Abstract
Background and Objectives: This scoping review explores the current state of the art of AI-based applications in the field of orthopedics, focusing on its implementation in diagnostic imaging and preoperative planning of knee joint procedures. Materials and Methods: The search was carried out using the recognized scholarly databases PubMed, Medline and Embase and was set to identify original research addressing AI applied to imaging in knee diagnosis and surgical planning, written in English and published up to January 2025. Results: The search produced 1612 papers, of which 36 were included in our review. All papers addressed AI applied to common imaging methods in clinical practice. Of these, thirty integrated AI-based tools with X-rays, one applied AI to X-rays to produce CT-like 3D reproductions, and two studies applied AI to MRI. Conclusions: Several AI tools have already been validated for enhancing the accuracy of measurements and detecting additional parameters in a shorter time compared to standard assessments. We expect these may soon be introduced into routine clinical practice to streamline a number of technical tasks and in some cases to replace the need for human intervention.
1. Introduction
Artificial intelligence (AI) is a broad and rapidly evolving field of computer science focused on the design and development of intelligent machines able to perform tasks that typically require human intelligence skills, including learning, reasoning, problem-solving and perception [1]. Within the field of AI, the greatest achievements and applications have been through data-driven approaches such as machine learning (ML) and deep learning (DL). These approaches involve developing algorithms able to ingest considerable amounts of data to discover and model patterns and relationships within these data directly, learning from mistakes and improving their performance in a way that resembles experiential learning [1]. Specifically, ML aims to teach machines how to perform specific tasks by extracting representative features from the data and then identifying patterns from these features via mathematical models and algorithms. Deep learning is a subset of ML, which employs artificial neural networks (NNs), originally thought to mimic the human brain, to extract complex numerical features typically not directly correlated with human experience and perception [2,3]. This differs from traditional ML, where features are manually hand-crafted or selected (e.g., inclusion of clinical variables, use of radiomic features from imaging, etc.) and thus preserve a certain degree of interpretability [3]. NN architectures typically used in the imaging domain can be broadly classified into convolutional neural networks (CNNs) and Vision Transformers (ViT), the former excelling in analyzing local image features with filters, the latter able to more effectively capture global context and relationships thanks to the attention mechanisms [4]. Recurrent Neural Networks (RNNs) are also frequently adopted architectures for the analysis of time series, thus finding application in image sequences/video analysis as well as surgical planning pathways [4]. Overall, DL methods require larger data for model development and often lack interpretability compared to ML, but their predictive capabilities proved to be extremely powerful and well-performing on numerous tasks [3].
In orthopedic medical imaging, AI finds vast applicability especially for tasks such as imaging quality enhancement, image classification and detection and segmentation.
Indeed, AI tools enable users to improve image quality by reducing noise, improving the image resolution, optimizing contrast, making imaging more reliable for diagnosis and planning.
Other AI algorithms can classify images into categories, enabling fast and automated identification of pathological condition. Finally, AI-based tools can accurately detect and outline anatomical structures or pathological areas within the image (image detection and segmentation), favoring a quantitative assessment of regions of interest (e.g., by measuring surface/volume) as well as supporting surgical planning.
The automation of these tasks via AI can impact several aspects of the patient journey and support the healthcare professionals in providing superior quality of care. With specific reference to the orthopedics field, AI has shown promising results in improving diagnostic accuracy, supporting clinical decision-making, optimizing and personalizing surgical and/or treatment planning and monitoring patients’ outcome [2,5]. In 2020, a study by Federer et al. [5] provided an overview of such applications in orthopedics. Since then, a growing body of research has followed with numerous publications. Here, we provide an updated mapping of the literature since 2020, with a specific focus on developments and use of AI applications in diagnostic imaging and preoperative planning of knee joint procedures.
2. Materials and Methods
The review process was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-SC) [6].
The Population, Intervention, Comparator, and Outcomes of Interest enabled the formulation of the following broad research questions: How is AI being used in the analysis of images for knee diagnosis and knee surgery planning? Is AI an optimum tool for improving diagnostic accuracy, surgical outcomes, and postoperative assessments?
A systematic literature search was carried out using scholarly databases without the aid of AI tools. The search consisted of a single search of PubMed, Medline, and Embase accessed through IRCCS Humanitas Research Hospital. The queries searched for this scoping review were: (artificial intelligence OR machine learning OR deep learning) AND orthopedics AND knee AND (imaging OR preoperative planning). No searches were performed to explore dissemination material or gray literature.
Following the manual removal of duplicates, two reviewers (L.B., M.B.M.R.) independently screened the titles and abstracts using the eligibility criteria. The only inclusion criterion was work presented as an original research article investigating AI applications in diagnostic imaging or preoperative planning for knee surgery, while criteria for exclusion were formats other than original articles, thus reviews, case reports, letters/editorials, book chapters, methodological papers and papers written in languages other than English. The full texts of all eligible articles were retrieved and reviewed independently by the same two reviewers (L.B., M.B.M.R.) to assess their appropriateness to the research question. Disagreements were discussed with the senior author (T.B.) and resolved by consensus. Finally, references of selected articles were checked to identify titles missed upon screening. Any studies meeting the inclusion criterion were integrated into the final selection.
The following data were extracted and recorded on an Excel sheet: imaging type, AI type, AI aim/application, and the main results. When needed, Excel sheet functions were used to create chart plots.
No methodological quality of the studies reviewed was assessed for this scoping review.
3. Results
3.1. Screening and Selection
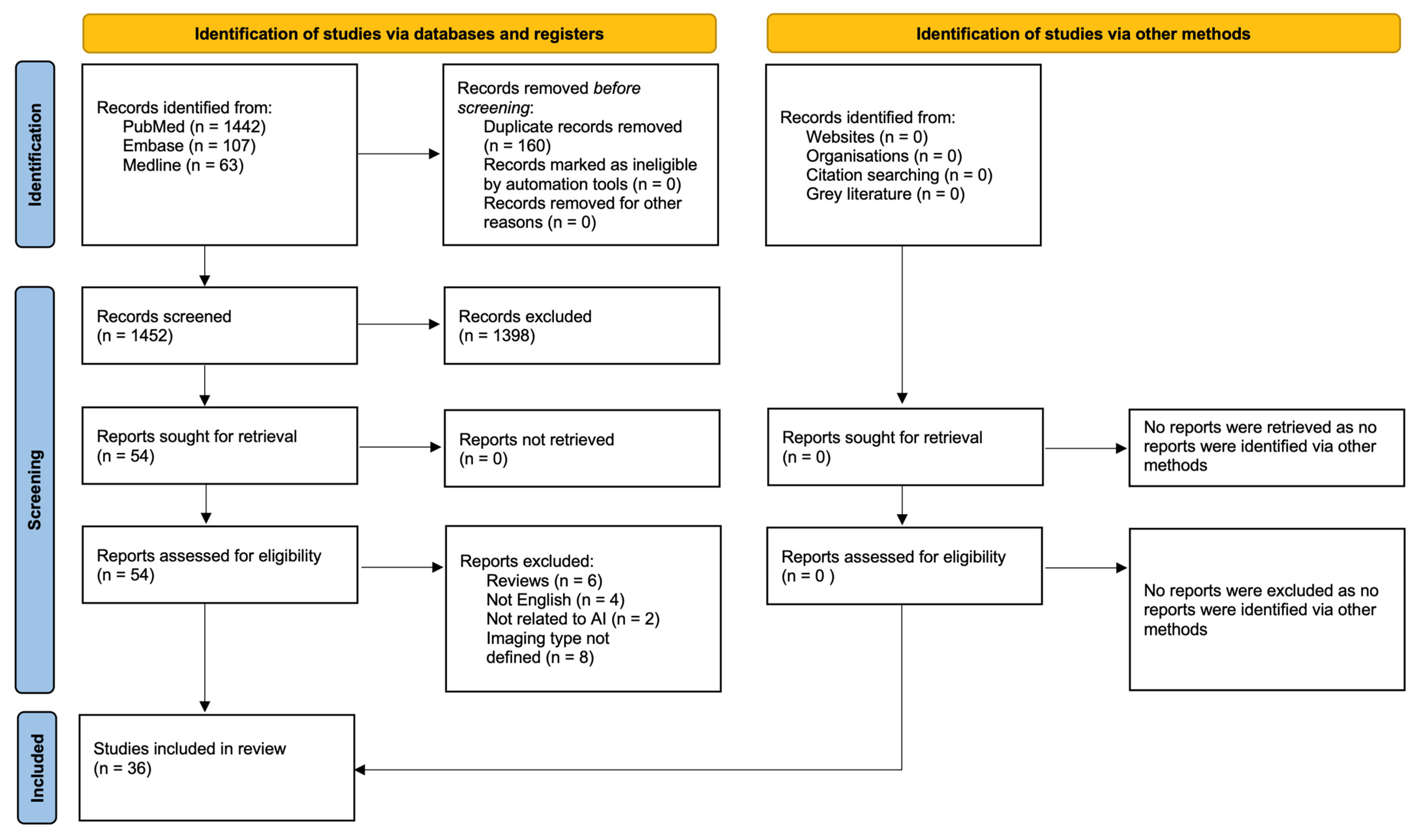
The systematic literature search yielded 1612 articles of which 160 were duplicates. In total, 1398 articles were excluded following title and abstract screening. The remaining 54 papers were all retrieved and later assessed according to the eligibility criteria, leaving 36 articles for review [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. The screening and review process is summarized in Figure 1. No further studies were identified via other methods.
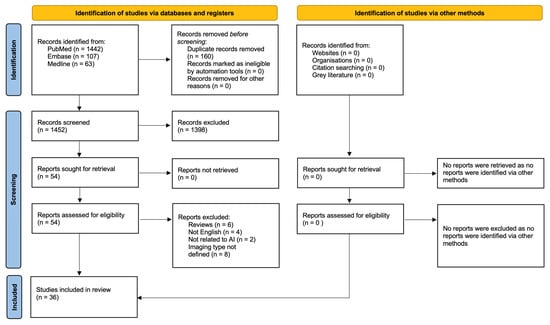
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources.
3.2. Overview of Papers
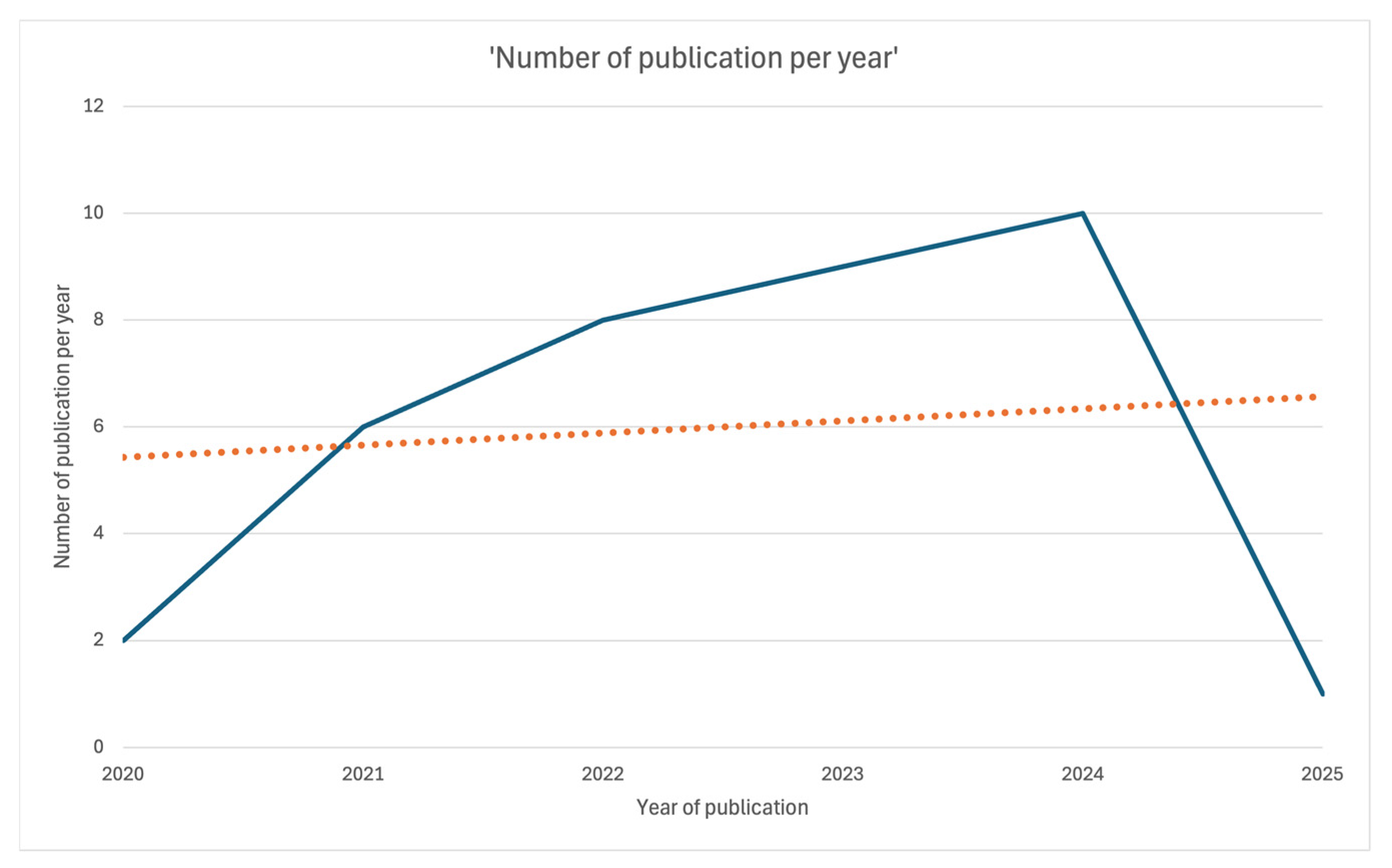
The present scoping review includes a total of 36 papers (Figure 1). All were published between 2020 and 2025: two in 2020 [7,21], six in 2021 [8,9,10,22,23,24], eight in 2022 [11,12,25,26,27,28,29,30], nine in 2023 [13,14,15,16,17,31,32,33,34], ten in 2024 [18,19,35,36,37,38,39,40,41,42], and one in January 2025 [25] (Figure 2).
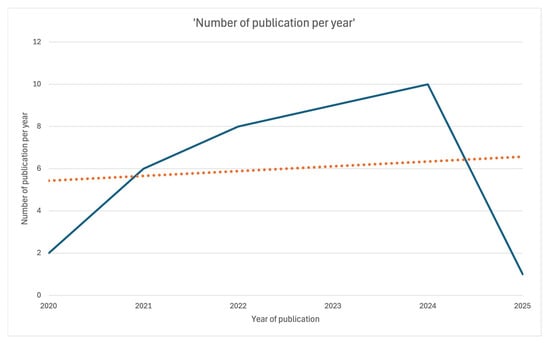
Figure 2.
Linear plot showing the number of publications per year over time.
The topics discussed in these papers included the type of AI technology, imaging technology, the operative setting and purposes for AI implementation. The articles reviewed and their main characteristics are summarized in Table 1 and Table 2, divided for ease of consultation according to their use in diagnostics or preoperative planning (Table 1 and Table 2).

Table 1.
Papers on AI tools applied to diagnoses of knee conditions.

Table 2.
Papers reviewed on AI tools applied to surgical planning.
3.3. Imaging Methods
All papers addressed AI applied to common imaging methods in clinical practice. The majority (n = 30) integrated AI-based tools with X-rays. Another one applied AI to X-rays to produce CT-like 3D reproductions [30]. Two studies applied AI to MRI [7,18]: one study employing a 5 min 3D quantitative double-echo steady-state (qDESS) sequence with automatic T2 mapping and deep learning super-resolution augmentation [7], the other applying automated femoral segmentation software to 3D high-resolution MRI to assess acute cartilage damage [18].
3.4. Range of AI Tool Applications
The AI tools in the papers reviewed were used to support operator assessments, either by enhancing imaging features or reducing operator-dependent factors (variability, misinterpretation) through automized assessments.
3.4.1. OA Diagnosis
Several studies proposed ways for reducing variability among measurements for OA parameters, so as to allow more accurate evaluation of individual patient risk for OA progression. Two studies applied AI measuring quantitative cartilage parameters of knee osteoarthritis [12,19]. One study by Smolle et al. describes the use of AI-enhanced X-rays by means of the Knee Osteoarthritis Labeling Assistant (KOALA), a software providing both metric assessments of anterior–posterior (AP) or PA knee X-rays and proposals for clinical OA grade. The system has proven to markedly increase reader agreement rates for KL grade, sclerosis, JSN and osteophyte Osteoarthritis Research Society International (OARSI) grades both among senior readers and between senior and junior readers [12]. Brejnebøl et al. measured the improvements in assessing knee OA on radiographs according to the established KL grading system using the AI tool RBknee (version 2.1; Radiobotics). AI assistance increased junior readers’ radiographic KOA grading performance and increased interobserver agreement for osteoarthritis grading across radiologists and orthopedics of various experience levels [19].
3.4.2. Fracture Detection
Another group of studies implemented ways for increasing fracture detections, especially difficult to identify from plain radiographs [8,9,10,18,30,33].
Three studies [8,9,10] explored AI-based technology to increase fracture recognition from X-rays. The 2021 study by Liu focused on the use of AI-based tools in the detection of tibial plateau fractures, reaching a level of accuracy comparable to a human operator—though at the time of the study, the tool was still unable to replace evaluation of orthopedic physicians [10]. The study by Guermazi et al. describes the development of an AI algorithm capable of interpreting full-size high spatial resolution and multiple radiographic views of the same patient and can be integrated into picture archiving and communications systems [9]. The system has been shown to improve fracture detection sensitivity by 10.4% and is appreciable for all body locations except for shoulder, clavicle and thoracolumbar spine [9]. The AI-based system developed by Lind et al. goes beyond detection and provides detailed fracture classification as by the 2018 AO/Orthopedic Trauma Association (AO-OTA) fracture and dislocation compendium [8]. Results showed a weighted mean AUC of 0.87 for proximal tibia fractures, 0.89 for patella fractures and 0.89 for distal femur fractures. Almost 3/4 of the area under the curve (AUC) estimates were above 0.8, out of which more than half reached an AUC of 0.9 or above [8].
Two studies applied deep learning models to detect cartilage fractures from MRI [7,18]. The study by Wang et al. describes a model that increases clinicians’ accuracy—especially among trainees—for anterior cruciate ligament (ACL) ruptures. The model achieved an area under the receiver operating characteristic curve of 0.987 and a sensitivity and specificity of 95.1% [18]. The study by Chaudhari et al. describes the use of a deep learning super-resolution augmentation algorithm to enhance a 5 min 3D qDESS MRI sequence that automatically generates T2 relaxation time maps [7], increasing sensitivity compared to conventional knee MRI for subtle cartilage lesions. Three studies applied AI to CT scans. Fernandes et al. created an AI algorithm able to convert 2D radiographs to 3D bone model reconstructions with excellent accuracy when comparing all measurement parameters from the CT scan and manual measurements (mean absolute error ≤ 1.98 mm) [17,30,33].
3.4.3. Limb Alignment
Eight papers describe the automatization of the assessment of the of lower-extremity alignment from full leg radiographs (FLRs) and of the calculation of main clinical angles by means of different software architectures and approaches [11,12,13,16,23].
Tack et al.’s study [23] is the first of these to describe a fully automated method for the quantification of hip–knee–ankle (HKA) alignment from FLRs (varus or valgus deformity), using the YOLOv4 And Resnet Landmark regression Algorithm (YARLA). The average deviation of landmarks manually placed by experts and automatically detected by their system was less than 2.0 ± 1.5 mm for all structures [23].
The AI-based system by Erne describes the automatized determination of four parameters in addition to hip–knee–ankle angle (mFAmTA) described by Tack [23], namely mMPTA, mLDFA, mLDTA, and FSAmTA.
Also, the AI-based systems developed by Simon et al. and by Larson et al. provide automatization of clinical angles (mechanical axis HKA and pelvic tilt) as well as additional measurements to those routinely collected with manual annotations, including bone length (femur, tibia, full length), laterality discrepancies, and position of orthopedic hardware; moreover, these systems are also able to generate radiologist-like reports [24,25]. Similarly, Alberti et al. proposed an algorithm for the standardization of Q-angle measurement from whole leg radiographs on a heterogeneous sample of patients, including patients at pediatric age and with bone pathologies [20].
The study by Bernard de Villeneuve et al. [29] described a first in its kind algorithm to help surgeons in preoperatively assessing lower limb deformities and evaluating the appropriate angle for osteotomy.
More recently, Tanner et al. described a detection-based DL algorithm that can detect the bilateral femoral head, knee, and ankle joints with high precision also in patients where the femoral head is difficult to view and calculates HKA angles in LLRs with comparable accuracy to that calculated by manual measurement [36].
Hoffmann et al. [39] tested the performance of a CNN implemented in a common planning software (mediCAD® 7.0; mediCAD Hectec GmbH) that allows analysis and preoperative planning. MediCAD-based analysis demonstrated excellent accuracy for overall lower limb alignment and leg length, but showed significant deviations in joint-level measurements, particularly in cases involving TKA [39].
Finally, Yang J. et al. [35] described a fully automated model that reliably assesses the alignment of lower limbs in patients with knee OA, with or without the presence of a prosthesis, on personal portable devices on LLRs with no need for image pre-processing.
Taken together, results from all the studies above report high accuracy between physician and AI-generated measurements, with similar outcomes in terms of ICC (ICC > 95%) [22,24,25,28,29]. The study by Bernard de Villeneuve et al. features the intra-observer and the interobserver variation on angle determination, which was within 1° [29]. All systems also evidence time saved compared to manual assessment (from 48 to 130 s).
3.4.4. Implant Size
Several studies describe AI-based systems to improve preoperative planning for implant size and positioning compared to conventional X-ray-based templating [33,38,40,41,42]. A few focused on the use of radiographic imaging.
Yu et al. describe a DL-based model using X-ray images alone with no additional information, yielding high predictive power for implant size, especially when using lateral images. The study, however, is based on radiographs from a single surgeon [41]. Park KB et al. [40] compared predictions of an automated preoperative model for TKA on X-rays with the conventional templating by experienced surgeons. Although the model produced only minor differences in terms of accuracy ± 1 levels of the surgeons, it led to a >22% higher agreement of exact accuracy rates for femoral and tibial implant sizes with the actual implant sizes [40]. Other studies described models that did not lead to significant improvements compared to current practice [42].
Others focused on implant description by CT imaging. Li et al. [33] described an AI-based preoperative planning system, AIJOINT, specifically validated for TKA. The system, which includes CT image processing, component planning, and PSI designing modules, requires much less time than traditional CT segmentation (3.74 ± 0.82 vs. 128.88 ± 17.31 min, p < 0.05) and PSI design (35.10 ± 3.98 vs. 159.52 ± 17.14 min, p < 0.05), and comparable time for size planning. Its accuracy in predicting the size of both femoral and tibial components was 92.9% vs. 42.9% and 47.6%, respectively (p < 0.05), of the conventional method in size planning [33]. The study by Liu Z et al., the first DL regression model for automated patellofemoral annotation trained on both physiologic and pathologic CT imaging, reported a mean absolute error between predicted and ground truth landmark coordinates, which was 0.20/0.26 cm in the healthy/arthroplasty cohort [17].
Lan et al. [38] tested an AI-based preoperative 3D planning technology already used in hip implants on prosthesis size and axial alignment planning specifically in TKA and compared it to traditional 2D template measurement technology. The accuracy of prosthesis size, VCA and HKA prediction in the AI group was significantly higher than that in the 2D group (p < 0.05). Moreover, WOMAC and AKS scores in the AI group at 3–12 months follow-up after surgery were better than those in the 2D group (p < 0.05) [38].
3.5. AI Models and Technologies
The reviewed studies highlight the predominance of DL approaches, with only one instance of a ML model [26]. CNNs are the most frequently used architectures, predominantly trained in a supervised fashion [7,10,14,15,17,18,21,22,23,25,27,28,29,31,33,34,35,36,39,41]. Only one study employed self-supervised pretraining, though still followed by supervised fine-tuning [17]. For segmentation tasks, well-established architectures such as UNet and Mask R-CNN dominate [19,22,28,31,33], with only one example of a transformer architecture [42], reflecting the limited adoption of this architecture in knee imaging so far. Interestingly, UNet is also often adopted for landmark localization, framing the position regression (detection) problem into a segmentation one [28,29]. Landmark localization is, however, mostly tackled as an object detection task, for which YOLO [23,36,40] and HRNet [33,35] represent the preferred architectures. RetinaNet was also employed for fracture detection [10] [Liu PR]. In most of these studies, the automated segmentation and/or landmark localization was paramount to the automation of quantitative assessment of alignment parameters [11,14,23,28,29,32,35,36,39] or disease grading [12,19]. AI was also used for classification tasks (e.g., implant classification, patient selection), with ResNet and EfficientNet being the most common AI architectures for this task [8,25,27,41]. Notably, a few studies reported the use of GradCAMs to generate explainability maps in support to ease the interpretation of the outputs of their models [14,21,34]. It is worth also noticing that some commercial AI tools are already present in the landscape and are involved in research studies that assess their usability and robustness, namely LAMA (Leg Angle Measurement Assistant) [11,16,32], KOALA (Knee Osteoarthritis Labeling Assistant) [12], PeekMed [13,30] and RBKnee [19].
Large-scale datasets like the Osteoarthritis Initiative (OAI) play a crucial role in model development, yet validation is often conducted on private cohorts. However, most studies originate from single-center datasets, limiting their generalizability. Notable exceptions are Wang, and Karnuta 2021 and 2023 [18,21,34], which leverage large multicentric cohorts at validation as well as training. Finally, no study explored the development and use of most recent advances with foundation models, indicating a gap in the current research landscape.
4. Discussion
The present review aimed to map the recent literature on developments and use of AI tools in diagnostic imaging and preoperative planning of knee joint procedures, identifying the most relevant research studies which have been published up to January 2025. Narrowing the search focus to knee surgery, our analysis identified a total of 36 original studies reporting specific applications of AI in an array of steps, from patient referral and candidate selection to preoperative planning. While in previous years studies prevalently described AI related to X-ray imaging of fracture detection and OA measurement [14], our findings reveal a broader scope of AI applications, especially in the areas of preoperative planning (patient selection and risk assessment) and diagnostic accuracy. Overall, the results of these studies are consistent in reporting higher levels of accuracy gained by applying AI tools to processes that traditionally rely on the specialist’s judgment and activity or on commonly used equipment and software. In practical terms, they highlight the gain in diagnostic accuracy (such as fracture detection and implant characterization) and great potential of streamlining a number of technical tasks, while allowing specialists to focus their attention and time on other activities requiring human agency and supervision. Indeed, in many of the papers published, the authors highlight specific areas where AI-integrated steps would take on a greater role, consequently freeing considerable resources (human, economic, intellectual, time, logistical resources, and so on) for allocation elsewhere. For example, the study by Lind et al. reports an AI-enhanced tool that provides highly accurate measurements to correctly classify fractures by the 2018 AO-OTA fracture classification system and even detect fractures which may be missed by traditional imaging interpretation [8]. The group of Simon et al. [24] found the DL algorithm LAMATM in leg length measurements to match human performance and even exceed it in terms of time and standardization of the technique. Moreover, it produced radiology reports that could potentially replace those drafted by radiologists. Measurement times with AI software were three times faster (saving around 2 min per patient) than manual annotation and were run asynchronously [8]. Finally, the study by Yang J et al. proposed an AI-based system that allows orthopedics to assess limb alignment from their mobile phones based on the availability of X-rays alone, making this extremely attractive for less equipped medical centers [35].
Noteworthy is the steady trend in studies applied to radiographic imaging, which to date remains the most cost-effective and widespread clinical support among most clinical settings.
From a perspective of technological implementation, almost all papers identified are validation studies, where the scope of studies remains narrowed on technical aspects such as efficacy measurements (process accuracy yield, sensitivity, specificity, reliability, replicability, and validity) by means of comparison with retrospective data. Only one specifically involved a prospective deployment of deep learning [17,43]. This represents the first step towards integration of AI technology in routine clinical activity. This should encourage a shift in approach towards its application in daily clinical practice.
No quality improvement assessments nor large-scale studies on clinical practice were found among those screened. Likewise, none covered in detail aspects such as economic evaluations, or studies on patient outcomes, pathways of care, and quality of life. In fact, the set of papers reviewed reflects the current stage of AI implementation, i.e., of ongoing development, technical validation, and testing. As many of these tools become embedded into clinical practice, we expect the types of studies published to shift in focus.
4.1. Strengths and Limitations
This review is a first attempt to provide an updated state of the art on the AI-based applications in the field of knee surgery in terms of diagnostic and treatment planning. One aspect worth noting on the findings of this scoping review is the studies being performed on a variety of databases of patient populations from geographical areas which may prevent the generalization of findings as well as a rapid widespread adoption of the algorithms reported.
4.2. Next Steps
Most of the studies analyzed in this review are single center and rely on limited validation or test datasets, which hampers the assessment of generalizability and real-world applicability of the proposed AI approaches. Particularly, only a few studies offer evaluations of the training set bias or clarify the range of applicability of their models in accordance with FAIR principles [8,12,18,19,23,31,34]. In relation to this, explainability remains underexplored, with only a handful of studies incorporating tools like GradCAM to aid in interpreting model outcomes [14,21,34]—yet interpretability will be critical for clinical adoption. Finally, some of the presented AI systems are closed source, with limited information on the underlying methodology [12,13,19], making it difficult to compare results across studies or assess the robustness of the approaches.
Considering these current common limitations, further research and development are necessary for refining AI algorithms, to address challenges related to biased training data, incomplete or unrepresentative datasets, under-representation of populations, implicit assumptions while creating the algorithm, and biased outcomes generated by the algorithm [44]. This involves careful examination and selection of training data, ongoing evaluation of algorithms’ performance, and the implementation of strategies to mitigate and correct biases. The most recent AI modeling strategies have not been fully explored in knee imaging (e.g., transformer-based architectures, large foundation models, vision-language models), leaving a research gap that will be filled in the coming years. However, research should primarily focus on broad spectrum validation of applicability and generalizability of these technologies. In accordance with FAIR principles, AI development should promote transparency and the continual improvement of algorithms by means of studies for validating the effectiveness of algorithms in larger and more diverse patient populations, as well as exploring potential applications beyond imaging and preoperative planning, like robotic-assisted surgery.
5. Conclusions
This scoping review shows the recent advances in the field of computer science brought by AI tools in the orthopedic practice, with significant potential for improving diagnostic accuracy, surgical outcomes, and postoperative assessments. As researchers and practitioners continue to explore the possibilities of AI, further advancements and standardization efforts will play a central role in realizing the full potential of AI as a helpful and standardized tool in ordinary practice.
Author Contributions
Conceptualization, L.B. and T.B.; methodology, L.B. and M.B.M.R.; formal analysis, L.B. and M.B.M.R.; investigation, L.B. and M.B.M.R.; data curation, L.B., M.B.M.R. and E.B.; writing—original draft preparation, L.B. and M.B.M.R.; writing—review and editing, L.B. and M.B.M.R., A.F., F.L.R. and T.B.; supervision, A.F. and T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contribution presented in this study is included in the article.
Acknowledgments
The authors would like to thank Manuella Walker for her support in the development and editing of the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Myers, T.G.; Ramkumar, P.N.; Ricciardi, B.F.; Urish, K.L.; Kipper, J.; Ketonis, C. Artificial Intelligence and Orthopaedics: An Introduction for Clinicians. J. Bone Jt. Surg. 2020, 102, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Poduval, M.; Ghose, A.; Manchanda, S.; Bagaria, V.; Sinha, A. Artificial Intelligence and Machine Learning: A New Disruptive Force in Orthopaedics. Indian J. Orthop. 2020, 54, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica 2021, 83, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Tang, Y.; Wang, C.; Landman, B.A. Transforming Medical Imaging with Transformers? A Comparative Review of Key Properties, Current Progresses, and Future Perspectives. Med. Image Anal. 2023, 85, 102762. [Google Scholar] [CrossRef]
- Federer, S.J.; Jones, G.G. Artificial intelligence in orthopaedics: A scoping review. PLoS ONE 2021, 16, e0260471. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chaudhari, A.S.; Grissom, M.J.; Fang, Z.; Sveinsson, B.; Lee, J.H.; Gold, G.E.; Hargreaves, B.A.; Stevens, K.J. Diagnostic Accuracy of Quantitative Multicontrast 5-Minute Knee MRI Using Prospective Artificial Intelligence Image Quality Enhancement. Am. J. Roentgenol. 2021, 216, 1614–1625. [Google Scholar] [CrossRef]
- Lind, A.; Akbarian, E.; Olsson, S.; Nåsell, H.; Sköldenberg, O.; Razavian, A.S.; Gordon, M. Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system. PLoS ONE 2021, 16, e0248809. [Google Scholar] [CrossRef]
- Guermazi, A.; Tannaoury, C.; Kompel, A.; Murakami, A.M.; Ducarouge, A.; Gillibert, A.; Li, X.; Tournier, A.; Lahoud, Y.; Jarraya, M.; et al. Improving Radiographic Fracture Recognition Performance and Efficiency Using Artificial Intelligence. Radiology 2022, 302, 627–636. [Google Scholar] [CrossRef]
- Liu, P.R.; Zhang, J.-Y.; Xue, M.D.; Duan, Y.Y.; Hu, J.L.; Liu, S.X.; Xie, Y.; Wang, H.L.; Wang, J.W.; Huo, T.T.; et al. Artificial Intelligence to Diagnose Tibial Plateau Fractures: An Intelligent Assistant for Orthopedic Physicians. Curr. Med. Sci. 2021, 41, 1158–1164. [Google Scholar] [CrossRef]
- Schwarz, G.M.; Simon, S.; Mitterer, J.A.; Frank, B.J.H.; Aichmair, A.; Dominkus, M.; Hofstaetter, J.G. Artificial intelligence enables reliable and standardized measurements of implant alignment in long leg radiographs with total knee arthroplasties. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Goetz, C.; Maurer, D.; Vielgut, I.; Novak, M.; Zier, G.; Leithner, A.; Nehrer, S.; Paixao, T.; Ljuhar, R.; et al. Artificial intelligence-based computer-aided system for knee osteoarthritis assessment increases experienced orthopaedic surgeons’ agreement rate and accuracy. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Z.; Abdelhameed, M.A.; AlMaeen, B.N.; Bernard de Villeneuve, F.; Fernandes, L.R.; Jacquet, C.; Ollivier, M. In slope-changing osteotomy one millimeter is not one degree: Results of an artificial intelligence-automated software analysis. Int. Orthop. 2023, 47, 915–920. [Google Scholar] [CrossRef]
- Bonnin, M.; Müller-Fouarge, F.; Estienne, T.; Bekadar, S.; Pouchy, C.; Ait Si Selmi, T. Artificial Intelligence Radiographic Analysis Tool for Total Knee Arthroplasty. J. Arthroplast. 2023, 38, S199–S207.e2. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; Yang, S.C.; Hur, J.H.; In, Y. Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs. Bioengineering 2023, 10, 632. [Google Scholar] [CrossRef]
- Pagano, S.; Muller, K.; Gotz, J.; Reinhard, J.; Schindler, M.; Grifka, J.; Maderbacher, G. The Role and Efficiency of an AI-Powered Software in the Evaluation of Lower Limb Radiographs Before and After Total Knee Arthroplasty. Clin. Med. 2023, 12, 5498. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, A.; Fauveau, V.; Lee, J.; Marcadis, P.; Fayad, Z.A.; Chan, J.J.; Gladstone, J.; Mei, X.; Huang, M. Learning for Automated Measurement of Patellofemoral Anatomic Landmarks. Bioengineering 2023, 10, 815. [Google Scholar] [CrossRef]
- Wang, D.; Liu, S.G.; Ding, G.A.; Sun, A.L.; Jiang, D.; Jiang, J.; Zhao, J.Z.; Chen, D.S.; Ji, G.; Li, N.; et al. A Deep Learning Model Enhances Clinicians’Diagnostic Accuracy to More Than 96% for Anterior Cruciate Ligament Ruptures on Magnetic Resonance Imaging. Arthroscopy 2024, 40, 1197–1205. [Google Scholar] [CrossRef]
- Brejnebol, M.; Lenskjold, A.; Ziegeler, K.; Ruitenbeek, H.; Müller, F.C.; Nybing, J.U.; Visser, J.J.; Schiphouwer, L.M.; Jasper, J.; Bashian, B.; et al. Interobserver Agreement and Performance of Concurrent AI Assistance for Radiographic Evaluation of Knee Osteoarthritis. Radiology 2024, 312, e233341. [Google Scholar] [CrossRef]
- Alberti, L.; LoIacono, D.; Favaro, A.; Bondi, A.; Bertolino, L.; Bonanzinga, T. Artificial intelligence applied to q-angle measurement: Preliminary results on an algorithmbased on bounding box. Joints 2025, 3, e1397. [Google Scholar]
- Karnuta, J.M.; Luu, B.C.; Roth, A.L.; Haeberle, H.S.; Chen, A.F.; Iorio, R.; Schaffer, J.L.; Mont, M.A.; Patterson, B.M.; Krebs, V.E.; et al. Artificial Intelligence to Identify Arthroplasty Implants from Radiographs of the Knee. J. Arthroplast. 2021, 36, 935–940. [Google Scholar] [CrossRef]
- Schock, J.; Truhn, D.; Abrar, D.B.; Merhof, D.; Conrad, S.; Post, M.; Mittelstrass, F.; Kuhl, C.; Nebelung, S. Automated Analysis of Alignment in Long-Leg Radiographs by Using a Fully Automated Support System Based on Artificial Intelligence. Radiol. Artif. Intell. 2021, 3, e200198. [Google Scholar] [CrossRef] [PubMed]
- Tack, A.; Preim, B.; Zachov, S. Fully automated Assessment of Knee Alignment from Full-Leg X-Rays employing a “YOLOv4 and Resnet Landmark regression Algorithm” (YARLA): Data from the Osteoarthritis Initiative. Comput. Methods Programs Biomed 2021, 205, 106080. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Schwartz, G.; Aichmair, A.; Frank, B.J.H.; Hummer, A.; DiFranco, M.D.; Dominkus, M.; Hofstaetter, J.G. Fully automated deep learning for knee alignment assessment in lower extremity radiographs: A cross-sectional diagnostic study. Skelet. Radiol. 2022, 51, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Nguyen, C.; Do, B.; Kaul, A.; Larson, A.; Wang, S.; Wang, E.; Bultman, E.; Stevens, K.; Pai, J.; et al. Artificial Intelligence System for Automatic Quantitative Analysis and Radiology Reporting of Leg Length Radiographs. J. Digit. Imaging 2022, 35, 1494–1505. [Google Scholar] [CrossRef]
- Lambrechts, A.; Wirix-Speetjens, R.; Maes, F.; Van Huffel, S. Artificial Intelligence Based Patient-Specific Preoperative Planning Algorithm for Total Knee Arthroplasty. Front. Robot. AI 2022, 9, 840282. [Google Scholar] [CrossRef]
- Houserman, D.J.; Berend, K.R.; Lombardi, A.V., Jr.; Fischetti, C.E.; Duhaime, E.P.; Jain, A.; Crawford, D.A. The Viability of an Artificial Intelligence/Machine Learning Prediction Model to Determine Candidates for Knee Arthroplasty. J. Arthroplast. 2022, 38, 2075–2080. [Google Scholar] [CrossRef]
- Erne, F.; Grover, P.; Dreischarf, M.; Reumann, M.K.; Saul, D.; Histing, T.; Nüssler, A.K.; Springer, F.; Scholl, C. Automated Artificial Intelligence-Based Assessment of Lower Limb Alignment Validated on Weight-Bearing Pre- and Postoperative Full-Leg Radiographs. Diagnostics 2022, 12, 2679. [Google Scholar] [CrossRef]
- Bernard de Villeneuve, F.; Jacquet, C.; El Kadim, B.; Donnez, M.; Coue, O.; Poujade, T.; Khakha, R.; Argenson, J.N.; Ollivier, M. An artificial intelligence based on a convolutional neural network allows a precise analysis of the alignment of the lower limb. Int. Orthop. 2023, 47, 511–518. [Google Scholar] [CrossRef]
- Fernandes, L.R.; Arce, C.; Martinho, G.; Campos, J.P.; Meneghini, R.M. Accuracy, Reliability, and Repeatability of a Novel Artificial Intelligence Algorithm Converting Two-Dimensional Radiographs to Three-Dimensional Bone Models for Total Knee Arthroplasty. J. Arthroplast. 2022, 38, 2032–2036. [Google Scholar] [CrossRef]
- Steele, J.R.; Jang, S.J.; Brilliant, Z.R.; Mayman, D.J.; Sculco, P.K.; Jerabek, S.A.; Vigdorchik, J.M. Deep Learning Phenotype Automation and Cohort Analyses of 1946 Knees Using the Coronal Plane Alignment of the Knee Classification. J. Arthroplast. 2023, 38, S215–S221.e1. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Mitterer, J.A.; Vallant, S.M.; Simon, S.; Hanak-Hammerl, F.; Schwarz, G.M.; Klasan, A.; Hofstaetter, J.G. Gender-specific distribution of knee morphology according to CPAK and functional phenotype classification: Analysis of 8739 osteoarthritic knees prior to total knee arthroplasty using artificial intelligence. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Chen, X.; Xu, H.; Zhang, Y.; Qian, W. Development and Validation of an Artificial Intelligence Preoperative Planning and Patient-Specific Instrumentation System for Total Knee Arthroplasty. Bioengineering 2023, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Karnuta, J.M.; Shaikh, H.J.F.; Murphy, M.P.; Brown, N.M.; Pearle, A.D.; Nawabi, D.H.; Chen, A.F.; Ramkumar, P.N. Artificial Intelligence for Automated Implant Identification in Knee Arthroplasty: A Multicenter External Validation Study Exceeding 3.5 Million Plain Radiographs. J. Arthroplast. 2023, 38, 2004–2008. [Google Scholar] [CrossRef]
- Yang, J.; Ren, P.; Xin, P.; Wang, Y.; Ma, Y.; Liu, W.; Wang, Y.; Wang, Y.; Zhang, G. Automatic measurement of lower limb alignment in portable devices based on deep learning for knee osteoarthritis. J. Orthop. Surg. 2024, 19, 232. [Google Scholar] [CrossRef]
- Tanner, I.L.; Ye, K.; Moore, M.S.; Rechenmacher, A.J.; Ramirez, M.M.; George, S.Z.; Bolognesi, M.P.; Horn, M.E. Developing a Computer Vision Model to Automate Quantitative Measurement of Hip-Knee-Ankle Angle in Total Hip and Knee Arthroplasty Patients. J. Arthroplast. 2024, 39, 2225–2233. [Google Scholar] [CrossRef]
- Tandel, J.; Shetty, V.; Wagh, Y.; Shekhar, S.; Wagh, A.; Parvathy, J.M.; Karade, V.; Maurya, A. Evaluating axial alignment and knee phenotypes in a young Indian population, using X-rays converted to three-dimensional bone models, and their relevance in total knee arthroplasty. Knee 2024, 48, 197–206. [Google Scholar] [CrossRef]
- Lan, Q.; Li, S.; Zhang, J.; Guo, H.; Yan, L.; Tang, F. Reliable prediction of implant size and axial alignment in AI-based preoperative planning for total knee arthroplasty. Sci. Rep. 2024, 14, 16972. [Google Scholar] [CrossRef]
- Hoffmann, C.; Göksu, F.; Klöpfer-Krämer, I.; Watrinet, J.; Blum, P.; Hungerer, S.; Schröter, S.; Stuby, F.; Augat, P.; Fürmetz, J. High accuracy in lower limb alignment analysis usingconvolutional neural networks, with improvements needed for joint-level metrics. Knee Surg. Sports Traumatol. Arthrosc. 2024, 1–7. [Google Scholar] [CrossRef]
- Park, K.B.; Kim, M.S.; Yoon, D.K.; Jeon, Y.D. Clinical validation of a deep learning-based approach for preoperative decision-making in implant size for total knee arthroplasty. J. Orthop. Surg. Res. 2024, 19, 637. [Google Scholar] [CrossRef]
- Yu, Y.; Cho, Y.J.; Park, S.; Kim, Y.H.; Goh, T.S. Development of an artificial intelligence model for predicting implant size in total knee arthroplasty using simple X-ray images. J. Orthop. Surg. Res. 2024, 19, 516. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.E.; Kim, B.; Lee, S.; Lee, J.J.; Ro, D.H. A deep learning based automatic two-dimensional digital templating model for total knee arthroplasty. Knee Surg. Relat. Res. 2024, 36, 38. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.S.; Sandino, C.M.; Cole, E.K.; Larson, D.B.; Gold, G.E.; Vasanawala, S.S.; Lungren, M.P.; Hargreaves, B.A.; Langlotz, C.P. Prospective Deployment of Deep Learning in MRI: A Framework for Important Considerations, Challenges, and Recommendations for Best Practices. Magn. Reson. Imaging 2021, 54, 357–371. [Google Scholar] [CrossRef]
- Felländer-Tsai, L. AI ethics, accountability, and sustainability: Revisiting the Hippocratic oath. Acta Orthop. 2020, 91, 1–2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).