A Comparative Longitudinal Study Analyzing Vaginal Microbiota Differences Between Term and Preterm Pregnancies in Korean Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Vaginal Sample Collection
2.2. DNA Extraction and Probe Design
2.2.1. DNA Extraction
2.2.2. Primer and Probe Design
2.3. Target Plasmid Preparation for Standard Curve Production
2.4. Optimization of Simplex and Multiplex qPCR Assays
2.4.1. Specificity and Accuracy of qPCR Assays
2.4.2. Sensitivity of qPCR Assays
2.5. Quantification of Vaginal Bacteria by Real-Time PCR (qPCR)
2.6. Statistical Analyses
3. Results
3.1. The Characteristics of the Study Participants
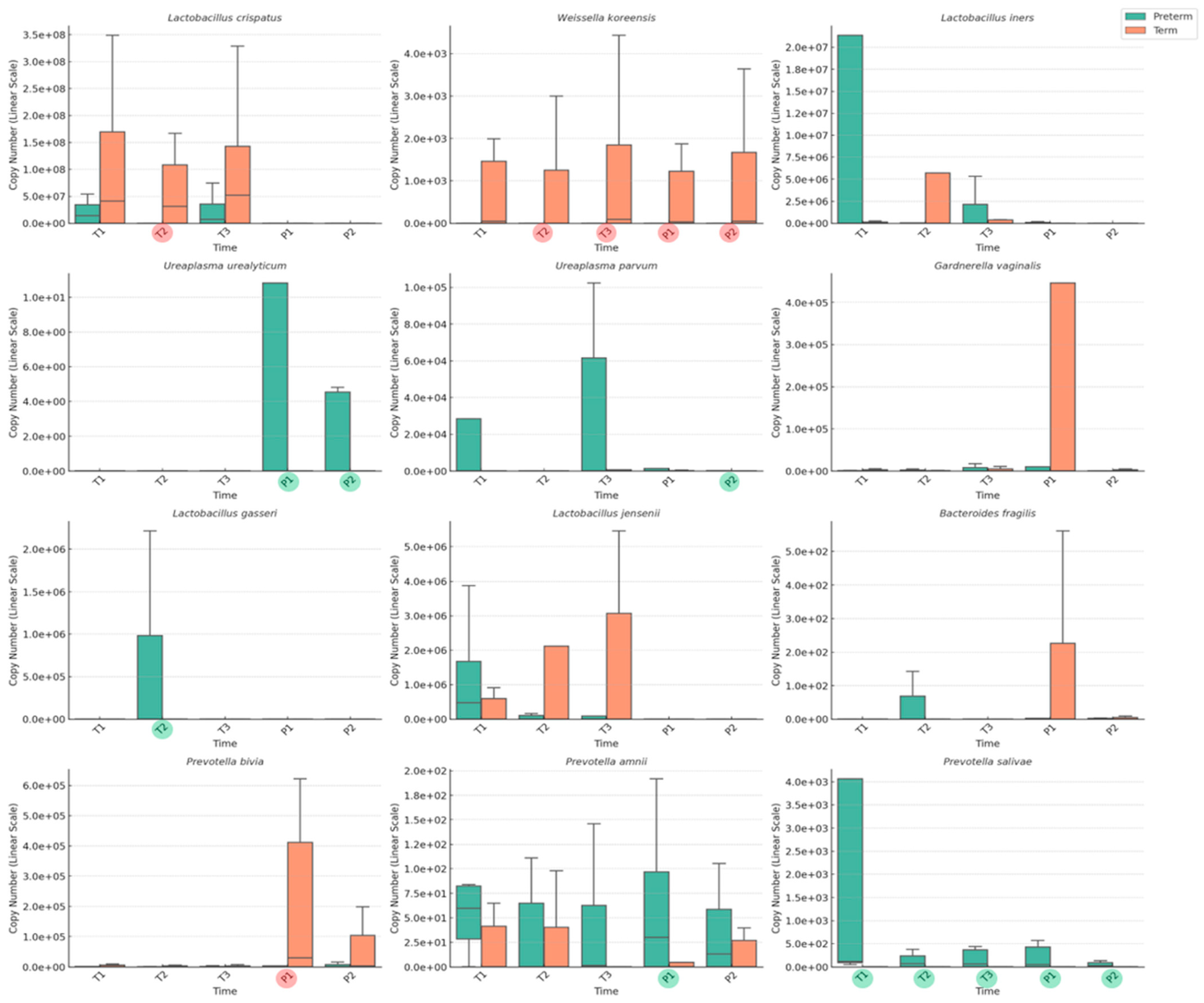
3.2. Longitudinal Analysis of Vaginal Microbiome in Term and Preterm Birth Groups
3.3. Difference in Vaginal Microbiome Between Term and Preterm Birth Groups
3.4. Correlation Analysis of Vaginal Microbiome and Cervical Length During Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 75, 10. [Google Scholar] [CrossRef] [PubMed]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Liu, J.; Liu, M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr. 2022, 176, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jo, M.-W.; Bae, S.-H.; Yoon, S.-J.; Lee, J.Y. Measuring the burden of disease due to preterm birth complications in Korea Using Disability-Adjusted Life Years (DALY). Int. J. Environ. Res. Public Health 2019, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm birth lifetime costs in the United States in 2016: An update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef]
- Henderson, J.; Carson, C.; Redshaw, M. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: A population-based survey. BMJ Open 2016, 6, e012676. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 17–42. [Google Scholar] [CrossRef]
- Ferreira, A.; Bernardes, J.; Goncalves, H. Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review. J. Clin. Med. 2023, 12, 4360. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M.; Morrotti, R.; Avila, C.; Oyarzun, E.; Insunza, A.; Parra, M.; Behnke, E.; Montiel, F.; Cassell, G.H. Infection and labor: VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am. J. Obstet. Gynecol. 1992, 166, 129–133. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Fernandes, A.D.; Di Bella, J.M.; Hammond, J.-A.; Reid, G.; Gloor, G.B. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 2013, 1, 12. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Moon, J.; Kang, N.; Kim, Y.-H.; You, Y.-A.; Kwon, E.; Ansari, A.; Hur, Y.M.; Park, T.; Kim, Y.J. Predicting preterm birth through vaginal microbiota, cervical length, and WBC using a machine learning model. Front. Microbiol. 2022, 13, 912853. [Google Scholar] [CrossRef]
- Broeders, S.; Huber, I.; Grohmann, L.; Berben, G.; Taverniers, I.; Mazzara, M.; Roosens, N.; Morisset, D. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci. Technol. 2014, 37, 115–126. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, L.; Tong, J.; Xu, C. Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J. Obstet. Gynaecol. Res. 2009, 35, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Rappé, M.S.; Giovannoni, S.J. The uncultured microbial majority. Annu. Rev. Microbiol. 2003, 57, 369–394. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F. Exploring the global vaginal microbiome and its impact on human health. Microb. Pathog. 2021, 160, 105172. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 1–19. [Google Scholar]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Severgnini, M.; Morselli, S.; Camboni, T.; Ceccarani, C.; Laghi, L.; Zagonari, S.; Patuelli, G.; Pedna, M.F.; Sambri, V.; Foschi, C. A deep look at the vaginal environment during pregnancy and puerperium. Front. Cell. Infect. Microbiol. 2022, 12, 838405. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Zagonari, S.; Patuelli, G.; Zhu, C.; Foschi, C.; Morselli, S.; Pedna, M.F.; Sambri, V.; Marangoni, A. Vaginal metabolic profiles during pregnancy: Changes between first and second trimester. PLoS ONE 2021, 16, e0249925. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, H.N.; Lee, K.A. Longitudinal changes of vaginal microbiome during pregnancy and puerperium. J. Korean Soc. Obstet. Gynecol. 2023, 109, 435. [Google Scholar]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy vaginal microbiota and influence of probiotics across the female life span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Baud, A.; Hillion, K.-H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 2023, 13, 9061. [Google Scholar] [CrossRef]
- Hyman, R.W.; Fukushima, M.; Jiang, H.; Fung, E.; Rand, L.; Johnson, B.; Vo, K.C.; Caughey, A.B.; Hilton, J.F.; Davis, R.W. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014, 21, 32–40. [Google Scholar] [CrossRef]
- Kumar, M.; Murugesan, S.; Singh, P.; Saadaoui, M.; Elhag, D.A.; Terranegra, A.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T. Vaginal microbiota and cytokine levels predict preterm delivery in Asian women. Front. Cell. Infect. Microbiol. 2021, 11, 639665. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, G.; Lim, Y.-H. Elucidating the mechanism of Weissella-dependent lifespan extension in Caenorhabditis elegans. Sci. Rep. 2015, 5, 17128. [Google Scholar] [CrossRef]
- You, Y.A.; Kwon, E.J.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Vaginal microbiome profiles of pregnant women in Korea using a 16S metagenomics approach. Am. J. Reprod. Immunol. 2019, 82, e13124. [Google Scholar] [CrossRef]
- George, S.D.; Van Gerwen, O.T.; Dong, C.; Sousa, L.G.; Cerca, N.; Elnaggar, J.H.; Taylor, C.M.; Muzny, C.A. The Role of Prevotella Species in Female Genital Tract Infections. Pathogens 2024, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gin, C.; Fettweis, J.; Foxman, B.; Gelaye, B.; MacIntyre, D.A.; Subramaniam, A.; Fraser, W.; Tabatabaei, N.; Callahan, B. Meta-analysis reveals the vaginal microbiome is a better predictor of earlier than later preterm birth. BMC Biol. 2023, 21, 199. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Erez, O.; Pacora, P.; Nicolaides, K.H. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: Updated indirect comparison meta-analysis. Am. J. Obstet. Gynecol. 2018, 219, 10–25. [Google Scholar] [CrossRef]
- Jain, V.; McDonald, S.D.; Mundle, W.R.; Farine, D. Guideline No. 398: Progesterone for prevention of spontaneous preterm birth. J. Obstet. Gynaecol. Can. 2020, 42, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Shennan, A.; Story, L.; on behalf of the Royal College of Obstetricians, Gynaecologists. Cervical cerclage: Green-top guideline no. 75. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1178–1210. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Candiani, M.; Farina, A. Spontaneous Preterm Birth Phenotyping Based on Cervical Length and Immune-Mediated Factors. JAMA Netw. Open 2024, 7, e244559. [Google Scholar] [CrossRef]
- Walther-António, M.R.; Jeraldo, P.; Berg Miller, M.E.; Yeoman, C.J.; Nelson, K.E.; Wilson, B.A.; White, B.A.; Chia, N.; Creedon, D.J. Pregnancy’s stronghold on the vaginal microbiome. PLoS ONE 2014, 9, e98514. [Google Scholar] [CrossRef]
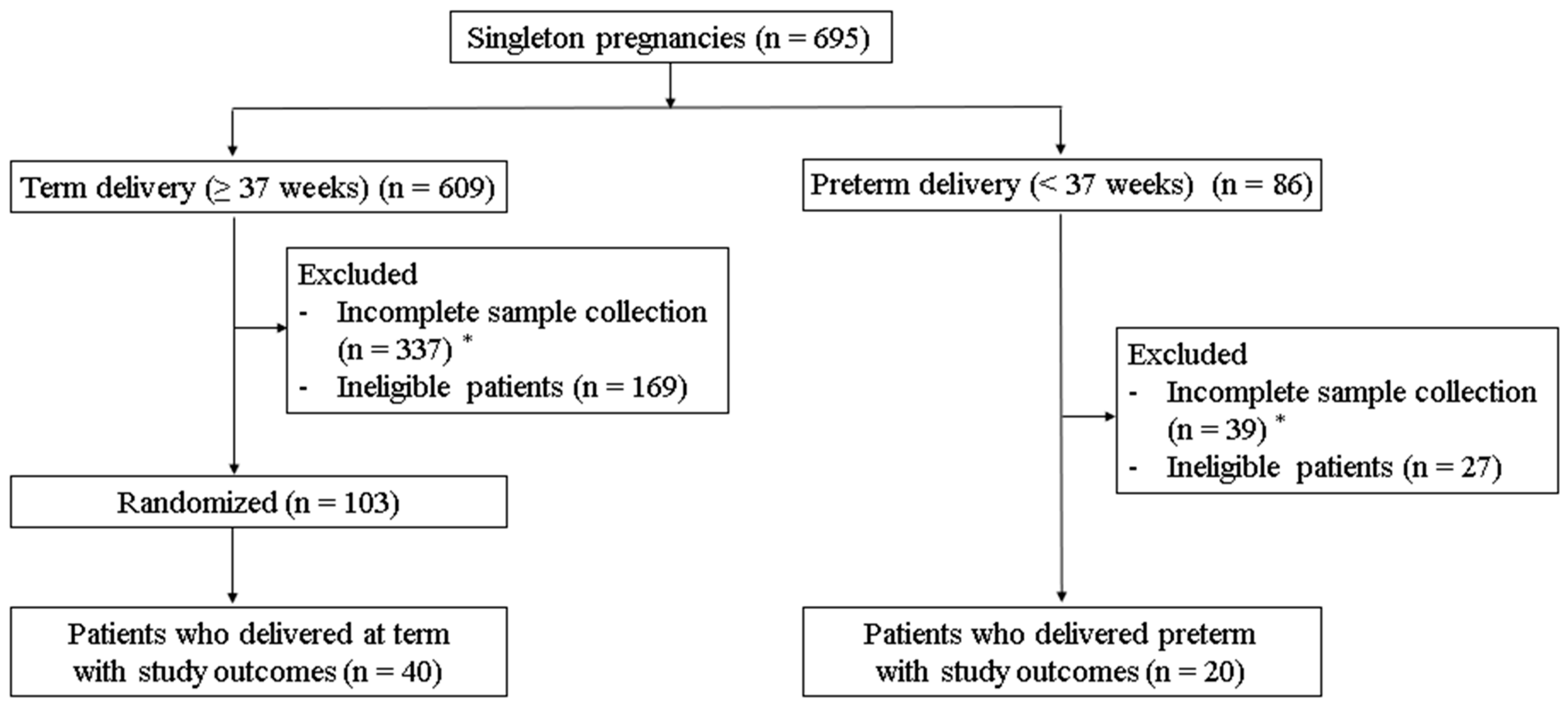

| Characteristics | Term Birth (n = 40) | Preterm Birth (n = 20) | p-Value |
|---|---|---|---|
| Age (years, mean ± SD) | 33.0 ± 3.8 | 34.4 ± 3.8 | 0.191 |
| Pre-pregnancy BMI (kg/m2, mean ± SD) | 21.9 ± 3.1 | 24.8 ± 5.9 | 0.119 |
| Parity, n (%) | |||
| 1 | 20 (50.0) | 13 (65.0) | 0.296 |
| 2 | 18 (45.0) | 5 (25.0) | |
| 3 | 2 (5.0) | 2 (10.0) | |
| Preterm history, n (%) | |||
| Yes | 1 (2.5) | 1 (5.0) | 0.611 |
| No | 39 (97.5) | 19 (95.0) | |
| Method of conception, n (%) | |||
| Natural pregnancy | 32 (80.0) | 13 (65.0) | 0.206 |
| IVF-ET (ART) | 8 (20.0) | 7 (35.0) | |
| Gestational age at sampling (weeks, median (range)) | |||
| First trimester | 11.6 (11.3–12.5) | 12.4 (12.0–12.6) | 0.362 |
| Second trimester | 24.3 (23.0–25.0) | 23.7 (20.6–24.9) | 0.994 |
| Third trimester | 36.3 (35.4–37.0) | 35.1 (34.5–35.3) | 0.000 * |
| Postpartum timing at sampling (days, median (range)) | |||
| First sampling | 9.0 (8.0–10.8) | 9.0 (8.0–11.3) | 0.189 |
| Second sampling | 43.0 (40.3–45.8) | 40.5 (36.3–48.5) | 0.294 |
| Use of Lactobacillus supplements, n (%) | 21 (52.5) | 6 (30.0) | 0.220 |
| Use of antibiotics or antifungals, n (%) a | 12 (30.0) | 6 (30.0) | 1.000 |
| White blood cell count (103/µL, mean ± SD) b | 8.8 ± 2.0 | 10.0 ± 2.6 | 0.083 |
| C-reactive protein (mg/dL, median (range)) b | 0.12 (0.08–0.25) | 0.21 (0.06–0.43) | 0.393 |
| CL in the second trimester (mm, mean ± SD) | 43.0 ± 6.0 | 38.3 ± 8.1 | 0.005 * |
| CL in the third trimester (mm, mean ± SD) | 30.9 ± 9.1 | 19.7 ± 11.2 | 0.009 * |
| Mode of delivery | |||
| Vaginal delivery, n (%) | 14 (35.0) | 5 (25.0) | 0.624 |
| Cesarean delivery, n (%) | 26 (65.0) | 15 (75.0) | |
| Gestation age at delivery (weeks, median (range)) | 38.5 (38.0–39.4) | 35.8 (34.2–36.4) | 0.000 * |
| Birth weight (g, mean ± SD) | 3232.5 ± 306.8 | 2421.5 ± 792.2 | 0.000 * |
| Sex | |||
| Female, n (%) | 20 (50.0) | 10 (50.0) | 1.000 |
| Male, n (%) | 20 (50.0) | 10 (50.0) | |
| Apgar score at 1 min (median (range)) | 9.0 (8.0–9.0) | 9.0 (7.0–9.0) | 0.227 |
| Apgar score at 5 min (median (range)) | 10.0 (10.0–10.0) | 10.0 (8.3–10.0) | 0.047 * |
| Trimester | Bacteria | Correlation Coefficient † | p-Value | Confidence Interval (95%) |
|---|---|---|---|---|
| T2 | Lactobacillus cripatus | 0.006 | 0.98 | - |
| T2 | Weissella koreensis | 0.336 | 0.16 | - |
| T2 | Lactobacillus iners | 0.009 | 0.97 | −0.40~0.42 |
| T2 | Ureaplasma urealyticum | 0.260 | 0.28 | - |
| T2 | Ureaplasma parvum | 0.187 | 0.44 | - |
| T2 | Bacteriodes fragilis | −0.542 | 0.02 * | −0.79~−0.07 |
| T3 | Lactobacillus crispatus | 0.117 | 0.75 | −0.56~−0.89 |
| T3 | Weissella koreensis | 0.389 | 0.27 | - |
| T3 | Lactobacillus iners | 0.054 | 0.88 | −0.68~0.86 |
| T3 | Ureaplasma urealyticum | 0.406 | 0.24 | - |
| T3 | Ureaplasma parvum | 0.109 | 0.78 | −0.62~0.85 |
| T3 | Bacteroides fragilis | −0.305 | 0.32 | - |
| T3 | Prevotella bivia | 0.750 | 0.01 * | 0.22~0.95 |
| T3 | Prevotella salivae | −0.693 | 0.03 * | −0.94~−0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, G.; Lee, K.A.; Kim, S.J.; Oh, K.Y.; Lee, S.; Lee, H.C.; Kim, S.Y.; Park, M.H. A Comparative Longitudinal Study Analyzing Vaginal Microbiota Differences Between Term and Preterm Pregnancies in Korean Women. Medicina 2025, 61, 752. https://doi.org/10.3390/medicina61040752
Nam G, Lee KA, Kim SJ, Oh KY, Lee S, Lee HC, Kim SY, Park MH. A Comparative Longitudinal Study Analyzing Vaginal Microbiota Differences Between Term and Preterm Pregnancies in Korean Women. Medicina. 2025; 61(4):752. https://doi.org/10.3390/medicina61040752
Chicago/Turabian StyleNam, Gina, Kyung A. Lee, Soo Jung Kim, Kwan Young Oh, Sunghee Lee, Hyun Chul Lee, So Yoon Kim, and Mi Hye Park. 2025. "A Comparative Longitudinal Study Analyzing Vaginal Microbiota Differences Between Term and Preterm Pregnancies in Korean Women" Medicina 61, no. 4: 752. https://doi.org/10.3390/medicina61040752
APA StyleNam, G., Lee, K. A., Kim, S. J., Oh, K. Y., Lee, S., Lee, H. C., Kim, S. Y., & Park, M. H. (2025). A Comparative Longitudinal Study Analyzing Vaginal Microbiota Differences Between Term and Preterm Pregnancies in Korean Women. Medicina, 61(4), 752. https://doi.org/10.3390/medicina61040752






