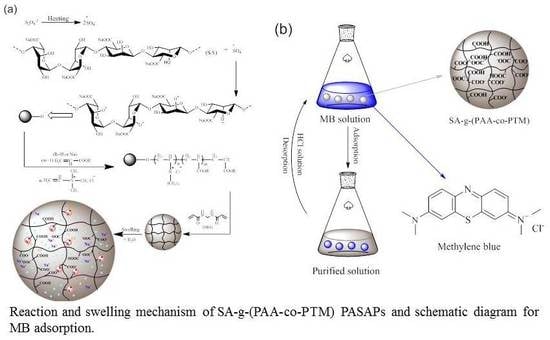
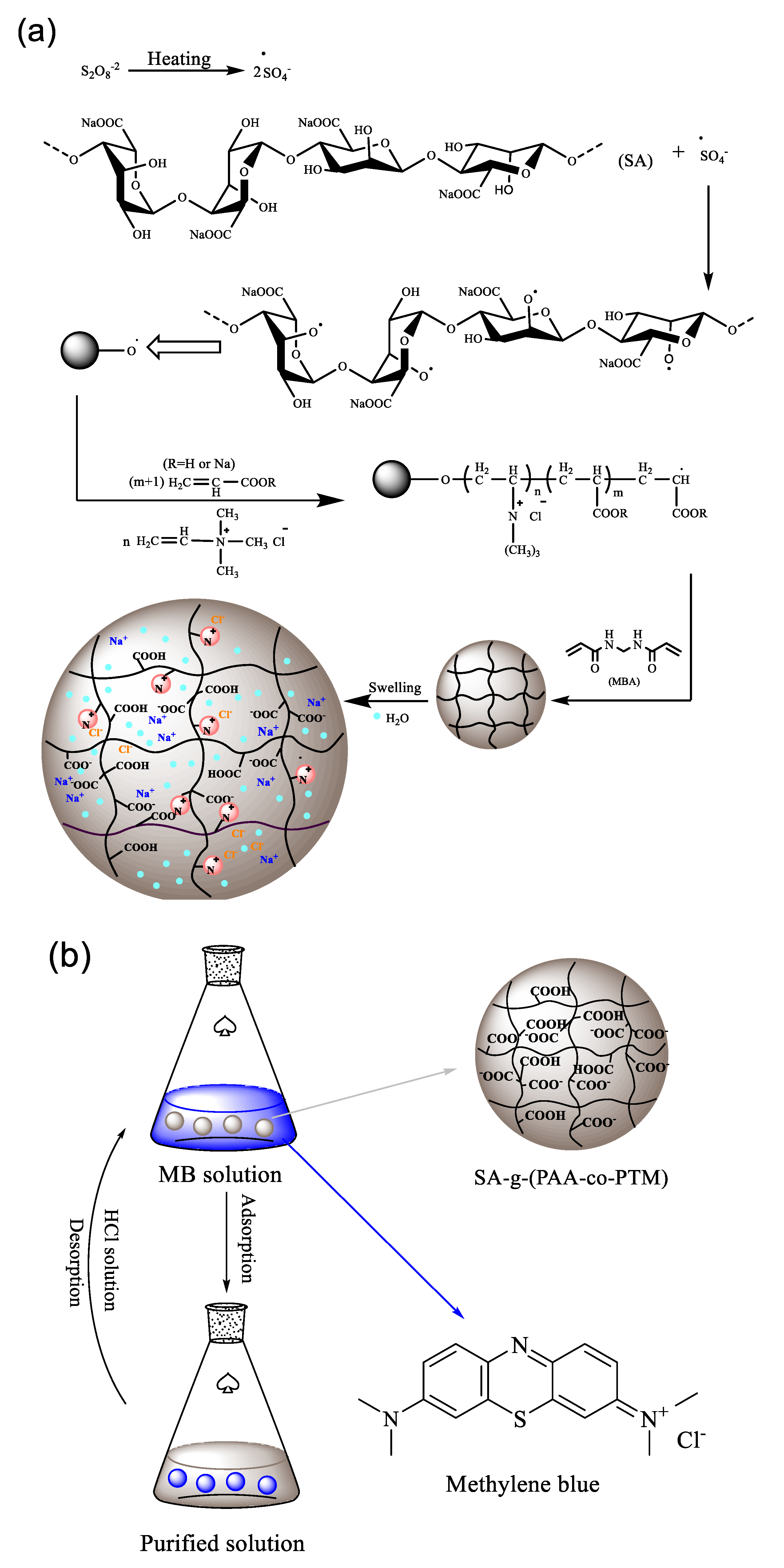
Preparation of the Sodium Alginate-g-(Polyacrylic Acid-co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of SA-g-(PAA-co-PTM) PASAP
2.1.1. FTIR
2.1.2. XRD Analysis
2.1.3. Morphologies of SA-g-(PAA-co-PTM) PASAP
2.1.4. TGA Analysis
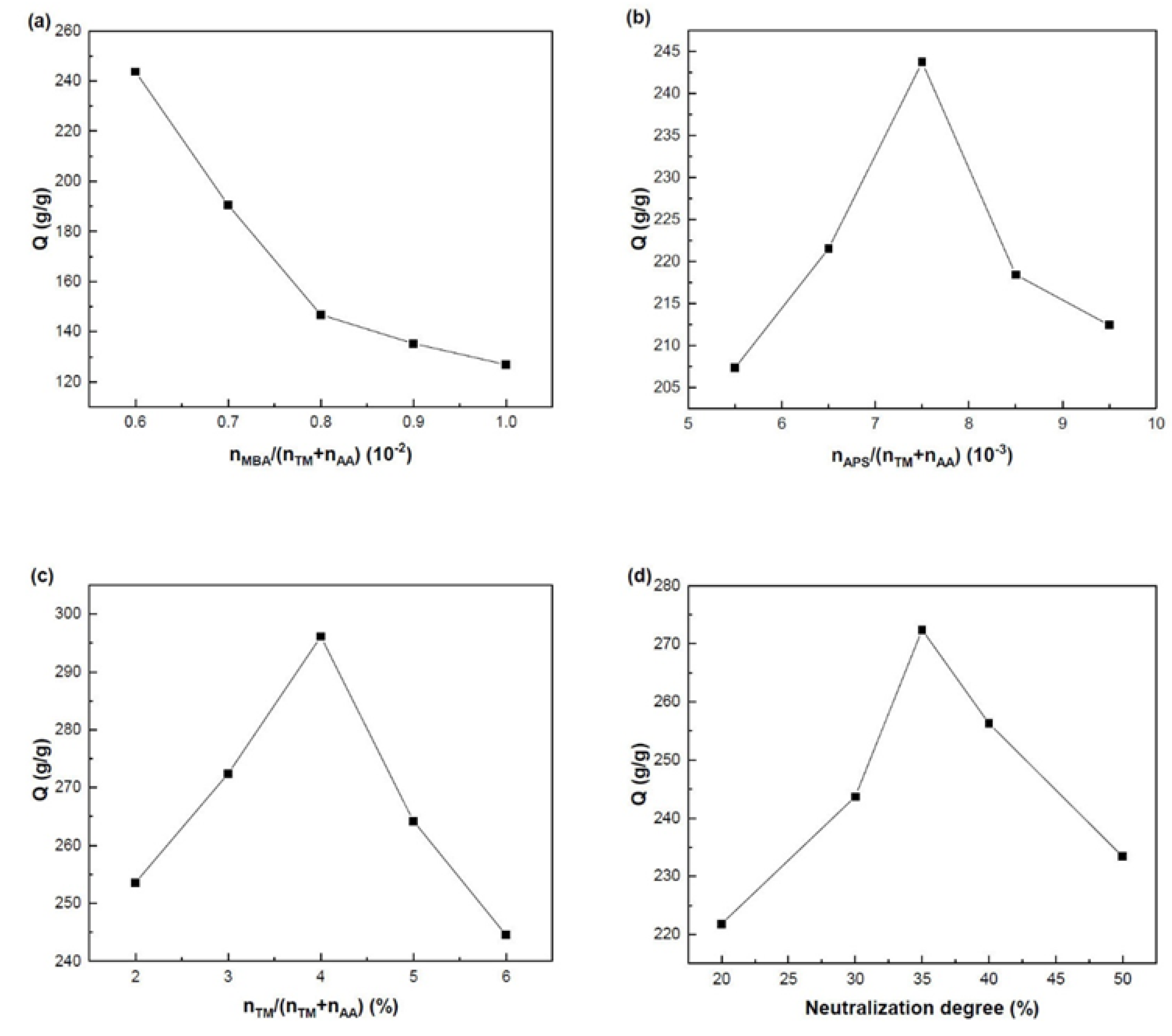
2.2. Optimization of Synthesis Conditions
2.2.1. Effects of the Amount of Cross-Linker
2.2.2. Effects of the Amount of Initiator
2.2.3. Effects of the Molar Ratio between the Monomers
2.2.4. Effects of the Neutralization Degree of AA
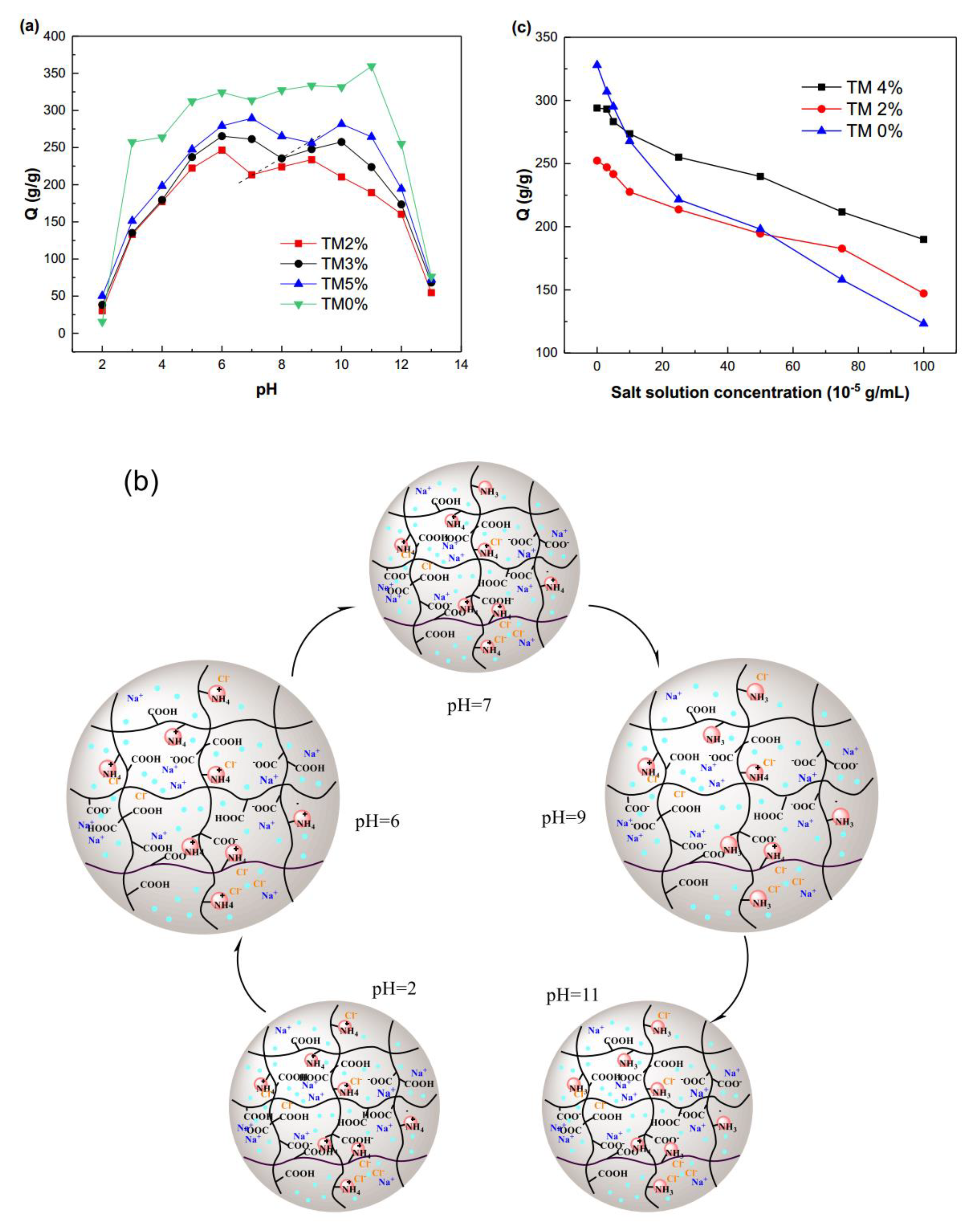
2.3. Effects of Solution Property on the Swelling Properties of SA-g-(PAA-co-PTM) PASAP
2.3.1. pH
2.3.2. Ionic Strength
2.4. Dye Adsorption Properties
2.4.1. Effects of TM Content
2.4.2. Effects of Solution pH
2.4.3. Adsorption Kinetics
2.4.4. Adsorption Isotherm
2.4.5. Thermodynamics of Adsorption
2.4.6. Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of SA-g-(PAA-co-PTM) PASAP
3.3. Structural Characterization and Swelling Properties Study of SA-g-(PAA-co-PTM) PASAP
3.3.1. FT-IR Spectroscopy
3.3.2. X-ray Diffraction Studies (XRD)
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Thermal Gravimetric Analysis (TGA)
3.3.5. Determination of the Swelling Properties of SA-g-(PAA-co-PTM) PASAP
3.4. Dye Adsorption Properties of SA-g-(PAA-co-PTM) PASAP
3.4.1. Adsorption Kinetics
3.4.2. Adsorption Isotherms
3.4.3. Recycling Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, E.; Shang, S.; Tao, X.M.; Jiang, S.; Chiu, K. Regeneration and reuse of highly polluting textile dyeing effluents through catalytic ozonation with carbon aerogel catalysts. J. Clean. Prod. 2016, 137, 1055–1065. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Du, Q.; Zhang, L.; Yang, X.; Wu, S.; Xia, Y.; Wang, Z.; Xia, L.; Cao, A. Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning. Carbohydr. Polym. 2014, 102, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Dinh, D.M.; Hsieh, Y. Lo Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Z. A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J. Memb. Sci. 2017, 524, 214–224. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Nagarpita, M.V.; Roy, P.; Shruthi, S.B.; Sailaja, R.R.N. Synthesis and swelling characteristics of chitosan and CMC grafted sodium acrylate-co-acrylamide using modified nanoclay and examining its efficacy for removal of dyes. Int. J. Biol. Macromol. 2017, 102, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, D.; Zhang, M.; Wu, P.; Jin, H.; Li, J.; Wang, X.; Ge, H.; Wang, Z.; Lou, H. Fabrication and application of magnetic starch-based activated hierarchical porous carbon spheres for the efficient removal of dyes from water. Mater. Chem. Phys. 2016, 174, 179–186. [Google Scholar] [CrossRef]
- Tang, M.; Li, X.; Gao, C.; Li, X.; Qiu, H. Adsorption performance of CuFe2O4/rGO nanocomposites towards organic dye. Mater. Chem. Phys. 2017, 185, 114–121. [Google Scholar] [CrossRef]
- Olusegun, S.J.; de Sousa Lima, L.F.; Mohallem, N.D.S. Enhancement of adsorption capacity of clay through spray drying and surface modification process for wastewater treatment. Chem. Eng. J. 2018, 334, 1719–1728. [Google Scholar] [CrossRef]
- Bello, K.; Sarojini, B.K.; Narayana, B.; Rao, A.; Byrappa, K. A study on adsorption behavior of newly synthesized banana pseudo-stem derived superabsorbent hydrogels for cationic and anionic dye removal from effluents. Carbohydr. Polym. 2018, 181, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Korpe, S.; Erdoǧan, B.; Bayram, G.; Ozgen, S.; Uludag, Y.; Bicak, N. Crosslinked DADMAC polymers as cationic super absorbents. React. Funct. Polym. 2009, 69, 660–665. [Google Scholar] [CrossRef]
- Deen, G.R.; Wei, T.T.; Fatt, L.K. New stimuli-responsive polyampholyte: Effect of chemical structure and composition on solution properties and swelling mechanism. Polymer (United Kingdom) 2016, 104, 91–103. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Li, C.; Yue, Z.; Zhai, M.; Li, J. Antipolyelectrolyte swelling of amphiphilic hydroxypropyl methylcellulose phthalate gels. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 356, 89–96. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Liu, Y.; Tan, H. Preparation and study on the volume phase transition properties of novel carboxymethyl chitosan grafted polyampholyte superabsorbent polymers. J. Taiwan Inst. Chem. Eng. 2016, 59, 569–577. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Gao, B.; Zhao, C.; Zhang, S.; Chen, N. Enhancement of textile-dyeing sludge dewaterability using a novel cationic polyacrylamide: Role of cationic block structures. RSC Adv. 2017, 7, 11626–11635. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1953; pp. 101–102. [Google Scholar]
- Pourjavadi, A.; Harzandi, A.M.; Hosseinzadeh, H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Z.; Du, Y. Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomaterials 2004, 25, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.F.; Tang, H.L.; Tan, H.M. Study of carboxymethyl chitosan based polyampholyte superabsorbent polymer I. Optimization of synthesis conditions and pH sensitive property study of carboxymethyl chitosan-g-poly(acrylic acid-co-dimethyldiallylammonium chloride) superabsorbent polymer. Carbohydr. Polym. 2010, 81, 365–371. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly(sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Gao, M.; Gawel, K.; Stokke, B.T. Polyelectrolyte and antipolyelectrolyte effects in swelling of polyampholyte and polyzwitterionic charge balanced and charge offset hydrogels. Eur. Polym. J. 2014, 53, 65–74. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of Methylene Blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Marrakchi, F.; Khanday, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010, 101, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Varghese, L.R.; Das, N. Enhanced TDS removal using cyclodextrinated, sulfonated and aminated forms of bead-membrane duo nanobiocomposite via sophorolipid mediated complexation. Desalination 2015, 360, 35–44. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem. Eng. J. 2008, 39, 521–530. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Bahrami, H. Preparation, characterization and dye adsorption properties of biocompatible composite (alginate/titania nanoparticle). Desalination 2011, 275, 93–101. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Yu, C.; Geng, J.; Zhuang, Y.; Zhao, J.; Chu, L.; Luo, X.; Zhao, Y.; Guo, Y. Preparation of the chitosan grafted poly (quaternary ammonium)/Fe3O4 nanoparticles and its adsorption performance for food yellow 3. Carbohydr. Polym. 2016, 152, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Panic, V.V.; Madzarevic, Z.P.; Volkov-Husovic, T.; Velickovic, S.J. Poly (methacrylic acid) based hydrogels as sorbents for removal of cationic dye basic yellow 28: Kinetics, equilibrium study and image analysis. Chem. Eng. J. 2013, 217, 192–204. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]





| Material | Decomposition Step | Temperature Range (°C) | Maximum Decomposition Temperature (°C) | Weight Loss Percentage (%) | Total Weight Loss Percentage (%) | Temperature at 5% Weight Loss (°C) | Temperature at 10% Weight Loss (°C) |
|---|---|---|---|---|---|---|---|
| SA | 1 | 200–283 | 251 | 40.02 | 40.02 | 60.20 | 102.00 |
| SA-g-(PAA-co-PTM) | 1 | 210–300 | 252 | 18.91 | 62.05 | 158.23 | 229.67 |
| 2 | 300–404 | 371 | 30.23 | ||||
| 3 | 404–477 | 427 | 12.91 |
| Adsorbents | Adsorption Capacity (mg/g) | Sources |
|---|---|---|
| SA-g-(PAA-co-PTM) (TM2%) | 1445.4 | Present work |
| Fox nutshell activated carbon | 968.74 | [7] |
| ĸ-carrageenan/graphene oxide gel beads | 658.4 | [8] |
| Bamboo-based activated carbon | 454.2 | [24] |
| Keratin nanofibrous membranes | 170 | [25] |
| Modified Ball clay | 100 | [26] |
| Chitosan/sepiolite composite | 40.986 | [27] |
| Models | Parameters | Dye Concentrations(mg/L) | ||
|---|---|---|---|---|
| 2600 | 2800 | 3000 | ||
| Pseudo-first-order model | (min−1) | 2.508 | 6.866 | 3.944 |
| 0.9814 | 0.9851 | 0.9428 | ||
| Pseudo-second-order model | (g/(mg min)) | 1.00 × 10−4 | 5.86 × 10−5 | 4.04 × 10−5 |
| 0.9999 | 0.9999 | 0.9997 | ||
| Weber Morris model | (mg2/(g2 min)) | 7.00 × 104 | 1.01 × 105 | 7.94 × 104 |
| (mg2/(g2 min)) | 3.24 × 103 | 6.85 × 103 | 1.04 × 104 | |
| (mg2/(g2 min)) | 6.53 × 102 | 9.85 × 102 | 1.79 × 103 | |
| 4.83 × 106 | 4.70 × 106 | 4.68 × 106 | ||
| 0.8333 | 0.8222 | 0.9527 | ||
| Boyd model | (min−1) | 0.043 | 0.043 | 0.027 |
| 0.9470 | 0.9299 | 0.9574 | ||
| Model | Parameters | 308 K | 318 K | 328 K |
|---|---|---|---|---|
| Langmuir | KL (L/mg) | 0.0236 | 0.0208 | 0.0205 |
| R2 | 0.9976 | 0.9994 | 0.9990 | |
| RL | 0.0208 | 0.0235 | 0.0238 | |
| Freundlich | KF (mg/g) | 1205.4 | 1098.9 | 1182.6 |
| bF | 0.126 | 0.135 | 0.114 | |
| R2 | 0.9728 | 0.9548 | 0.9744 | |
| Dubinin-Radushkevich | K (J2/mol2) | −2.015 × 10−4 | −6.973 × 10−4 | −4.743 × 10−4 |
| R2 | 0.5941 | 0.8802 | 0.6788 | |
| E (kJ/mol) | 49.81 | 26.78 | 32.47 |
| T(K) | ΔG° (kJ/mol) | TΔS° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/(mol K)) |
|---|---|---|---|---|
| 308 | −23.75 | 17.69 | −6.02 | 57.44 |
| 318 | −24.19 | 18.27 | ||
| 328 | −24.91 | 18.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Zhao, Y.; Wang, H.; Wang, Y.; Zhu, H.; Chen, Y.; Chen, S.; Jin, S.; Yang, Z.; Li, P.; et al. Preparation of the Sodium Alginate-g-(Polyacrylic Acid-co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property. Mar. Drugs 2018, 16, 476. https://doi.org/10.3390/md16120476
Tang S, Zhao Y, Wang H, Wang Y, Zhu H, Chen Y, Chen S, Jin S, Yang Z, Li P, et al. Preparation of the Sodium Alginate-g-(Polyacrylic Acid-co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property. Marine Drugs. 2018; 16(12):476. https://doi.org/10.3390/md16120476
Chicago/Turabian StyleTang, Shuxian, Ying Zhao, Haitao Wang, Yuqiao Wang, Hexiang Zhu, Yu Chen, Shusen Chen, Shaohua Jin, Ziming Yang, Puwang Li, and et al. 2018. "Preparation of the Sodium Alginate-g-(Polyacrylic Acid-co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property" Marine Drugs 16, no. 12: 476. https://doi.org/10.3390/md16120476
APA StyleTang, S., Zhao, Y., Wang, H., Wang, Y., Zhu, H., Chen, Y., Chen, S., Jin, S., Yang, Z., Li, P., & Li, S. (2018). Preparation of the Sodium Alginate-g-(Polyacrylic Acid-co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property. Marine Drugs, 16(12), 476. https://doi.org/10.3390/md16120476