Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233
Abstract
:1. Introduction
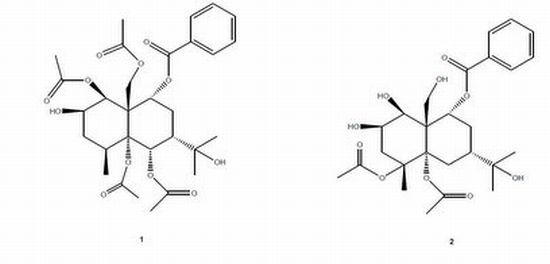
2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC a,b, mult. | δH c, mult. (J in Hz) | δC a,b, mult. | δH c, mult. (J in Hz) | |
| 1 | 70.9, CH | 5.50 d (3.5) | 70.9, CH | 5.50 d (3.5) |
| 2 | 68.5, CH | 5,38, m | 68.4, CH | 5.36, m |
| 3a | 30.3, CH2 | 1.75, dd (14.5, 2.0) | 39.0, CH2 | 1.83 dd (14.0, 2.0) |
| 3b | 2.13, dd (14.5, 3.2) | 2.09, dd (14.0, 3.0) | ||
| 4 | 32.5, CH | 2.22, m | 68.8, C | |
| 5 | 88.8, C | 88.8, C | ||
| 6a | 76.9, CH | 5.88, d (1.0) | 30.7, CH2 | 2.18, m |
| 6b | 1.80, m | |||
| 7 | 47.9, CH | 2.19, m | 42.4, CH | 2.06, m |
| 8a | 34.2, CH2 | 2.40, m | 32.2, CH2 | 2.16, m |
| 8b | 2.16, m | 2.02, m | ||
| 9 | 68.8, CH | 5.34, m | 68.8, CH | 5.69, m |
| 10 | 52.7, C | 50.9, CH | ||
| 11 | 82.4, C | 83.1, C | ||
| 12 | 25.5, CH3 | 1.41, s | 24.1, CH3 | 1.44, s |
| 13 | 30.1, CH3 | 1.42, s | 29.1, CH3 | 1.29, s |
| 14a | 64.4, CH2 | 5.00, d (12.7) | 65.5, CH2 | 5.11, d (12.5) |
| 14b | 4.25, d (12.7) | 4.79, d (12.5) | ||
| 15 | 17.3, CH3 | 1.12, d (7.5) | 26.0, CH3 | 1.37, s |
| 16 | 164.4, C | 164.6, C | ||
| 17 | 128.8, C | 128.7, C | ||
| 18/22 | 129.5, CH | 7.90, dd (8.0, 2.0) | 129.5, CH | 7.93, dd (8.0, 2.0) |
| 19/21 | 128.5, CH | 7.52, t (8.0) | 128.5, CH | 7.53, t (8.0) |
| 20 | 133.6, CH | 7.65, t (8.0) | 133.5, CH | 7.64, t (8.0) |
| 23 | 169.9, C | 168.3, C | ||
| 24 | 20.8, CH3 | 2.19, s | 20.7, CH3 | 1.98, s |
| 25 | 168.6, C | 169.3, C | ||
| 26 | 20.0, CH3 | 1.46, s | 20.0, CH3 | 1.38, s |
| 27 | 169.4, C | |||
| 28 | 20.8 CH3 | 2.20, s | ||
| 29 | 169.6, C | |||
| 30 | 21.0, CH3 | 2.09, s | ||


3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Cultivation and Stress Applications
3.3. Extraction and Isolation
3.4. Tyrosinase Inhibition Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Saleema, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Yamazaki, H.; Rotinsulu, H.; Kaneko, T.; Murakami, K.; Fujiwara, H.; Ukai, K.; Namikoshi, M. A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs 2012, 10, 2691–2697. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef]
- Zhang, L.; An, R.; Wang, J.; Sun, N.; Zhang, S.; Hu, J.; Kuai, J. Exploring novel bioactive compounds from marine microbes. Curr. Opin. Microbiol. 2005, 8, 276–281. [Google Scholar]
- Jensen, P.R.; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological perspectives. Annu. Rev. Microbiol. 1994, 48, 559–584. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, K. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Park, H.B.; Kwon, H.C.; Lee, C.-H.; Yang, H.O. Glionitrin A, an antibiotic−antitumor metabolite derived from competitive interaction between abandoned mine microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, W.; Wu, B. Methodological aspects about determination of plant defensive phenolics in response to stress. Curr. Anal. Chem. 2013, 9, 352–359. [Google Scholar] [CrossRef]
- Xu, J.; Ebada, S.F.; Proksch, P. Pestalotiopsis, a highly creative genus: Chemistry and bioactivity of secondary metabolites. Fung. Divers. 2010, 44, 15–31. [Google Scholar] [CrossRef]
- Wei, M.-Y.; Li, D.; Shao, C.L.; Deng, D.-S.; Wang, C.Y. (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Strobel, G.; Yang, X.; Sears, J.; Kramer, R.; Sidhu, R.S.; Hess, W.M. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 1996, 142, 435–440. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Chen, X.; Guo, L.; Che, Y. Pestalofones A–E, bioactive cyclohexanone derivatives from the plant endophytic fungus Pestalotiopsis fici. Bioorg. Med. Chem. 2009, 17, 606–613. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Jiang, L.; Chen, X.; Guo, L.; Che, Y. Chloropupukeananin, the first chlorinated pupukeanane derivative, and its precursors from Pestalotiopsis fici. Org. Lett. 2008, 10, 1397–1400. [Google Scholar] [CrossRef]
- Liu, L.; Bruhn, T.; Guo, L.; Götz, D.C.G.; Brun, R.; Stich, A.; Che, Y.; Bringmann, G. Chloropupukeanolides C–E: Cytotoxic pupukeanane chlorides with a spiroketal skeleton from Pestalotiopsis fici. Chem. Eur. J. 2011, 17, 2604–2613. [Google Scholar]
- Vamos-Vigyazo, L. Polyphenol oxidase and peroxidase in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Wu, X. New lignan glucosides with tyrosinase inhibitory activity from exocarp of Castanea henryi. Carbohydr. Res. 2012, 355, 45–49. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, B.; Wu, X.; Sun, M.; Li, M. Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713-2721. https://doi.org/10.3390/md11082713
Wu B, Wu X, Sun M, Li M. Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Marine Drugs. 2013; 11(8):2713-2721. https://doi.org/10.3390/md11082713
Chicago/Turabian StyleWu, Bin, Xiaodan Wu, Min Sun, and Minhui Li. 2013. "Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233" Marine Drugs 11, no. 8: 2713-2721. https://doi.org/10.3390/md11082713
APA StyleWu, B., Wu, X., Sun, M., & Li, M. (2013). Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Marine Drugs, 11(8), 2713-2721. https://doi.org/10.3390/md11082713