Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan
Abstract
:1. Introduction
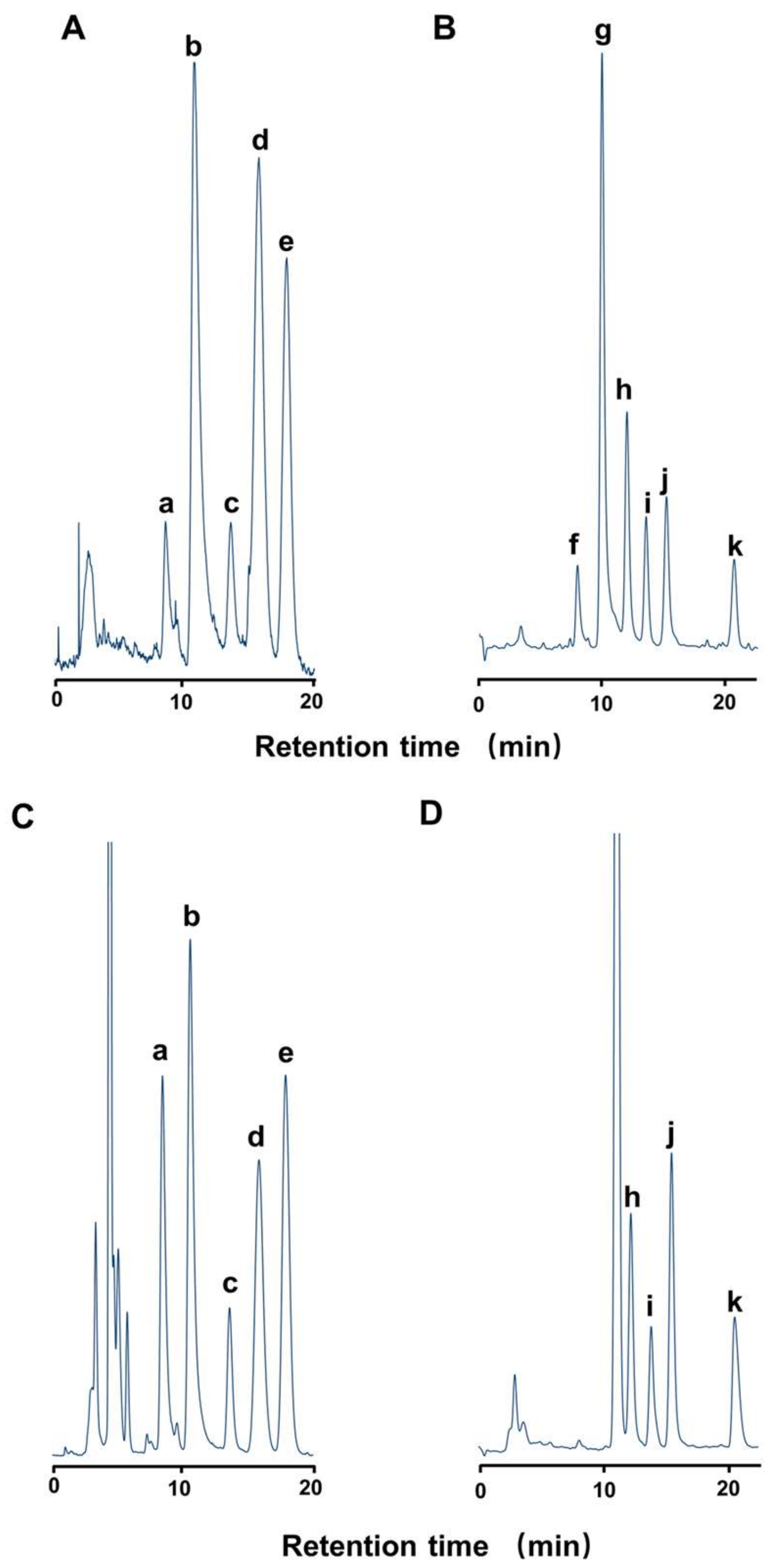
2. Results
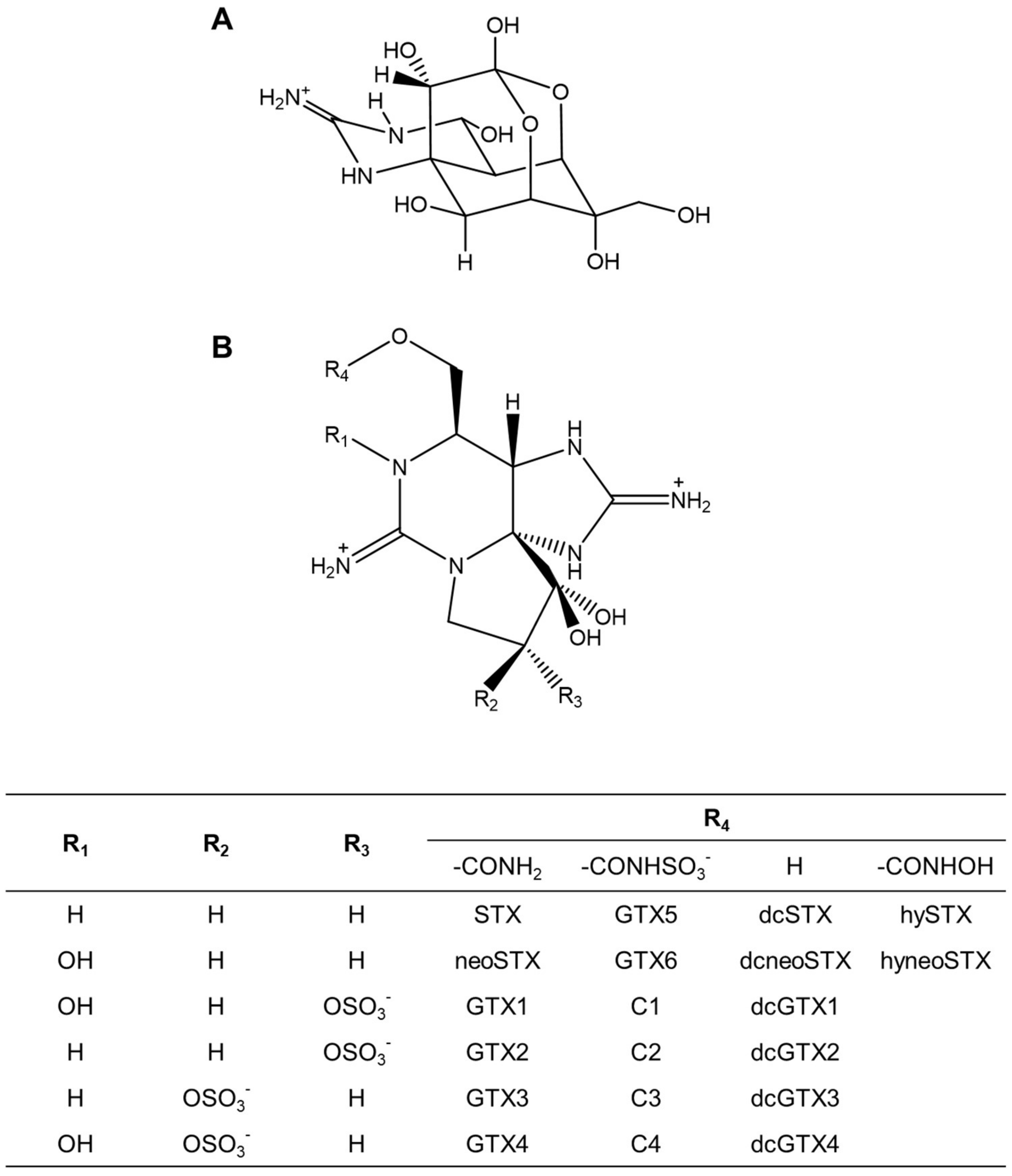
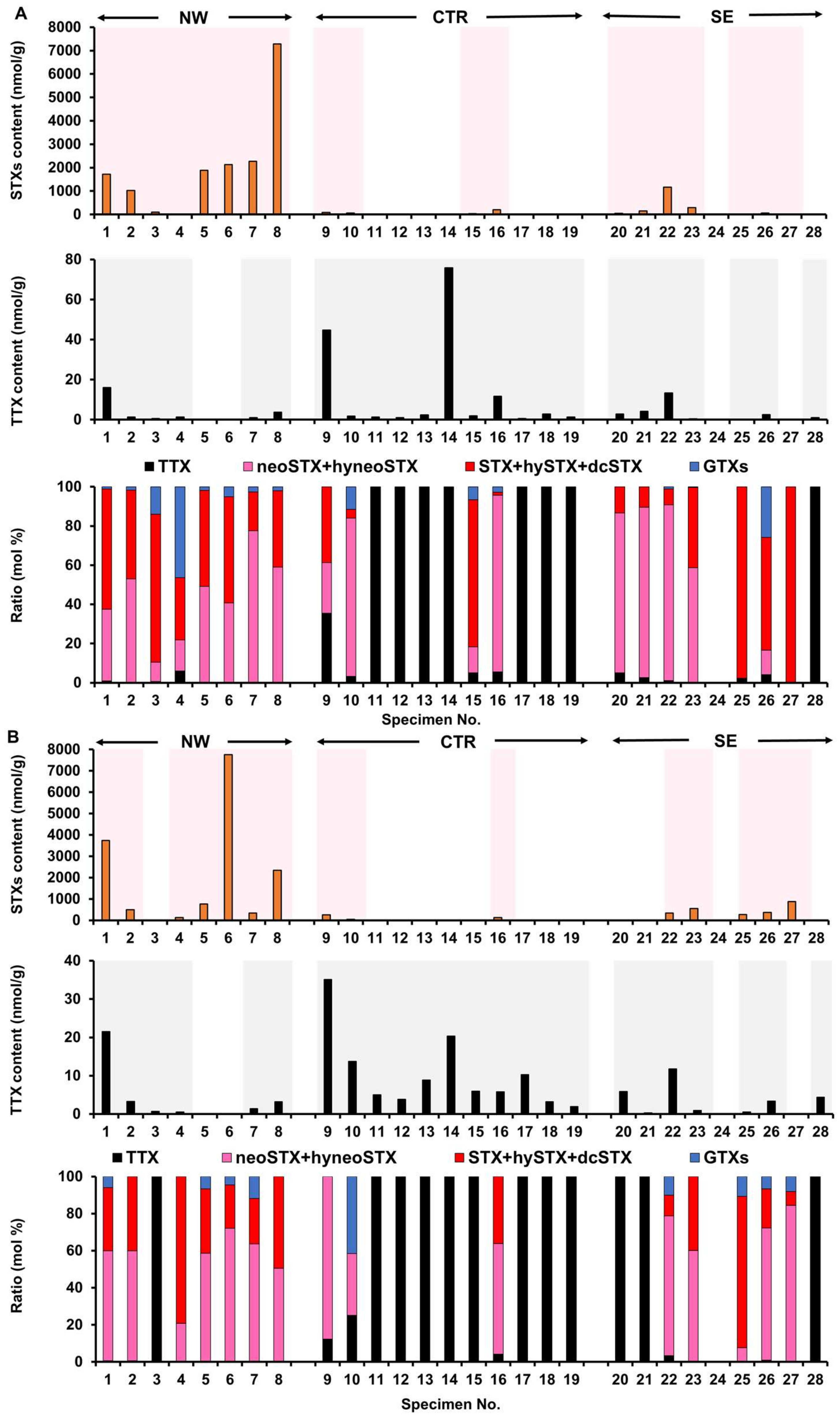
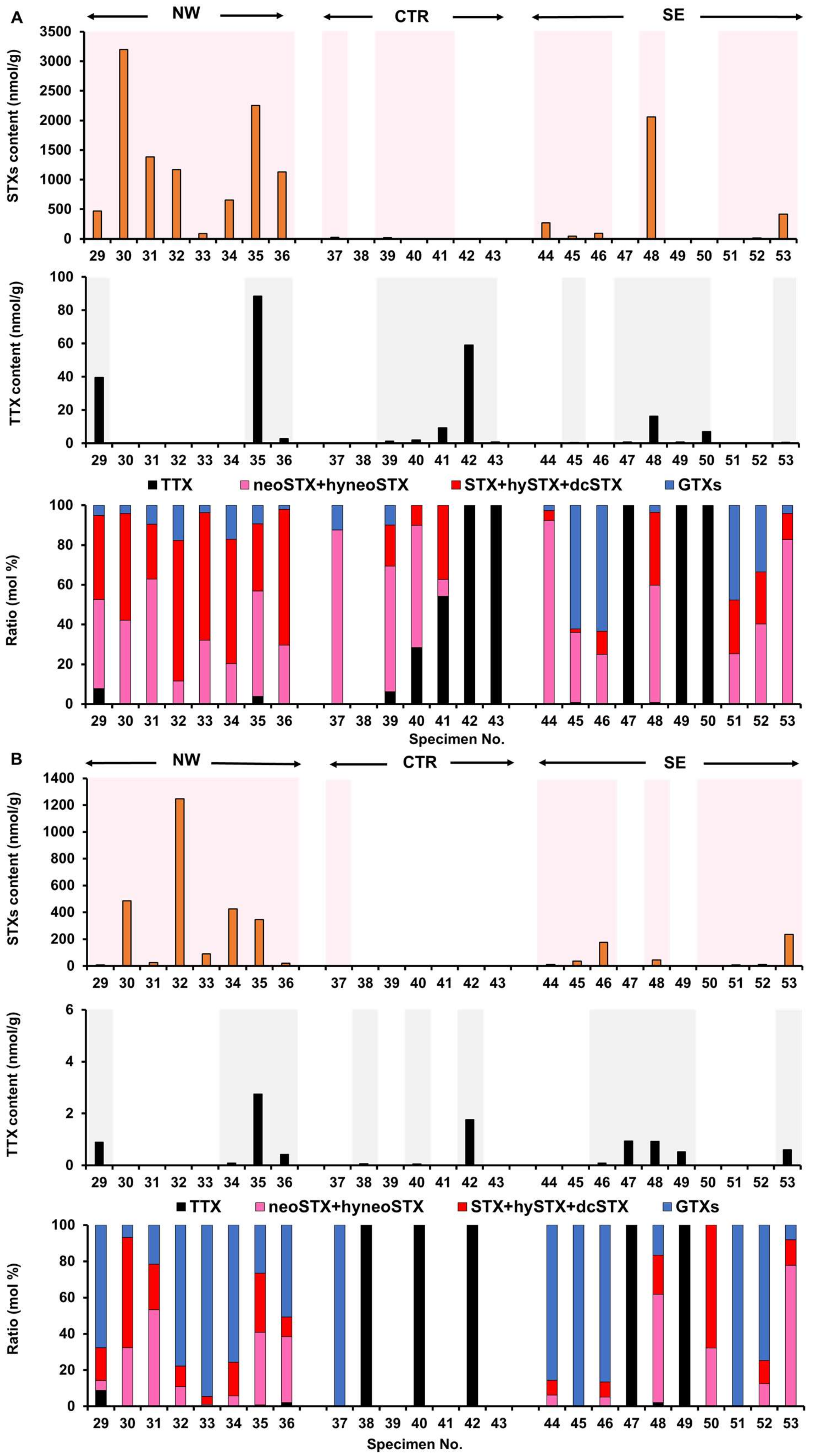
2.1. Toxin Profile of Z. aeneus Collected in 2018
2.2. Toxin Profile of Z. aeneus Collected in 2019
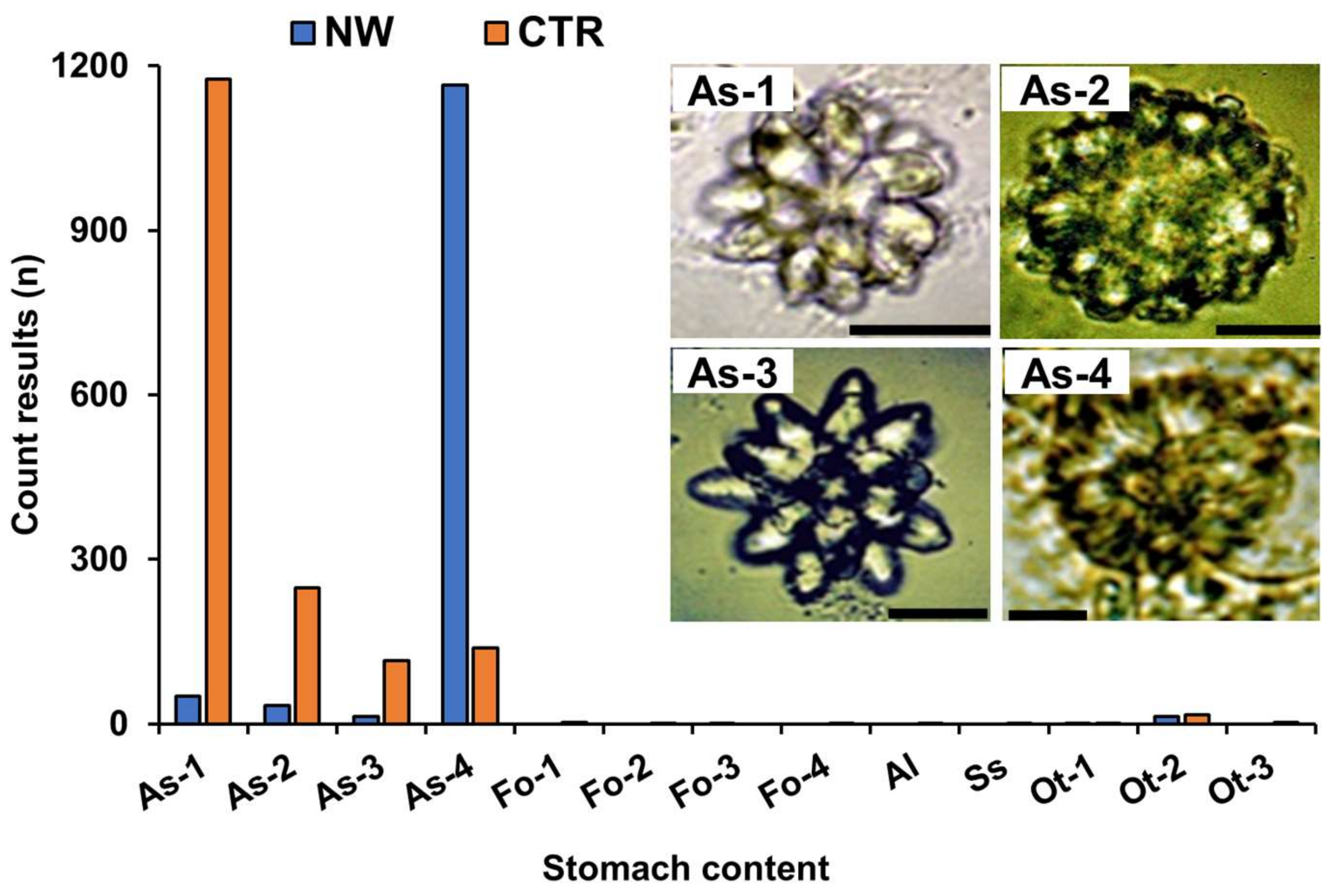
2.3. Analysis of Stomach Contents
3. Discussion
4. Materials and Methods
4.1. Crab Specimens
4.2. Toxin Extraction
4.3. Toxin Quantification
4.3.1. High-performance Liquid Chromatography with Fluorometric Detection (HPLC-FLD) for STXs
4.3.2. Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) for TTX
4.4. Stomach Content Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noguchi, T.; Arakawa, O.; Daigo, K.; Oikawa, H.; Asakawa, M.; Miyazawa, K. Different intoxication mechanism between paralytic shellfish toxin (PST)-and/or tetrodotoxin-contaminated xanthid crabs and PST-contaminated edible shore crabs in Japan and their food poisonings. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 575–589. [Google Scholar]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; KoNosu, S.; Inoue, A.; Saisho, T.; Miyake, S. Screening of toxic crabs in the Ryukyu and Amami Islands. Bull. Jap. Soc. Sci. Fish. 1969, 35, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Konosu, S.; Inoue, A.; Noguchi, T.; Hashimoto, Y. A further examination on the toxicity of three species of xanthid crab. Bull. Jap. Soc. Sci. Fish. 1969, 35, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Koyama, K.; Noguchi, T.; Uzu, A.; Hashimoto, K. Individual, local, and size-dependent variations in toxicity of the xanthid crab Zosimus aeneus. Bull. Jap. Soc. Sci. Fish. 1983, 49, 1273–1279. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, L.E.; Endean, R. Paralytic shellfish toxins in the xanthid crab Atergatis floridus collected from Australian coral reefs. J. Wilderness Med. 1991, 2, 118–126. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Jeng, S.-S.; Hwang, D.-F. Seasonal and regional variations of toxicity in the xanthid crab Zosimus aeneus in Taiwan. Fish. Sci. 1997, 63, 313–314. [Google Scholar] [CrossRef] [Green Version]
- Sagara, T.; Taniyama, S.; Takatani, T.; Nishibori, N.; Nishio, S.; Noguchi, T.; Arakawa, O. Toxicity and toxin profiles of xanthid crabs collected around Nakanoshima in the Tokara Islands, Japan. J. Food Hyg. Soc. Jpn. 2009, 50, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, M.; Gomez-Delan, G.; Tsuruda, S.; Shimomura, M.; Shida, Y.; Taniyama, S.; Barte-Quilantang, M.; Shindo, J. Toxicity assessment of the xanthid crab Demania cultripes from Cebu Island, Philippines. J. Toxicol. 2010, 2010, 172367. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, M.; Tsuruda, S.; Ishimoto, Y.; Shimomura, M.; Kishimoto, K.; Shida, Y.; Barte-Quilantang, M.; Gomez-Delan, G. Paralytic toxin profiles of xanthid crab Atergatis floridus collected on reefs of Ishigaki Island, Okinawa Prefecture, Japan and Camotes Island, Cebu Province, Philippines. Sci. J. Clin. Med 2014, 3, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, O.; Noguchi, T.; Onoue, Y. Paralytic shellfish toxin profiles of xanthid crabs Zosimus aeneus and Atergatis floridus collected on reefs of Ishigaki Island. Fish. Sci. 1995, 61, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Arakawa, O.; Daigo, K.; Hashimoto, K. Local differences in toxin composition of a xanthid crab Atergatis floridus inhabiting Ishigaki Island, Okinawa. Toxicon 1986, 24, 705–711. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Hwang, D.-F.; Chai, T.-J.; Jeng, S.-S. Occurrence of tetrodotoxin and paralytic shellfish poison in the Taiwanese crab Lophozozymus pictor. Toxicon 1995, 33, 1669–1673. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Hwang, D.-F.; Chai, T.-J.; Jeng, S.-S. Toxicity and toxic components of two xanthid crabs, Atergatis floridus and Demania reynaudi, in Taiwan. Toxicon 1997, 35, 1327–1335. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin--distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011, 2011, 276939. [Google Scholar] [CrossRef] [Green Version]
- Kotaki, Y.; Tajiri, M.; Oshima, Y.; Yasumoto, T. Identification of a calcareous red alga as the primary source of paralytic shellfish toxins in coral reef crabs and gastropods. Bull. Jap. Soc. Sci. Fish. 1983, 49, 283–286. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nagai, H.; Yasumura, D.; Michishita, T.; Endo, A.; Yotsu, M.; Kotaki, Y. Interspecies distribution and possible origin of tetrodotoxin. Ann. N. Y. Acad. Sci. 1986, 479, 44–51. [Google Scholar] [CrossRef]
- Monniot, C.; Monniot, F.; Laboute, P. Coral reef ascidians of New Caledonia; ORSTOM: Paris, France, 1991. [Google Scholar]
- Varol, O.; Houghton, S.D. A review and classification of fossil didemnid ascidian spicules. J. Micropalaeontol. 1996, 15, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Hirose, M.; Tochikubo, T.; Hirose, E. Taxonomic significance of tunic spicules in photosymbiotic ascidians: A quantitative and molecular evaluation. J. Mar. Biol. Assoc. U.K. 2010, 90, 1065. [Google Scholar] [CrossRef]
- Noguchi, T.; Uzu, A.; Koyama, K.; Maruyama, j.; Nagashima, Y.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Bull. Jap. Soc. Sci. Fish. 1983, 49, 1887–1892. [Google Scholar] [CrossRef] [Green Version]
- Yasumura, D.; Oshima, Y.; Yasumoto, T.; Alcala, A.C.; Alcala, L.C. Tetrodotoxin and paralytic shellfish toxins in Philippine crabs. Agric. Biol. Chem. 1986, 50, 593–598. [Google Scholar] [CrossRef]
- Arakawa, O.; Noguchi, T.; Shida, Y.; Onoue, Y. Occurrence of carbamoyl-N-hydroxy derivatives of saxitoxin and neosaxitoxin in a xanthid crab Zosimus aeneus. Toxicon 1994, 32, 175–183. [Google Scholar] [CrossRef]
- Arakawa, O.; Noguchi, T.; Onoue, Y. Transformation of gonyautoxins in the xanthid crab Atergatis floridus. Fish. Sci. 1998, 64, 334–337. [Google Scholar] [CrossRef]
- Kotaki, Y.; Oshima, Y.; Yasumoto, T. Bacterial transformation of paralytic shellfish toxins in coral reef crabs and a marine snail. Bull. Jap. Soc. Sci. Fish. 1985, 51, 1009–1013. [Google Scholar] [CrossRef]
- Arakawa, O.; Noguchi, T.; Shida, Y.; Onoue, Y. Occurrence of 11-oxotetrodotoxin and 11-nortetrodotoxin-6 (R)-ol in a Xanthid Crab Atergatis floridus Collected at Kojima, Ishigaki Island. Fish. Sci. 1994, 60, 769–771. [Google Scholar] [CrossRef]
- Gao, W.; Kanahara, Y.; Yamada, M.; Tatsuno, R.; Yoshikawa, H.; Takatani, T.; Arakawa, O. Contrasting toxin selectivity between the marine pufferfish Takifugu pardalis and the freshwater pufferfish Pao suvattii. Toxins 2019, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Jeon, J.-k.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar] [CrossRef]
- Saisho, T.; Noguchi, T.; Koyama, K.; Uzu, A.; Kikuta, T.; Hashimoto, K. Examination of stomach contents in xanthid crabs. Bull. Jap. Soc. Sci. Fish. 1983, 49, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Monniot, F.; Clement, P.; Souron, J.-P. The ubiquity of fluorine amidst the taxonomic and mineralogical diversity of ascidian spicules. Biochem. Syst. Ecol. 1995, 23, 129–137. [Google Scholar] [CrossRef]
- Ferreira, E.P.; Alves, C.F.; Sanjinés, A.E.S.; Alves, M.C. Ascidian spicules of Quaternary sediments from the lower slope of the Campos Basin (Brazil). Quat. Int 2019, 508, 116–124. [Google Scholar] [CrossRef]
- Nagashima, Y.; Noguchi, T.; Maruyama, j.; Hashimoto, K.; Kamimura, s. Occurrence of paralytic shellfish poisons in an ascidian Holocynthia roretzi. Bull. Jap. Soc. Sci. Fish. 1984, 50, 331–334. [Google Scholar] [CrossRef] [Green Version]
- Freitas, J.C.; Ogata, T.; Veit, C.H.; Kodama, M. Occurrence of tetrodotoxin and paralytic shellfish toxins in Phallusia nigra (Tunicata, Ascidiacea) from the Brazilian coast. J. Venom. Anim. Toxins 1996, 2, 28–38. [Google Scholar] [CrossRef]
- Roje-Busatto, R.; Ujević, I. PSP toxins profile in ascidian Microcosmus vulgaris (Heller, 1877) after human poisoning in Croatia (Adriatic Sea). Toxicon 2014, 79, 28–36. [Google Scholar] [CrossRef]
- Oshima, Y. Post-column devivatization HPLC method for analysis of PSP. J. AOAC Inter. 1995, 78, 528–532. [Google Scholar] [CrossRef]
- Zhu, H.; Sonoyama, T.; Yamada, M.; Gao, W.; Tatsuno, R.; Takatani, T.; Arakawa, O. Co-Occurrence of Tetrodotoxin and Saxitoxins and Their Intra-Body Distribution in the Pufferfish Canthigaster valentini. Toxins 2020, 12, 436. [Google Scholar] [CrossRef]
- Tsutsui, H.; Jordan, R.W. Modified cleaning method for biomineralized components. J. Micropalaeontol. 2018, 37, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Lozar, F.; Riaux-Gobin, C.; Iwankow, G.; Jordan, R.W. Coccolith assemblages from holothurian gastrointestinal tracts. J. Nannoplankton Res. 2019, 4, 63–72, https://doi.org/10670/1.uzqtan. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tsutsui, H.; Yamawaki, N.; Morii, Y.; Nishihara, G.N.; Itoi, S.; Arakawa, O.; Takatani, T. Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan. Mar. Drugs 2021, 19, 670. https://doi.org/10.3390/md19120670
Zhang Y, Tsutsui H, Yamawaki N, Morii Y, Nishihara GN, Itoi S, Arakawa O, Takatani T. Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan. Marine Drugs. 2021; 19(12):670. https://doi.org/10.3390/md19120670
Chicago/Turabian StyleZhang, Yuchengmin, Hideto Tsutsui, Nobuhiro Yamawaki, Yasuhiro Morii, Gregory N. Nishihara, Shiro Itoi, Osamu Arakawa, and Tomohiro Takatani. 2021. "Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan" Marine Drugs 19, no. 12: 670. https://doi.org/10.3390/md19120670
APA StyleZhang, Y., Tsutsui, H., Yamawaki, N., Morii, Y., Nishihara, G. N., Itoi, S., Arakawa, O., & Takatani, T. (2021). Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan. Marine Drugs, 19(12), 670. https://doi.org/10.3390/md19120670





