3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes
Abstract
1. Introduction
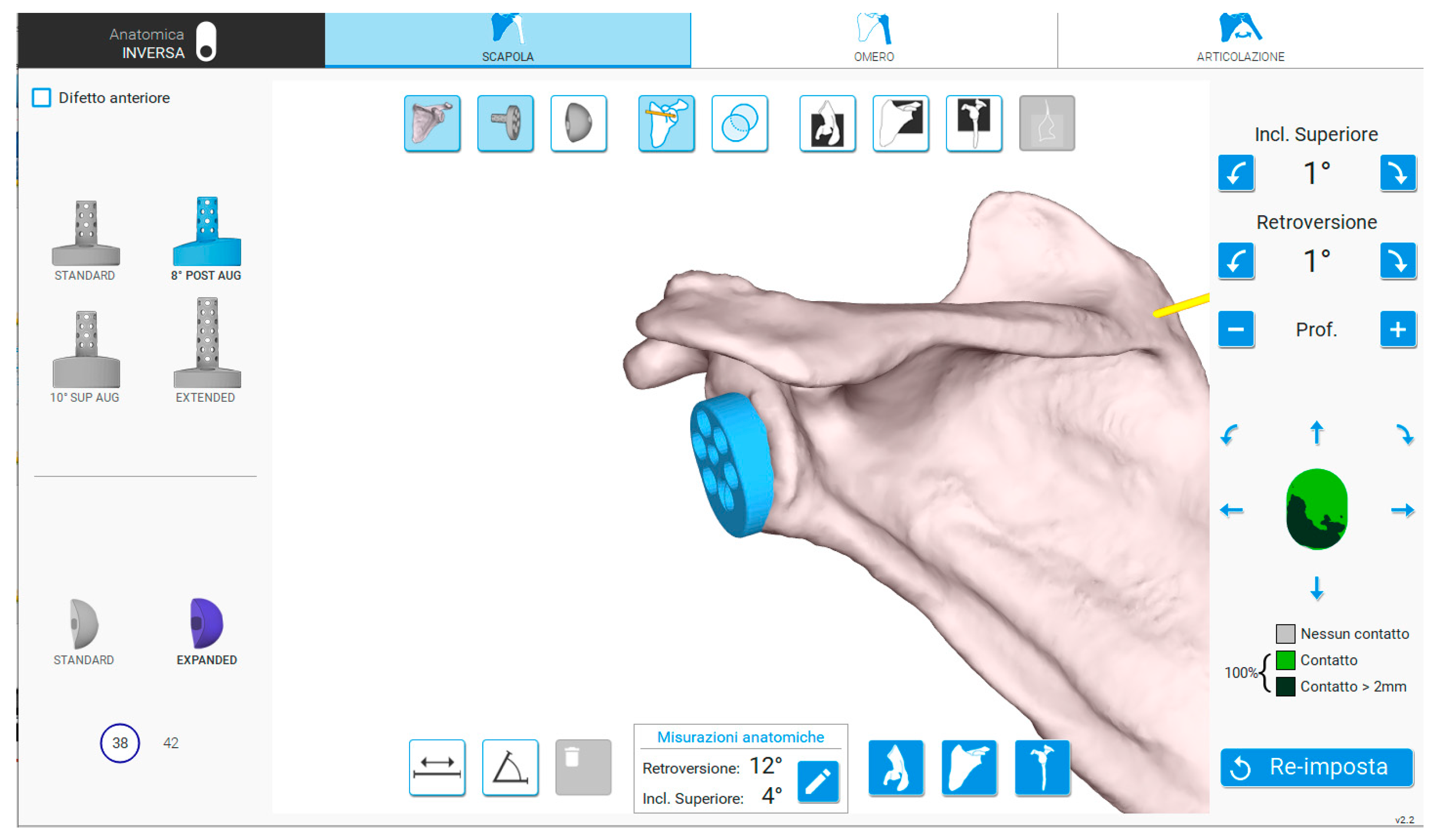
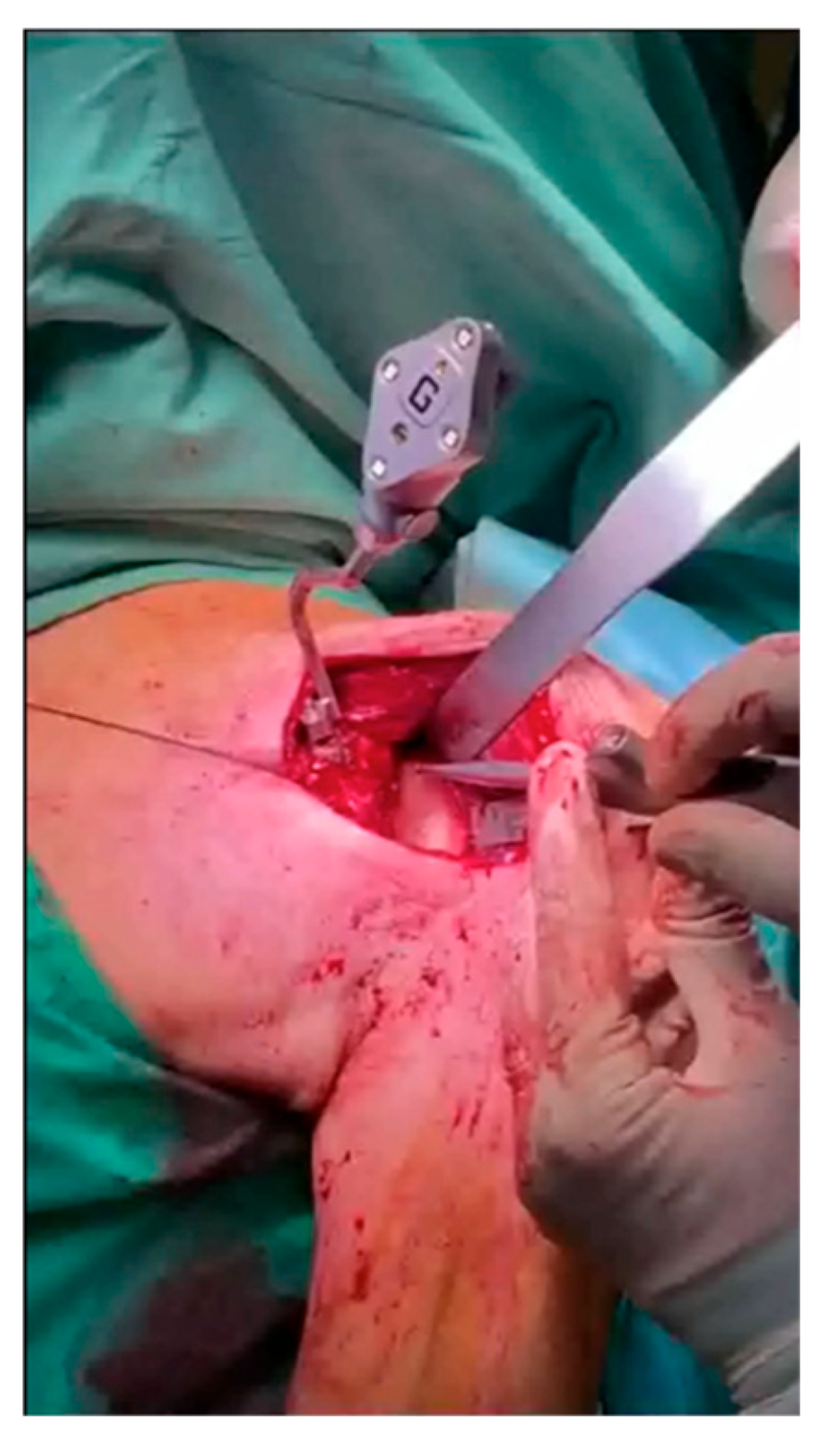
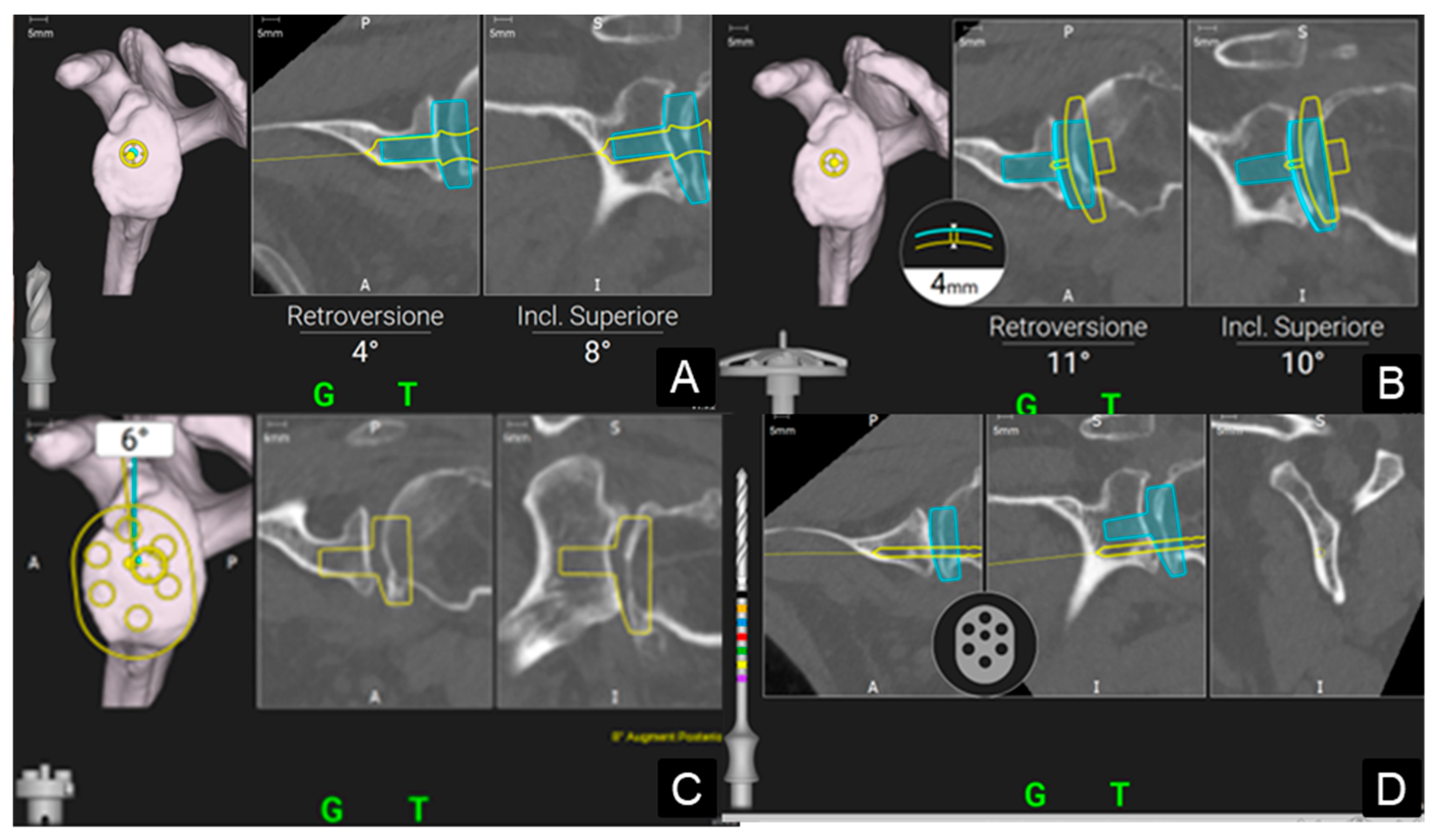
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 3D CT | three-dimensional computed tomography |
| CONV | conventional group |
| DASH score | Disability of the Arm, Shoulder, and Hand |
| GPS® | Guided Personalized Surgery |
| NAV | Navigated group |
| PJI | periprosthetic joint infection |
| RSA | reverse shoulder arthroplasty |
| SD | standard deviation |
References
- Holzgrefe, R.E.; Hao, K.A.; Panther, E.J.; Schoch, B.S.; Roche, C.P.; King, J.J.; Wright, J.O.; Wright, T.W. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: A matched cohort study. J. Shoulder Elb. Surg. 2023, 32, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Boufadel, P.; Lopez, R.; Fares, M.Y.; Daher, M.; Dhytadak, D.; Gulotta, L.V.; Abboud, J.A. Intraoperative Navigation in Reverse Shoulder Arthroplasty: Advantages and Future Prospects. Clin. Orthop. Surg. 2024, 16, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.E.; Ricchetti, E.T.; Huffman, G.R.; Iannotti, J.P.; Glaser, D.L. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J. Shoulder Elb. Surg. 2013, 22, 1298–1308. [Google Scholar] [CrossRef]
- Gaj, E.; Pagnotta, S.M.; Berlinberg, E.J.; Patel, H.H.; Picconi, O.; Redler, A.; De Carli, A. Intraoperative navigation system use increases accuracy of glenoid component inclination but not functional outcomes in reverse total shoulder arthroplasty: A prospective comparative study. Arch. Orthop. Trauma. Surg. 2024, 144, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Youderian, A.R.; Greene, A.T.; Polakovic, S.V.; Davis, N.Z.; Parsons, M.; Papandrea, R.F.; Jones, R.B.; Byram, I.R.; Gobbato, B.B.; Wright, T.W.; et al. Two-year clinical outcomes and complication rates in anatomic and reverse shoulder arthroplasty implanted with Exactech GPS intraoperative navigation. J. Shoulder Elb. Surg. 2023, 32, 2519–2532. [Google Scholar] [CrossRef]
- Burns, D.M.; Frank, T.; Whyne, C.M.; Henry, P.D.G. Glenoid component positioning and guidance techniques in anatomic and reverse total shoulder arthroplasty: A systematic review and meta-analysis. Shoulder Elb. 2019, 11, 16–28. [Google Scholar] [CrossRef]
- Xu, J.J.; Molokwu, B.O.; Shabbir-Hussain, R.; de Casson, F.B.; Elwell, H.; Polakovic, V.S.; Myerson, C.L.; Zuckerman, J.D.; Virk, M.S. Utilization Trends of the ExactechGPS® Computer-Assisted Navigation System in Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2025; in press. [Google Scholar] [CrossRef]
- Peri, G.; Troiano, E.; Colasanti, G.B.; Mondanelli, N.; Giannotti, S. Custom-made Glenoid Baseplate and Intra-Operative Navigation in Complex Revision Reverse Shoulder Arthroplasty: A Case Report. J. Shoulder Elb. Arthroplast. 2024, 8, 24715492231218183. [Google Scholar] [CrossRef]
- Tarallo, L.; Micheloni, G.M.; Giorgini, A.; Donà, A.; Porcellini, G. The use of computer navigation in reverse shoulder arthroplasty revision: A case report. Acta Biomed. 2023, 94, e2023176. [Google Scholar] [CrossRef]
- Andriollo, L.; Pietramala, S.; Polizzi, A.; Niccoli, G.; Zattoni, G.; Morea, V. Computer-Assisted Navigation in Reverse Shoulder Arthroplasty: Surgical Experience and Clinical Outcomes. J. Clin. Med. 2024, 13, 2512. [Google Scholar] [CrossRef]
- Moreschini, F.; Colasanti, G.B.; Cataldi, C.; Mannelli, L.; Mondanelli, N.; Giannotti, S. Pre-Operative CT-Based Planning Integrated With Intra-Operative Navigation in Reverse Shoulder Arthroplasty: Data Acquisition and Analysis Protocol, and Preliminary Results of Navigated Versus Conventional Surgery. Dose-Response 2020, 18, 1559325820970832. [Google Scholar] [CrossRef] [PubMed]
- De Falco, L.; Troiano, E.; Cesari, M.; Aiuto, P.; Peri, G.; Nuvoli, N.; Fortina, M.; Mondanelli, N.; Giannotti, S. Intra-operative local plus systemic tranexamic acid significantly decreases post-operative bleeding and the need for allogeneic blood transfusion in total knee arthroplasty. Med. Glas. 2021, 18, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Gerber, C.; Emery, R.J.H.; Søjbjerg, J.O.; Gohlke, F.; Boileau, P. A review of the Constant score: Modifications and guidelines for its use. J. Shoulder Elb. Surg. 2008, 17, 355–361. [Google Scholar] [CrossRef]
- Katolik, L.I.; Romeo, A.A.; Cole, B.J.; Verma, N.N.; Hayden, J.K.; Bach, B.R. Normalization of the Constant score. J. Shoulder Elb. Surg. 2005, 14, 279–285. [Google Scholar] [CrossRef]
- Fabre, T.; Piton, C.; Leclouerec, G.; Gervais-Delion, F.; Durandeau, A. Entrapment of the suprascapular nerve. J. Bone Joint Surg. Br. 1999, 81, 414–419. [Google Scholar] [CrossRef]
- Gummesson, C.; Ward, M.M.; Atroshi, I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet. Disord. 2006, 7, 44. [Google Scholar] [CrossRef]
- Rockwood, C., Jr.; Jensen, K. X-ray evaluation of shoulder problems. In The Shoulder, 2nd ed.; Rockwood, C.A., Jr., Matsen, F.A., III, Wirth, M.A., Harryman, D.T., Eds.; WB Saunders: Philadelphia, PA, USA, 1998; pp. 199–231. [Google Scholar]
- Young, B.L.; Cantrell, C.K.; Hamid, N. Classifications in brief: The Nerot-sirveaux classification for scapular notching. Clin. Orthop. Relat. Res. 2018, 476, 2454–2457. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Beck, L.; Stertz, I.; Chalmers, P.N. Preoperative three-dimensional computer planning for reverse total shoulder arthroplasty and bone grafting for severe glenoid deformity. Shoulder Elb. 2021, 13, 492–501. [Google Scholar] [CrossRef]
- Guareschi, A.S.; Gannon, S.T.; Welsh, M.E.; Reis, R.J.; Wright, T.W.; King, J.J.; Papandrea, R.F.; Simovitch, R.W.; Friedman, R.J.; Eichinger, J.K. Outcomes following humeral head autograft glenoid reconstruction in primary reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2025, 34, 1051–1060. [Google Scholar] [CrossRef]
- Merolla, G.; Giorgini, A.; Bonfatti, R.; Micheloni, G.M.; Negri, A.; Catani, F.; Tarallo, L.; Paladini, P.; Porcellini, G. BIO-RSA vs. metal-augmented baseplate in shoulder osteoarthritis with multiplanar glenoid deformity: A comparative study of radiographic findings and patient outcomes. J. Shoulder Elb. Surg. 2023, 32, 2264–2275. [Google Scholar] [CrossRef]
- Pastor, M.F.; Kieckbusch, M.; Kaufmann, M.; Ettinger, M.; Wellmann, M.; Smith, T. Reverse shoulder arthroplasty for fracture sequelae: Clinical outcome and prognostic factors. J. Orthop. Sci. 2019, 24, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.P.; Gutbrod, J.; Cahill, M.; Shi, L. Optimal Treatment of Proximal Humeral Fractures in the Elderly: Risks and Management Challenges. Orthop. Res. Rev. 2023, 15, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Antonios, T.; Bakti, N.; Phadkhe, A.; Guliharc, A.; Singhb, B. Outcomes following arthroplasty for proximal humeral fractures. J. Clin. Orthop. Trauma 2020, 11, S31–S36. [Google Scholar] [CrossRef]
- Sasanuma, H.; Iijima, Y.; Saito, T.; Kanaya, Y.; Yano, Y.; Fukushima, T.; Nakama, S.; Takeshita, T. Clinical results of reverse shoulder arthroplasty for comminuted proximal humerus fractures in elderly patients: A comparison between nonporous stems versus trabecular metal stems. JSES Int. 2020, 4, 952–958. [Google Scholar] [CrossRef]
- Troiano, E.; Peri, G.; Calò, I.; Colasanti, G.B.; Mondanelli, N.; Giannotti, S. A novel “7 sutures and 8 knots” surgical technique in reverse shoulder arthroplasty for proximal humeral fractures: Tuberosity healing improves short-term clinical results. J. Orthop. Traumatol. 2023, 24, 1–9. [Google Scholar] [CrossRef]
- Morea, V.; Polizzi, A.; Niccoli, G.; Zattoni, G.; Andriollo, L. Reverse Shoulder Megaprosthesis for Massive Proximal Humeral Bone Loss in Fracture Outcome Settings: A Report of Two Cases and Literature Review. Cureus 2024, 16, e54276. [Google Scholar] [CrossRef]
- Hones, K.M.; King, J.J.; Schoch, B.S.; Struk, A.M.; Farmer, K.W.; Wright, T.W. The in vivo impact of computer navigation on screw number and length in reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, e629–e635. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, Y.S.; Potter, H.G.; Endo, Y.; Sivakumaran, T.; Lim, T.K.; Chun, T.J. Reverse total shoulder arthroplasty: An imaging overview. Skeletal. Radiol. 2001, 49, 19–30. [Google Scholar] [CrossRef]
- Kozak, T.; Bauer, S.; Walch, G.; Al-Karawi, S.; Blakeney, W. An update on reverse total shoulder arthroplasty: Current indications, new designs, same old problems. EFORT Open Rev. 2021, 6, 189–201. [Google Scholar] [CrossRef]
- Colasanti, G.B.; Moreschini, F.; Cataldi, C.; Mondanelli, N.; Giannotti, S. GPS guided reverse shoulder arthroplasty: An anatomic dissection study. Acta Bio Med. 2020, 91, 204–208. [Google Scholar] [CrossRef]
- Wang, A.W.; Hayes, A.; Gibbons, R.; Mackie, K.E. Computer navigation of the glenoid component in reverse total shoulder arthroplasty: A clinical trial to evaluate the learning curve. J. Shoulder Elb. Surg. 2020, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, G.B.; Troiano, E.; De Sensi, A.G.; Di Sarno, L.; Renieri, A.; Mondanelli, N.; Giannotti, S. A Reverse Shoulder Arthroplasty Implantation with Custom-Made Humerus and Intraoperative GPS Navigation in a Rare Case of Unilateral Hip and Shoulder Dysplasia Associated with a Bone Marrow Mosaic PTEN Truncating Variant: Case Report. J. Shoulder Elb. Arthroplast. 2023, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, A.; Tarallo, L.; Novi, M.; Porcellini, G. Computer-Assisted Surgery in Reverse Shoulder Arthroplasty: Early Experience. Indian J. Orthop. 2021, 55, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Lung, T.S.; Cruickshank, D.; Grant, H.J.; Rainbow, M.J.; Bryant, T.J.; Bicknell, R.T. Factors contributing to glenoid baseplate micromotion in reverse shoulder arthroplasty: A biomechanical study. J. Shoulder Elb. Surg. 2019, 28, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Sprowls, G.R.; Wilson, C.D.; Stewart, W.; Hammond, K.A.P.; Baruch, N.H.; Ward, R.A.; Robin, B.N. Intraoperative navigation and preoperative templating software are associated with increased glenoid baseplate screw length and use of augmented baseplates in reverse total shoulder arthroplasty. JSES Int. 2020, 5, 102–108. [Google Scholar] [CrossRef]
- Saraglis, G.; Singh, H.; Charfare, Z.; Olujinmi, Z.J.; Devecseri, G.; Agbaje, A.; Malal, J.G. Mid-term Results Following Reverse Shoulder Arthroplasty and the Role of Navigation in the Management of Glenoid Bone Loss. Cureus 2024, 16, e54633. [Google Scholar] [CrossRef]
- Velasquez Garcia, A.; Abdo, G. Does computer-assisted navigation improve baseplate screw configuration in reverse shoulder arthroplasty? A systematic review and meta-analysis of comparative studies. J. Orthop. 2022, 36, 29–35. [Google Scholar] [CrossRef]
- Velasquez Garcia, A.; Abdo, G.; Sanchez-Sotelo, J.; Morrey, M.E. The Value of Computer-Assisted Navigation for Glenoid Baseplate Implantation in Reverse Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev. 2023, 11, e23.00038. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Rettig, S.A.; Virk, M.S.; Zuckerman, J.D. Impact of preoperative 3-dimensional planning and intraoperative navigation of shoulder arthroplasty on implant selection and operative time: A single surgeon’s experience. J. Shoulder Elb. Surg. 2020, 29, 2564–2570. [Google Scholar] [CrossRef]

| NAV | CONV | p | |
|---|---|---|---|
| Demographics | |||
| Gender (male/female) | 16/37 | 6/21 | 0.24 |
| Age (years); mean ± SD (range) | 74.6 ± 6 (58–84) | 73.8 ± 5 (62–82) | 0.41 |
| Follow-up (months); mean ± SD (range) | 30 ± 19 (24–71) | 54.5 ± 32 (24–108) | 0.002 |
| Intraoperative characteristics | |||
| Surgical time (min); mean ± SD (range) | 98 ± 22 (55–150) | 91 ± 22 (55–145) | 0.19 |
| Augmented baseplates | 36% | 25% | 0.9 |
| Screws’ number; mean ± SD (range) | 2 ± 0.5 (2–5) | 2.6 ± 0.8 (2–4) | 0.0047 |
| Screws length (mm); mean ± SD (range) | 36 ± 5 (22–46) | 31 ± 4 (18–42) | <0.00005 |
| Functional outcomes (points); mean ± SD (range) | |||
| Constant Score | 66 ± 16 (25–88) | 68 ± 16 (32–91) | 0.56 |
| Δ Constant Score | 10 ± 10 (0–50) | 10 ± 9.6 (0–30) | 0.96 |
| DASH Score | 18 ± 18 (0.8–84.1) | 26.4 ± 23 (0.8–71.7) | 0.28 |
| Complications | |||
| Blood transfusion | 7.5% | 7.4% | 0.18 |
| Others Revision | 9.4% 5.6% | 11% 11% | 1 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troiano, E.; Masini, A.; Colasanti, G.B.; Drago, C.; Giannotti, S.; Mondanelli, N. 3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes. Medicina 2025, 61, 749. https://doi.org/10.3390/medicina61040749
Troiano E, Masini A, Colasanti GB, Drago C, Giannotti S, Mondanelli N. 3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes. Medicina. 2025; 61(4):749. https://doi.org/10.3390/medicina61040749
Chicago/Turabian StyleTroiano, Elisa, Azzurra Masini, Giovanni Battista Colasanti, Caterina Drago, Stefano Giannotti, and Nicola Mondanelli. 2025. "3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes" Medicina 61, no. 4: 749. https://doi.org/10.3390/medicina61040749
APA StyleTroiano, E., Masini, A., Colasanti, G. B., Drago, C., Giannotti, S., & Mondanelli, N. (2025). 3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes. Medicina, 61(4), 749. https://doi.org/10.3390/medicina61040749






