Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications
Abstract
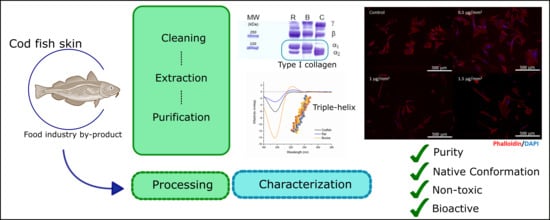
:1. Introduction
2. Results and Discussion
2.1. Electrophoretic Profiling of Acid-Soluble Collagen Extracted from Codfish Skin
2.2. Fourier-Transformed Infrared (FTIR) Spectroscopy Analysis
2.3. Extract Purity
2.4. Circular Dichroism (CD)
2.5. Amino Acid Composition
2.6. Denaturation Temperature
2.7. Heavy Metals
2.8. Cell Viability
2.9. Cell Adhesion
3. Materials and Methods
3.1. Preparation of Codfish Skin
3.2. Extraction of Acid-Soluble Collagen from Codfish Skin
3.3. Codfish Extract Purity
3.4. Sodium Dozdecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5. Fourier Transformed Infrared (FTIR) Spectroscopy
3.6. Protein Conformation
3.7. Denaturation Temperature
3.8. Amino Acid Content
3.9. Heavy Metals Quantification
3.10. Cell Culture
3.11. MTS Metabolic Activity Assay
3.12. Cell Adhesion on Collagen Coatings
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediat. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Tani, S.; Sano, A.; Fuijoka, K. Microstructure and release characteristics of the minipellet, a collagen based drug delivery system for controlled release of protein drugs. J. Control. Release 1999, 62, 313–324. [Google Scholar] [CrossRef]
- Albu, M.G.; Titorencu, I.; Ghica, M. Collagen-based drug delivery systems for tissue engineering. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTechOpen: London, UK, 2011. [Google Scholar]
- Pati, F.; Datta, P.; Adhikari, B.; Dhara, S.; Ghosh, K.; Mohapatra, P.K.D. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. J. Biomed. Mater. Res. Part A 2012, 100, 1068–1079. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yanagiguchi, K.; Yamada, S. Fish collagen as a scaffold. Austin J. Biomed. Eng. 2015, 2, 1029. [Google Scholar]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and carbon dioxide: Green solvents for the extraction of collagen/gelatin from marine sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Silva, J.C.; Barros, A.A.; Aroso, I.M.; Fassini, D.; Silva, T.H.; Reis, R.L.; Duarte, A.R.C. Extraction of collagen/gelatin from the marine demosponge chondrosia reniformis (nardo, 1847) using water acidified with carbon dioxide—Process optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular characterization of a nonfibrillar collagen from the marine sponge chondrosia reniformis nardo 1847 and positive effects of soluble silicates on its expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfi, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge chondrosia reniformis nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfi, S.; Mussino, F.; Ferrando, S.; Gallus, L.; Giovine, M. Molecular cloning, characterization, and expression analysis of a prolyl 4-hydroxylase from the marine sponge chondrosia reniformis. Mar. Biotechnol. 2015, 17, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges axinella cannabina and suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Wysokowski, M.; Zoltowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of poriferan origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.C.; Shao, Z.Y.; Li, C.B.; Yu, L.J.; Raja, M.A.; Liu, C.G. Isolation, characterization and evaluation of collagen from jellyfish rhopilema esculentum kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef]
- Exposito, J.Y.; Larroux, C.; Cluzel, C.; Valcourt, U.; Lethias, C.; Degnan, B.M. Demosponge and sea anemone fibrillar collagen diversity reveals the early emergence of a/c clades and the maintenance of the modular structure of type v/xi collagens from sponge to human. J. Biol. Chem. 2008, 283, 28226–28235. [Google Scholar] [CrossRef]
- Nowack, H.; Nordwig, A. Sea-anemone collagen—Isolation and characterization of cyanogen-bromide peptides. Eur. J. Biochem. 1974, 45, 333–342. [Google Scholar] [CrossRef]
- Benayahu, D.; Sharabi, M.; Pomeraniec, L.; Awad, L.; Haj-Ali, R.; Benayahu, Y. Unique collagen fibers for biomedical applications. Mar. Drugs 2018, 16, 102. [Google Scholar] [CrossRef]
- Cozza, N.; Bonani, W.; Motta, A.; Migliaresi, C. Evaluation of alternative sources of collagen fractions from loligo vulgaris squid mantle. Int. J. Biol. Macromol. 2016, 87, 504–513. [Google Scholar] [CrossRef]
- Coelho, R.C.G.; Marques, A.L.P.; Oliveira, S.M.; Diogo, G.S.; Pirraco, R.P.; Moreira-Silva, J.; Xavier, J.C.; Reis, R.L.; Silva, T.H.; Mano, J.F. Extraction and characterization of collagen from antarctic and sub-antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng. C 2017, 78, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.L.; Liu, X.; Wang, N.P.; Sun, J. Squid type ii collagen as a novel biomaterial: Isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting stat1 signaling in pro-inflammatory macrophages. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 89, 283–294. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Cocce, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Carnevali, M.D.C.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ovaska, M.; Bertalan, Z.; Miksic, A.; Sugni, M.; Di Benedetto, C.; Ferrario, C.; Leggio, L.; Guidetti, L.; Alava, M.J.; La Porta, C.A.M.; et al. Deformation and fracture of echinoderm collagen networks. J. Mech. Behav. Biomed. Mater. 2017, 65, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, I.C.; Emson, R.H.; Young, C.M. Smart collagen in sea lilies. Nature 1993, 366, 519–520. [Google Scholar] [CrossRef]
- Bao, Z.X.; Sun, Y.; Rai, K.; Peng, X.Y.; Wang, S.L.; Nian, R.; Xian, M. The promising indicators of the thermal and mechanical properties of collagen from bass and tilapia: Synergistic effects of hydroxyproline and cysteine. Biomater. Sci. 2018, 6, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Duarte, A.R.C.; Reis, R.L.; Silva, T.H. Bioinspiring chondrosia reniformis (nardo, 1847) collagen-based hydrogel: A new extraction method to obtain a sticky and self-healing collagenous material. Mar. Drugs 2017, 15, 380. [Google Scholar] [CrossRef]
- Bernhardt, A.; Paul, B.; Gelinsky, M. Biphasic scaffolds from marine collagens for regeneration of osteochondral defects. Mar. Drugs 2018, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, S.; Moreira-Silva, J.; Silva, T.H.; Perez-Martin, R.I.; Sotelo, C.G.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Porous hydrogels from shark skin collagen crosslinked under dense carbon dioxide atmosphere. Macromol. Biosci. 2013, 13, 1621–1631. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Wang, Y.; Regenstein, J.M. Effect of edta, hcl, and citric acid on ca salt removal from asian (silver) carp scales prior to gelatin extraction. J. Food Sci. 2009, 74, C426–C431. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (chiloscyllium punctatum) and blacktip shark (carcharhinus limbatus). LWT Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Fengxiang, Z.; Anning, W.; Zhihua, L.; Shengwen, H.; Lijun, S. Preparation and characterisation of collagen from freshwater fish scales. Food Nutr. Sci. 2011, 2011. [Google Scholar]
- Jeong, H.S.; Venkatesan, J.; Kim, S.K. Isolation and characterization of collagen from marine fish (thunnus obesus). Biotechnol. Bioprocess Eng. 2013, 18, 1185–1191. [Google Scholar] [CrossRef]
- Muralidharan, N.; Jeya Shakila, R.; Sukumar, D.; Jeyasekaran, G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (odonus niger) and their characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Gad, A.; Abu-Hussein, A.E.H.G.; Habib, S.I.; Badr, N.A.; Hashem, A.A. Chemical and biological evaluation of egyptian nile tilapia (oreochromis niloticas) fish scale collagen. Int. J. Biol. Macromol. 2015, 79, 618–626. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- International, A. (Ed.) Standard guide for characterization of type i collagen as starting material for surgical implants and substrates for tissue engineered medical products (temps). In F 2212-08; ASTM Inetrnational: West Conshohocken, MD, USA, 2008. [Google Scholar]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Skierka, E.; Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of baltic cod (gadus morhua). Food Chem. 2007, 105, 1302–1306. [Google Scholar] [CrossRef]
- Żelechowska, E.; Sadowska, M.; Turk, M. Isolation and some properties of collagen from the backbone of baltic cod (gadus morhua). Food Hydrocoll. 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Kimura, S.; Ohno, Y. Fish type i collagen: Tissue-specific existence of two molecular forms, (α1)2α2 and α1α2α3, in alaska pollack. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 88, 409–413. [Google Scholar] [CrossRef]
- Matsui, R.; Ishida, M.; Kimura, S. Characterization of an α3 chain from the skin type i collagen of chum salmon (oncoorhynchus keta). Comp. Biochem. Physiol. Part B Comp. Biochem. 1991, 99, 171–174. [Google Scholar] [CrossRef]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterisation of collagen extracted from the skin of striped catfish (pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Kiew, P.L.; Don, M.M. The influence of acetic acid concentration on the extractability of collagen from the skin of hybrid clarias sp. And its physicochemical properties: A preliminary study. Focus. Mod. Food Ind. 2013, 2, 123–128. [Google Scholar]
- Plepis, A.M.G.; Goissis, G.; Gupta, D.K.D. Dielectric and pyroelectric characterization of anionic and native collagen. Polym. Eng. Sci. 1996, 36, 2932–2938. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of drug delivering potential of type-i collagen from eel fish evenchelys macrura. J. Mater. Sci. Mater. Med. 2012, 23, 1729–1738. [Google Scholar] [CrossRef]
- Friess, W.; Lee, G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials 1996, 17, 2289–2294. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of collagen from skin, scale and bone of carp (cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish collagen. In Food Biochemistry and Food Processing; Simpson, B.K., Ed.; John Wiley & Sons, Inc.: Sussex, UK, 2012; pp. 365–387. [Google Scholar]
- Piez, K.A.; Gross, J. Amino acid composition of some fish collagens—Relation between composition and structure. J. Biol. Chem. 1960, 235, 995–998. [Google Scholar] [PubMed]
- Ramachan, G.N.; Bansal, M.; Bhatnaga, R.S. Hypothesis on role of hydroxyproline in stabilizing collagen structure. Biochim. Biophys. Acta 1973, 322, 166–171. [Google Scholar] [CrossRef]
- Persikov, A.V.; Ramshaw, J.A.M.; Kirkpatrick, A.; Brodsky, B. Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry 2005, 44, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Fallas, J.A.; Gauba, V.; Hartgerink, J.D. Solution structure of an abc collagen heterotrimer reveals a single-register helix stabilized by electrostatic interactions. J. Biol. Chem. 2009, 284, 26851–26859. [Google Scholar] [CrossRef] [PubMed]
- Piez, K.A. Extracellular Matrix Biochemistry; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987. [Google Scholar] [CrossRef]
- Usp chapter <232>, elemental impurities—Limits. In Second Supplement to USP 35—NF 30; United States Pharmacopeia: Rockville, MD, USA, 2012.
- International Organization for Standardization. Dentistry: Water-Basedcements—Part 1: Powder/Liquid Acid-Base Cements; 9917-1; ISO: Geneva, Switzerland, 2007; pp. 1–23. [Google Scholar]
- U.S. Food and Drug Administration. Draft Guidance for Industry: Lead in Cosmetic Lip Products and Externally Applied Cosmetics: Recommended Maximum Level; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2016.
- Wang, P.; Henning, S.M.; Heber, D. Limitations of mtt and mts-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lepock, J.R. Measurement of protein stability and protein denaturation in cells using differential scanning calorimetry. Methods 2005, 35, 117–125. [Google Scholar] [CrossRef] [PubMed]



| Amino Acid | Rat | Bovine | Codfish |
|---|---|---|---|
| Alanine | 111.16 | 102.04 | 91.48 |
| Arginine | 42.24 | 32.86 | 30.45 |
| Aspartic acid | 45.32 | 36.65 | 38.82 |
| Cystein | 0.99 | 1.24 | 1.28 |
| Glutamic acid | 73.33 | 59.43 | 56.08 |
| Glycine | 333.18 | 296.44 | 266.12 |
| Histidine | 3.61 | 3.11 | 5.01 |
| Hydroxylysine | 9.33 | 8.86 | 6.65 |
| Hydroxyproline | 96.06 | 78.35 | 39.60 |
| Isoleucine | 7.48 | 6.74 | 5.61 |
| Leucine | 23.29 | 17.50 | 16.51 |
| Lysine | 27.07 | 22.20 | 19.62 |
| Methionine | 8.03 | 7.81 | 15.04 |
| N-isobutylglycine | 12.70 | 14.33 | 13.75 |
| Phenylalanine | 14.62 | 11.58 | 12.70 |
| Proline | 109.21 | 89.89 | 62.69 |
| Serine | 42.74 | 32.03 | 53.87 |
| Threonine | 18.79 | 13.20 | 16.89 |
| Tyrosine | 3.76 | 1.48 | 2.25 |
| Valinine | 17.08 | 12.86 | 12.02 |
| Collagen Origin | Temperature According to % of Native Collagen | |
|---|---|---|
| 30 | 10 | |
| Codfish | 15.77 ± 0.09 | 18.24 ± 0.40 |
| Rat | 39.65 ± 0.02 | 40.08 ± 0.01 |
| Bovine | 33.19 ± 0.02 | 35.34 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. https://doi.org/10.3390/md16120495
Carvalho AM, Marques AP, Silva TH, Reis RL. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Marine Drugs. 2018; 16(12):495. https://doi.org/10.3390/md16120495
Chicago/Turabian StyleCarvalho, Ana M., Alexandra P. Marques, Tiago H. Silva, and Rui L. Reis. 2018. "Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications" Marine Drugs 16, no. 12: 495. https://doi.org/10.3390/md16120495
APA StyleCarvalho, A. M., Marques, A. P., Silva, T. H., & Reis, R. L. (2018). Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Marine Drugs, 16(12), 495. https://doi.org/10.3390/md16120495