the acute SARS-CoV-2 infection, predictors of post COVID-19 in hospitalized patients. Microorganisms 2023, 11, 2396. [Google Scholar]
- Sa, V.C.; Silva, T.A.; Reis, C.M.; Cunha, F.Q.; Figueiredo, F.; Bocca, A.L. The pattern of immune cell infiltration in chromoblastomycosis: Involvement of macrophage inflammatory protein-1 alpha/CCL3 and fungi persistence. Rev. Inst. Med. Trop. São Paulo 2007, 49, 49–53. [Google Scholar] [CrossRef]
- Peric, A.; Baletic, N.; Sotirovic, J.; Spadijer-Mirkovic, C. Macrophage inflammatory protein-1 production and eosinophil infiltration in chronic rhinosinusitis with nasal polyps. Ann. Otol. Rhinol. Laryngol. 2015, 124, 266–272. [Google Scholar]


| No. | Authors | Study Location (Conduction Year) | Participants (Number) | Plasmodium Species | Type of Malaria (i.e., Uncomplicated, Severe) | Method for Malaria Detection | Blood Samples for MIP-1 Detection | Method for MIP-1 Detection | Qualitative Blood Levels of MIP-1α and MIP-1β |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abrams et al., 2003 [30] | Malawi (1998–2000) | Pregnant women (537) | P. falciparum | Not specified | Microscopic method | Plasma | ELISA (Quantikine sandwich ELISA kits, R&D Systems, Abingdon, Oxon, UK.) | MIP-1α: Significantly higher MIP-1α levels were observed in malaria-infected individuals than uninfected individuals. MIP-1β: Significantly higher MIP-1β levels were observed in malaria-infected individuals than uninfected individuals. |
| 2 | Armah et al., 2007 [31] | Ghana (2005) | Children: Cerebral malaria (9), severe malarial anemia (5), non-malaria deaths/non-malarial controls (5) | P. falciparum | Severe malaria | Microscopic method | Serum | Commercially available multiplex colorimetric bead-based cytokine immunoassay coupled with the Luminex™ system (Austin, TX, USA) and human-specific bead sets (BioRad, San Diego, CA, USA) | Blood samples: MIP-1α: No difference in MIP-1α levels between cerebral malaria, severe malarial anemia, and non-malarial controls. MIP-1β: No difference in MIP-1β levels between cerebral malaria, severe malarial anemia, and non-malarial controls. CSF samples: MIP-1α: No difference in MIP-1α levels between cerebral malaria, severe malarial anemia, and non-malarial controls. MIP-1β: Significantly higher MIP-1β levels were observed in severe malarial anemia compared to non-malaria cases. Significantly higher MIP-1β levels were observed in cerebral malaria compared to non-malaria cases. No difference in MIP-1β levels between cerebral malaria and severe malarial anemia cases. |
| 3 | Ataíde et al., 2015 [32] | Brazil (2012–2013) | Pregnant women (137): Infected (45), uninfected (92) | P. falciparum, P. vivax | Not specified | Microscopic method/PCR | Plasma | Commercially available Millipore kit HCYTOMAG-60K-07 (IL-1β, IL-10, IL-6, IL-8, MIP-1α, TNF-α, Luminex technology (Luminex® Corp., Austin, TX, USA)) | Significantly higher MIP-1α levels were observed in malaria-infected individuals compared to uninfected individuals. |
| 4 | Awandare et al., 2006 [33] | Ghana (2000–2001) | Children (1–10 years old) with acute malaria: Severe malaria without respiratory distress (38), severe malaria with respiratory distress (18), uncomplicated malaria (24) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | Quantikine ELISA assays (D1000, DTA50, D8050, DMA00, and DMB00; R&D Systems) | MIP-1α: Significantly lower MIP-1α levels were observed in cerebral malaria compared to uncomplicated malaria cases. No difference in MIP-1α between uncomplicated malaria and healthy controls. No difference in MIP-1α between severe malarial anemia and uncomplicated malaria cases. No difference in MIP-1α between cerebral malaria and healthy controls. MIP-1β: No difference in MIP-1β between cerebral malaria, severe malarial anemia, uncomplicated malaria, and healthy controls. |
| 5 | Berg et al., 2014 [34] | Mozambique (2011–2012) | Adults (>18 years): Patients with malaria (131), healthy controls (56) | P. falciparum (131), mixed P. falciparum/P. vivax infections (1), mixed P. falciparum/P. malariae infections | Severe malaria/uncomplicated malaria | Microscopic method/RDT/PCR | Plasma | Multiplex cytokine assay (Bio-Plex Human cytokine 27-plex panel; Bio-Rad Laboratories Inc., Hercules, CA, USA) | MIP-1α: No difference in MIP-1α between malaria patients and healthy controls. No difference in MIP-1α between severe malaria and uncomplicated malaria patients. MIP-1β: Significantly higher MIP-1β levels were observed in malaria patients compared to healthy controls. Significantly higher MIP-1β levels were observed in severe malaria compared to uncomplicated malaria patients. |
| 6 | Burgmann et al., 1995 [35] | Thailand | Adults (15–65 years): Patients with severe malaria (20), healthy controls (15) | P. falciparum | Severe malaria | Microscopic method | Serum | ELISA (Quantikine, R&D Systems) | MIP-1α at admission was higher than healthy controls (the significant difference between groups is unclear). |
| 7 | Chaisavaneeyakorn et al., 2003 [36] | Kenya | Pregnant women (98) | P. falciparum | Uncomplicated malaria | Microscopic method | Plasma | ELISA (R&D Systems Inc. (Minneapolis, MI, USA.) | MIP-1α: No significant difference in MIP-1α between HIV-negative PM-negative and HIV-negative PM-positive. MIP-1β: No significant difference in MIP-1β between HIV-negative PM-negative and HIV-negative PM-positive. |
| 8 | Cox-Singh et al., 2011 [37] | Malaysia (2006–2009) | Adults: P. knowlesi (94), P. vivax (20), P. falciparum (22) | P. knowlesi, P. vivax, P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method/PCR | Serum (plasma in 4 patients) | FluorokineH MAP MultiAnalyte profiling ELISA for LUMINEX Technology (R&D Systems, Inc.) with Bio-Rad Bio-Plex System (Bio-Rad Laboratories Inc.) | MIP-1β: Patients with P. knowlesi malaria had significantly lower MIP-1β levels than those infected with P. falciparum. No difference in MIP-1β between P. knowlesi and P. vivax malaria. No significant difference between the P. vivax and P. falciparum groups. |
| 9 | Dieye et al., 2016 [38] | Sénégal (2012–2014) | Adults: Non-cerebral malaria patients (17), cerebral malaria patients (27): (18 survivors, 9 died), control individuals (18) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Serum | Milliplex MAP kit for human cytokine/chemokine magnetic bead panel (catalog # HCYTMAG-60K-PX29; EMD Millipore Corporation, Billerica, MA, USA) | MIP-1α and MIP-1β levels were significantly higher in malaria patients (non-cerebral and/or cerebral malaria) compared to control individuals. MIP-1α and MIP-1β levels were significantly lower in the survivors compared to cerebral malaria patients who died. |
| 10 | Dobaño et al., 2020 [39] | Brazil, Colombia, Guatemala, India, Papua New Guinea (2008–2012) | Pregnant women: P. vivax-infected (54) and uninfected pregnant women (247) | P. vivax | Not specified | Microscopic method/PCR | Plasma | Cytokine Magnetic 30-Plex Panel (Invitrogen, Madrid, Spain) | MIP-1β levels were significantly higher in P. vivax–infected pregnant women compared to uninfected women (at recruitment). |
| 11 | Frimpong et al., 2022 [46] | Ghana | Children (76): Clinical malaria with no sepsis (33), non-malaria febrile control (20), non-malaria sepsis (23) | P. falciparum | Symptomatic malaria | Microscopic method/RDT | Plasma | Human cytokine magnetic 25-plex panel (Thermo Fisher Scientific Corporation, Waltham, MA, USA) | MIP-1α and MIP-1β levels were higher in malaria compared to febrile control (the authors did not show the significant difference). |
| 12 | Herbert et al., 2015 [40] | India (2008–2010) | Participants aged 13–72 years: Cerebral malaria (42), cerebral malaria patients with multiple organ dysfunction (41), severe non cerebral malaria (53), multiple organ dysfunction (9), uncomplicated malaria (37), severe sepsis patients (10), viral encephalitis patients (9), healthy subjects (21) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method/RDT/PCR | Plasma | Luminex multianalytic profiling (MILLIPLEX® MAP human cytokine/chemokine—Premixed 26 Plex, Millipore, USA) | MIP-1α: Significantly higher MIP-1α levels were observed in malaria patients compared to endemic controls. No difference in MIP-1α between severe non-cerebral malaria/multiorgan dysfunction and mild malaria. No difference in MIP-1α between cerebral malaria/cerebral malaria with multiorgan dysfunction and mild malaria. No difference in MIP-1α between severe non-cerebral malaria and multiorgan dysfunction. MIP-1β: No difference in MIP-1β between malaria patients compared to endemic controls. No difference in MIP-1β between severe non-cerebral malaria/multiorgan dysfunction and mild malaria. No difference in MIP-1β between cerebral malaria/cerebral malaria with multiorgan dysfunction and mild malaria. No difference in MIP-1β between severe non-cerebral malaria and multiorgan dysfunction. |
| 13 | Jain et al., 2008 [19] | India (2004–2006) | Participants aged < 18 and ≥ 18 years: Cerebral malaria survivors (48), cerebral malaria non-survivors (12), healthy controls (25), mild malaria (48) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | A multiplex bead-based cytokine immunoassay (MMA) coupled with the Luminex™ system (Austin, TX, USA) and human-specific bead sets (BioRad, San Diego, CA, USA) | MIP-1α: No difference in MIP-1α between cerebral malaria (survivors or non-survivors), mild malaria, and healthy controls. MIP-1β: Significantly higher MIP-1β levels were observed in mild malaria than healthy controls. Significantly higher MIP-1β levels were observed in cerebral malaria survivors compared to healthy controls. There was no difference in MIP-1β between cerebral malaria non-survivors compared to healthy controls. There was no difference in MIP-1β between cerebral malaria (survivors or non-survivors) and mild malaria. There was no difference in MIP-1β between cerebral malaria survivors and non-survivors. |
| 14 | John et al., 2006 [18] | Uganda | Children aged 4–12 years: Children with cerebral malaria (88), children with uncomplicated malaria (76), community controls (100) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Serum | Colorimetric bead assay using the Luminex system and human-specific bead sets (R&D Systems). | MIP-1α: Significantly higher MIP-1α in cerebral malaria than in community controls. There was no difference in MIP-1α between cerebral malaria and uncomplicated malaria. MIP-1β: A significantly higher MIP-1β in cerebral malaria than in community controls. There was no difference in MIP-1β between cerebral malaria and uncomplicated malaria. |
| 15 | Obeng-Aboagye et al., 2023 [41] | Ghana | Children (57); severe malaria (27), uncomplicated malaria (10), non-malaria-related fever (20) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | A human cytokine magnetic 25-plex panel (Thermo Fisher Scientific Corporation, Waltham, MA, USA) | Significantly higher MIP-1α/MIP-1β levels were observed in severe malaria compared to febrile controls. There was no difference in MIP-1α/MIP-1β between uncomplicated malaria and febrile controls. There was no difference in MIP-1α/MIP-1β between severe and uncomplicated malaria. |
| 16 | Ochiel et al., 2005 [17] | Gabon | Children aged 2–7 years: Severe malaria cases (10), mild malaria cases (10), healthy malaria-exposed subjects (23) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | Quantitative enzyme-linked immunosorbent assay (Biosource International, Camarillo, CA, USA) | MIP-1α/MIP-1β levels were significantly higher in mild malaria/severe malaria compared to healthy controls. MIP-1α/MIP-1β levels were higher in severe malaria than in mild malaria (but not significantly different between groups). |
| 17 | Ong’echa et al., 2011 [42] | Kenya | Children aged 3–30 months: Uncomplicated malaria (31), non-SMA (37), SMA (80) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | Human cytokine 25-plex antibody bead kit (BioSource International) | There was no difference in MIP-1α/MIP-1β between severe malarial anemia, non-severe malarial anemia, and uncomplicated malaria |
| 18 | Royo et al., 2023 [43] | Benin (2018) | Children aged 2–6 years: Cerebral malaria (70): survivors (50), died (20) | P. falciparum | Severe malaria | Microscopic method/PCR | Plasma | The Human Premixed Multi-Analyte Kit (LXSAHM-17, R&D Systems, Lille, France) | There was no difference in MIP-1α/MIP-1β (CCL3/CCL4) between cerebral malaria patients who survived and those who died. |
| 19 | Suguitan et al., 2003 [44] | Cameroon (1996–2000) | Pregnant women: Malaria positive (89), malaria negative (83) | P. falciparum | Not specified | Microscopic method | Plasma | ELISA (DuoSet ELISA Development System; R&D Systems) | There was no difference in MIP-1α/MIP-1β levels between infected and uninfected individuals. |
| 20 | Thuma et al., 2011 [45] | Zambia (2001–2005) | Children aged <6 years: Severe malarialanemia (72), cerebral malaria (28), uncomplicated malaria (66) | P. falciparum | Severe malaria/uncomplicated malaria | Microscopic method | Plasma | bead-based assay (Human Cytokine/Chemokine Multiplex Immunoassay kits (LINCO Research)) | There was no difference in MIP-1α levels between severe malarialanemia, cerebral malaria, and uncomplicated malaria patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuraeiad, S.; Kotepui, K.U.; Mahittikorn, A.; Anabire, N.G.; Masangkay, F.R.; Wilairatana, P.; Wangdi, K.; Kotepui, M. A Systematic Review and Meta-Analysis of MIP-1α and MIP-1β Chemokines in Malaria in Relation to Disease Severity. Medicina 2025, 61, 676. https://doi.org/10.3390/medicina61040676
Kuraeiad S, Kotepui KU, Mahittikorn A, Anabire NG, Masangkay FR, Wilairatana P, Wangdi K, Kotepui M. A Systematic Review and Meta-Analysis of MIP-1α and MIP-1β Chemokines in Malaria in Relation to Disease Severity. Medicina. 2025; 61(4):676. https://doi.org/10.3390/medicina61040676
Chicago/Turabian StyleKuraeiad, Saruda, Kwuntida Uthaisar Kotepui, Aongart Mahittikorn, Nsoh Godwin Anabire, Frederick Ramirez Masangkay, Polrat Wilairatana, Kinley Wangdi, and Manas Kotepui. 2025. "A Systematic Review and Meta-Analysis of MIP-1α and MIP-1β Chemokines in Malaria in Relation to Disease Severity" Medicina 61, no. 4: 676. https://doi.org/10.3390/medicina61040676
APA StyleKuraeiad, S., Kotepui, K. U., Mahittikorn, A., Anabire, N. G., Masangkay, F. R., Wilairatana, P., Wangdi, K., & Kotepui, M. (2025). A Systematic Review and Meta-Analysis of MIP-1α and MIP-1β Chemokines in Malaria in Relation to Disease Severity. Medicina, 61(4), 676. https://doi.org/10.3390/medicina61040676








