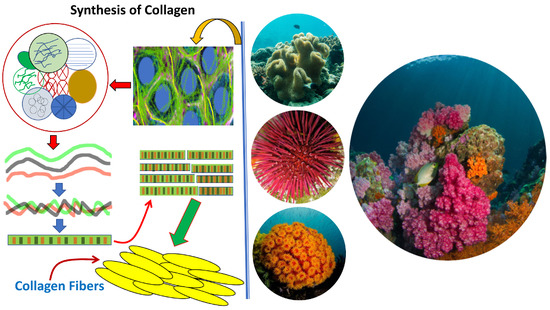
Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications
Abstract
1. Introduction
2. Current State of Collagen Research and its Medical Application
3. Purification Technique of Collagens from Marine Invertebrates
4. Future Applications of Invertebrate Collagens in Medical Field
5. Concluding Remarks
Conflicts of Interest
References
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; Sella, I.; Madhurapantula, R.S.; Antipova, O.; Mandelberg, Y.; Kashman, Y.; Benayahu, D.; Benayahu, Y. Molecular and ultrastructural studies of a fibrillar collagen from octocoral (Cnidaria). J. Exp. Biol. 2017, 220, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Benayahu, D.; Sharabi, M.; Pomeraniec, L.; Awad, L.; Haj-Ali, R.; Benayahu, Y. Unique Collagen Fibers for Biomedical Applications. Mar. Drugs 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the Marine Sponges Axinella cannabina and Suberites carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A. An Overview of the Medical Applications of Marine Skeletal Matrix Proteins. Mar. Drugs 2016, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Heinemann, S.; Heinemann, C.; Simon, P.; Bazhenov, V.V.; Shapkin, N.P.; Born, R.; Tabachnick, K.R.; Hanke, T.; Worch, H. Nanostructural organization of naturally occurring composites—Part I: Silica-collagen-based biocomposites. J. Nanomater. 2008, 623838. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 4, 6162. [Google Scholar] [CrossRef]
- Rodriguez-Pascual, F.; Slatter, D.A. Collagen cross-linking: Insights on the evolution of metazoan extracellular matrix. Sci. Rep. 2016, 6, 37374. [Google Scholar] [CrossRef]
- Rahman, M.A.; Fujimura, H.; Shinjo, R.; Oomori, T. Extracellular matrix protein in calcified endoskeleton: A potential additive for crystal growth and design. J. Cryst. Growth 2011, 324, 177–183. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Oomori, T. In vitro regulation of CaCO3 crystal growth by the highly acidic proteins of calcitic sclerites in soft coral, Sinularia polydactyla. Connect. Tissue Res. 2009, 50, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Oomori, T.; Worheide, G. Calcite formation in soft coral sclerites is determined by a single reactive extracellular protein. J. Biol. Chem. 2011, 286, 31638–31649. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A therapeutic potential for marine skeletal proteins in bone regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Hirabayashi, K.; Strychar, K.B.; Sammarco, P.W. Corals and their potential applications to integrative medicine. Evid. Based Complement. Altern. Med. 2014, 2014, 184959. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ben-Nissan, B. Marine Skeletons: Towards Hard Tissue Repair and Regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef]
- Webster, T.J.; Ahn, E.S. Nanostructured Biomaterials for Tissue Engineering Bone. In Tissue Engineering II: Basics of Tissue Engineering and Tissue Application; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 275–308. [Google Scholar]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Omura, Y.; Urano, N.; Kimura, S. Occurrence of fibrillar collagen with structure of (α1)2α2 in the test of sea urchin Asthenosoma ijimai. Comp. Biochem. Phys. B 1996, 115, 63–68. [Google Scholar] [CrossRef]
- Doherty, M.J.; Schlag, G.; Schwarz, N.; Mollan, R.A.; Nolan, P.C.; Wilson, D.J. Biocompatibility of xenogeneic bone, commercially available coral, a bioceramic and tissue sealant for human osteoblasts. Biomaterials 1994, 15, 601–608. [Google Scholar] [CrossRef]
- Guillemin, G.; Patat, J.L.; Fournie, J.; Chetail, M. The use of coral as a bone graft substitute. J. Biomed. Mater. Res. 2004, 21, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Marchac, D.; Sandor, G. Use of coral granules in the craniofacial skeleton. J. Craniofac. Surg. 1994, 5, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Helman, Y. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. Proc. Natl. Acad. Sci. USA 2007, 105, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Isa, Y.; Uehara, T. Studies on two closely related species of octocorallians: Biochemical and molecular characteristics of the organic matrices of endoskeletal sclerites. Mar. Biotechnol. 2006, 8, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Karl, K.; Nonaka, M.; Fujimura, H.; Shinjo, R.; Oomori, T.; Worheide, G. Characterization of the proteinaceous skeletal organic matrix from the precious coral Corallium konojoi. Proteomics 2014, 14, 2600–2606. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Garrone, R.; Exposito, J.Y. Distinct maturations of n-propeptide domains in fibrillar procollagen molecules involved in the formation of heterotypic fibrils in adult sea urchin collagenous tissues. J. Biol. Chem. 2004, 279, 9811–9817. [Google Scholar] [CrossRef]
- Bernhardt, A.; Paul, B.; Gelinsky, M. Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Mar. Drugs 2018, 16, 91. [Google Scholar] [CrossRef]
- Bairati, A. The Collagens of the Mollusca. In Biology of Invertebrate and Lower Vertebrate Collagens. NATO ASI Series (Series A: Life Sciences); Bairati, A., Garrone, R., Eds.; Springer: Boston, MA, USA, 1985. [Google Scholar]
- Maria, F.; Maria, F.; Andronescu, E.; Voicu, G.; Ficai, D.; Albu, M.G.; Ficai, A. Mollusc shell/collagen composite as potential biomaterial for bone substitutes. Rom. J. Mat. (RRM) 2010, 40, 359–364. [Google Scholar]
- Goh, K.L.; Holmes, D.F. Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef]
- Benedetto, C.D.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Clarke, S.; Walsh, P.; Maggs, C.; Buchanan, F. Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol. Adv. 2011, 29, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Adams, E. Invertebrate collagens. Science 1978, 202, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Engel, J. Versatile collagens in invertebrates. Science 1997, 277, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.F. The nature of collagen. In Comprehensive Biochemistry, Extracellular and Supporting Structures; Florkin, M., Stotz, E.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Exposito, J.Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R. Evolution of metazoan collagens. Prog. Mol. Subcell. Biol. 1999, 21, 119–139. [Google Scholar]
- Tanzer, M.L. The biological diversity of collagenous proteins. Trends Biochem. Sci. 1978, 3, 15–17. [Google Scholar] [CrossRef]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef]
- Hyun, C.H.; Joo, H.H.; Hee, K.J.; Yoo, S.J.; Su, J.Y.; Dae, H.L.; Hye, M.P. Preparing collagen useful in cosmetic composition, involves hydrolyzing fish by-products using enzyme. Korea Patent KR2013094989-A, 27 July 2013. [Google Scholar]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. Antiosteoporotic and antioxidant activities of diterpenoids from the Vietnamese soft corals Sinularia maxima and Lobophytum crassum. Med. Chem. Res. 2015, 24, 3551–3560. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Skeleton of Euplectella sp.: Structural Hierarchy from the Nanoscale to the Macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17beta-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef]
- Latire, T.; Legendre, F.; Bigot, N.; Carduner, L.; Kellouche, S.; Bouyoucef, M.; Carreiras, F.; Marin, F.; Lebel, J.-M.; Galéra, P.; et al. Shell Extracts from the Marine Bivalve Pecten maximus Regulate the Synthesis of Extracellular Matrix in Primary Cultured Human Skin Fibroblasts. PLoS ONE 2014, 9, e99931. [Google Scholar] [CrossRef] [PubMed]
- Junqua, S.; Robert, L.; Garrone, R. Biochemical and morphological studies on the collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R.; Huc, A.; Junqua, S. Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Diehl-Seifert, B.; Kurelec, B.; Zahn, R.K.; Dorn, A.; Jeričevic, B.; Uhlenbruck, G.; Müller, W.E.G. Attachment of sponge cells to collagen substrata: Effect of a collagen assembly factor. J. Cell Sci. 1985, 79, 271–285. [Google Scholar]
- Pallela, R.; Bojja, S.; Janapala, V.R. Biochemical and biophysical characterization of collagens of marine sponge, Ircinia fusca (Porifera: Demospongiae: Irciniidae). Int. J. Biol. Macromol. 2011, 49, 85–92. [Google Scholar] [CrossRef]
- Benayahu, Y.; Benayahu, D.; Kashman, Y.; Rudi, A.; Lanir, Y.; Sela, I.; Raz, E. Coral Derived Collagen and Methods of Farming Same. U.S. Patent US20110038914A1, 17 February 2011. [Google Scholar]
- Rahman, M.A.; Shinjo, R.; Oomori, T.; Worheide, G. Analysis of the proteinaceous components of the organic matrix of calcitic sclerites from the soft coral Sinularia sp. PLoS ONE 2013, 8, e58781. [Google Scholar] [CrossRef]
- Rahman, M.A.; Isa, Y. Characterization of proteins from the matrix of spicules from the alcyonarian, Lobophytum crassum. J. Exp. Mar. Biol. Ecol. 2005, 321, 71–82. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process in Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Lee, J.W.; Wang, S.; Henrotin, Y.; De Val, J.E.M.S.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W. Evaluation of Differentiated Bone Cells Proliferation by Blue Shark Skin Collagen via Biochemical for Bone Tissue Engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Arias-Moscoso, J.L.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.; Rouzaud-Sández, O.; Cardenas-Lopez, J.L.; Marquez-Rios, E.; Ezquerra-Brauer, J.M. Jumbo squid (Dosidicus gigas) mantle collagen: Extraction, characterization, and potential application in the preparation of chitosan-collagen biofilms. Bioresour. Technol. 2010, 101, 4212–4219. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.; Carnevali, M.D.C.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.H.; Kim, S.K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Venkatesan, J.; Janapala, V.R.; Kim, S.K. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. Biomed. Mater. Res. A. 2012, 100, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Mahato, A.; Thakur, N.L.; Joardar, S.N.; Mandal, B.B. In vitro and in vivo evaluation of the marine sponge skeleton as a bone mimicking biomaterial. Integr. Biol. (Camb). 2015, 7, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Granito, R.N.; Custódio, M.R.; Rennó, A.C. Natural marine sponges for bone tissue engineering: The state of art and future perspectives. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1717–1727. [Google Scholar] [CrossRef]
- Rahman, M.A.; Oomori, T. Structure, crystallization and mineral composition of sclerites in the alcyonarian coral. J. Cryst. Growth 2008, 310, 3528–3534. [Google Scholar] [CrossRef]
- Jiang, H.; Ramunno-Johnson, D.; Song, C.; Amirbekian, B.; Kohmura, Y.; Nishino, Y.; Takahashi, Y.; Ishikawa, T.; Miao, J. Nanoscale Imaging of Mineral Crystals inside Biological Composite Materials Using X-Ray Diffraction Microscopy. Phys. Rev. Lett. 2008, 100, 038103. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Da Sacco, L.; Masotti, A. Chitin and chitosan as multipurpose natural polymers for groundwater arsenic removal and AS2O3 delivery in tumor therapy. Mar. Drugs 2010, 8, 1518–1525. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Varum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.M.; Tupe, S.G.; Deshpande, M.V. Chitin synthase inhibitors as antifungal agents. Mini Rev. Med. Chem. 2013, 13, 222–236. [Google Scholar] [PubMed]
- Ruiz-Herrera, J.; San-Blas, G. Chitin synthesis as target for antifungal drugs. Curr. Drug Targets Infect. Disord. 2003, 3, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.A.; Liang, H.E.; Tager, A.M.; Luster, A.D.; van Rooijen, N.; Voehringer, D.; Locksley, R.M. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007, 447, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Natali, I.; Tempesti, P.; Carretti, E.; Potenza, M.; Sansoni, S.; Baglioni, P.; Dei, L. Aragonite crystals grown on bones by reaction of CO2 with nanostructured Ca(OH)2 in the presence of collagen. Implications in archaeology and paleontology. Langmuir 2014, 30, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L. Chitin-based materials in tissue engineering: Applications in soft tissue and epithelial organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef]
- Imhoff, J.M.; Garrone, R. Solubilization and characterization of Chondrosia reniformis sponge collagen. Connect. Tissue Res. 1983, 11, 193–197. [Google Scholar] [CrossRef]
- Ehrlich, H.; Hanke, T.; Simon, P.; Goebel, C.; Heinemann, S.; Born, R.; Worch, H. Demineralisation von natürlichen Silikat-basierten Biomaterialien: Neue Strategie zur Isolation organischer Gerüststrukturen. Biomaterialien 2005, 6, 297–302. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, M.; Wijgerde, T.; Sarà, A.; De Goeij, J.M.; Osinga, R. Development of an Integrated Mariculture for the Collagen-Rich Sponge Chondrosia reniformis. Mar. Drugs 2019, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Molloy, M.P.; Witzmann, F.A. Proteomics: Technologies and applications. Brief Funct. Genomics Proteomics 2002, 1, 23–39. [Google Scholar] [CrossRef]
- Rahman, M.A. The medicinal potential of promising marine organisms: A review. Blue Biotechnol. J. 2012, 1, 318–333. [Google Scholar]
- Dauphin, Y. Comparative studies of skeletal soluble matrices from some Scleractinian corals and molluscs. Int. J. Biol. Macromol. 2001, 28, 293–304. [Google Scholar] [CrossRef]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Peng, B.-R.; Lu, M.-C.; El-Shazly, M.; Wu, S.-L.; Lai, K.-H.; Su, J.-H. Aquaculture Soft Coral Lobophytum crassum as a Producer of Anti-Proliferative Cembranoids. Mar. Drugs 2018, 16, 15. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, Z.-D.; Gu, Y.-C.; Li, J.; Zhu, W.-L.; Guo, Y.-W. Further New Diterpenoids as PTP1B Inhibitors from the Xisha Soft Coral Sinularia polydactyla. Mar. Drugs 2018, 16, 103. [Google Scholar] [CrossRef]
- Chaugule, S.R.; Indap, M.M.; Chiplunkar, S.V. Marine Natural Products: New Avenue in Treatment of Osteoporosis. Front. Mar. Sci. 2017, 4, 384. [Google Scholar] [CrossRef]
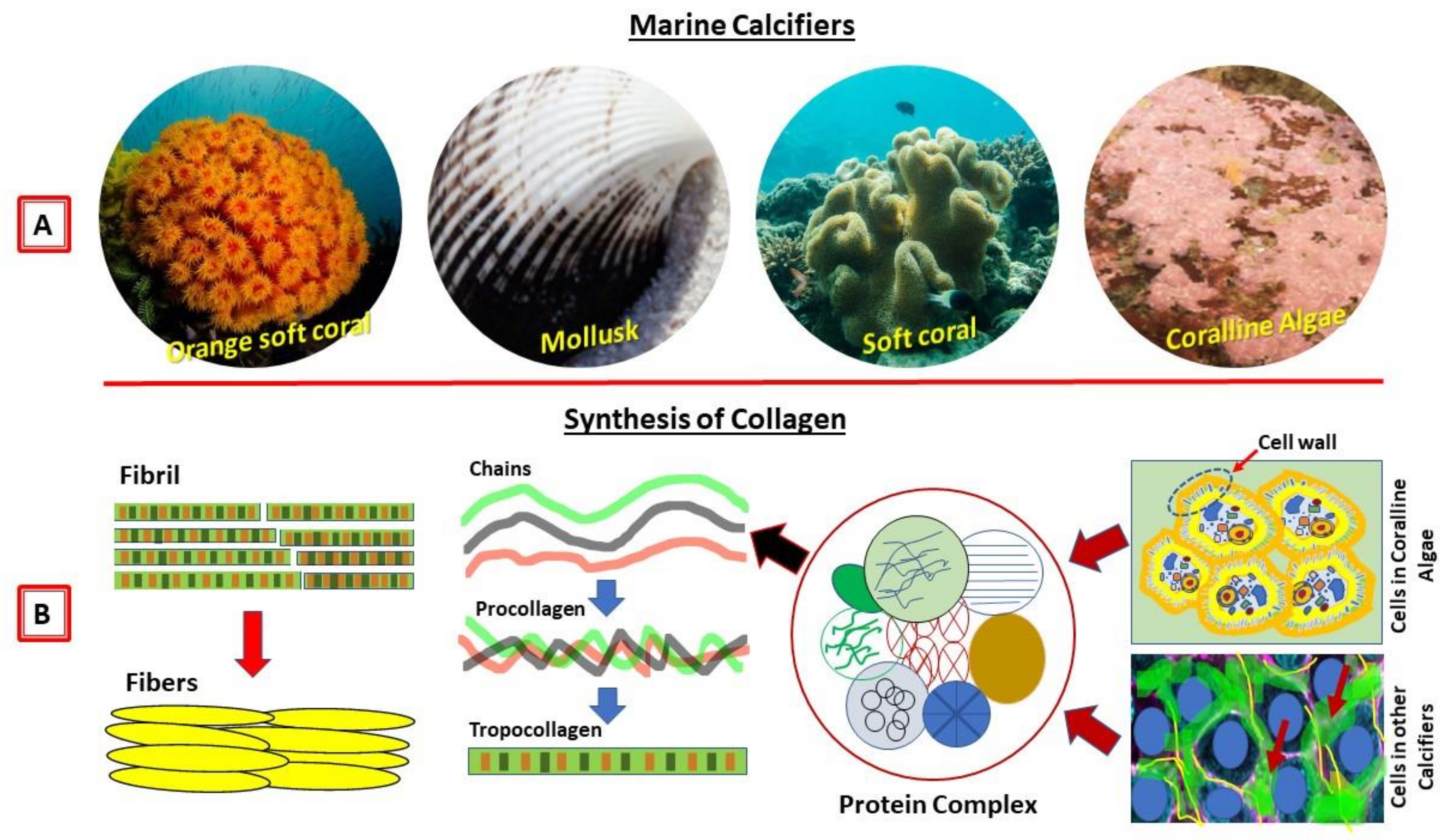
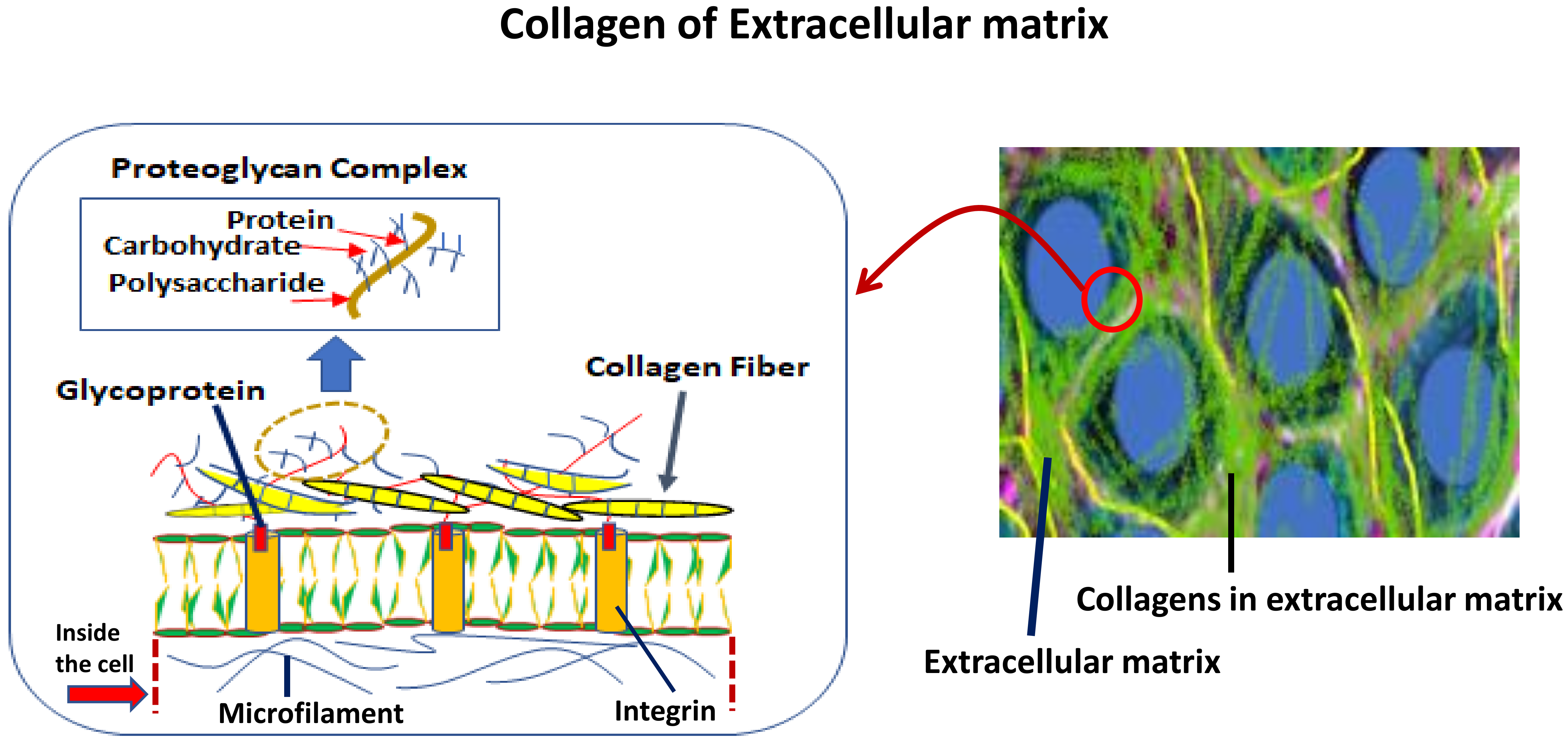
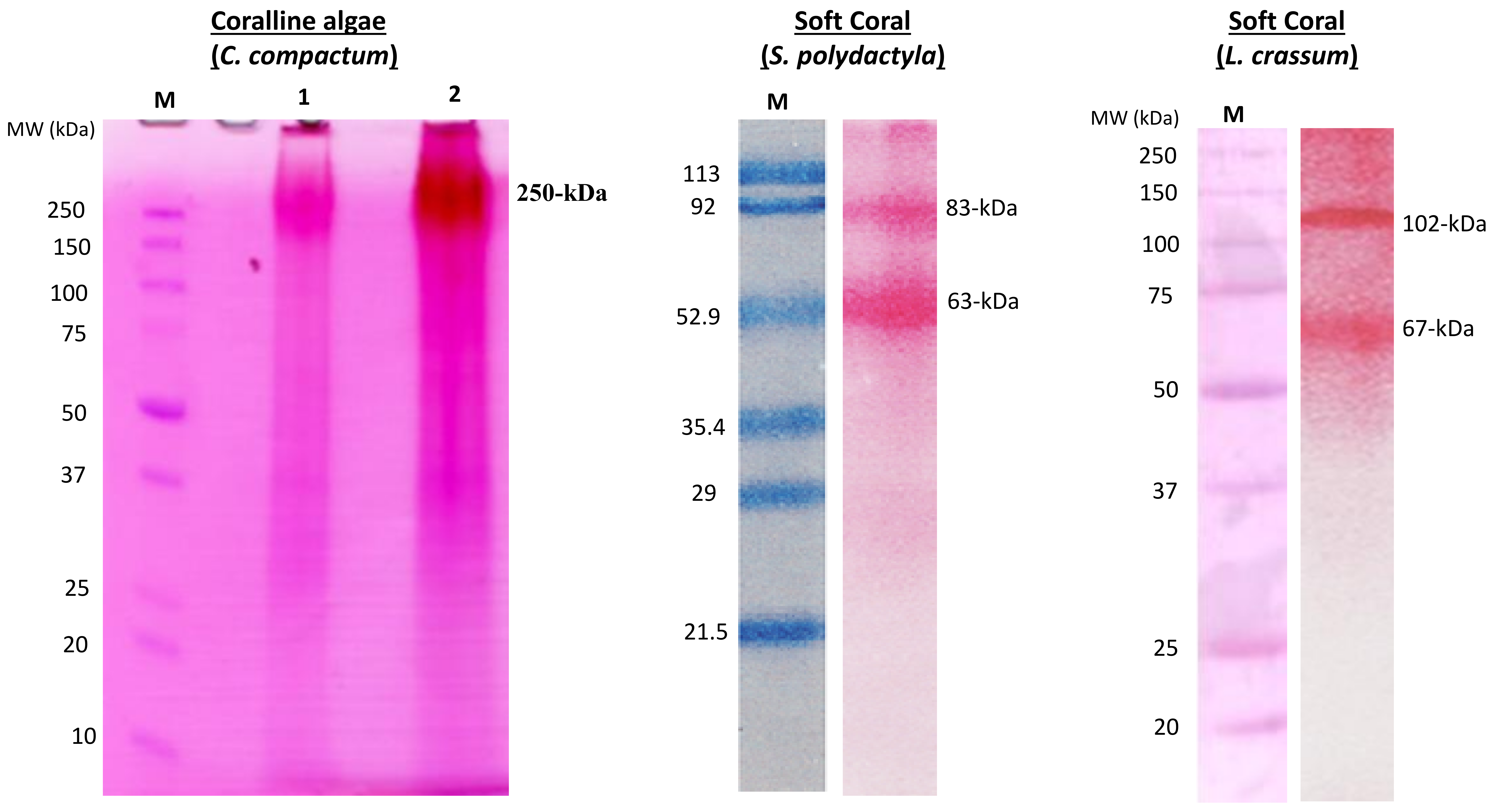


© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2019, 17, 118. https://doi.org/10.3390/md17020118
Rahman MA. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Marine Drugs. 2019; 17(2):118. https://doi.org/10.3390/md17020118
Chicago/Turabian StyleRahman, M. Azizur. 2019. "Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications" Marine Drugs 17, no. 2: 118. https://doi.org/10.3390/md17020118
APA StyleRahman, M. A. (2019). Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Marine Drugs, 17(2), 118. https://doi.org/10.3390/md17020118