Abstract
Glycogen synthase kinase 3β (GSK-3β) is a widely investigated molecular target for numerous diseases, and inhibition of GSK-3β activity has become an attractive approach for the treatment of diabetes. Meridianin C, an indole-based natural product isolated from marine Aplidium meridianum, has been reported as a potent GSK-3β inhibitor. In the present study, applying the structural-based optimization strategy, the pyrimidine group of meridianin C was modified by introducing different substituents based on the 2-aminopyrimidines-substituted pyrazolo pyridazine scaffold. Among them, compounds B29 and B30 showed a much higher glucose uptake than meridianin C (<5%) and the positive compound 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8, 16%), with no significant toxicity against HepG2 cells at the same time. Furthermore, they displayed good GSK-3β inhibitory activities (IC50 = 5.85; 24.4 μM). These results suggest that these meridianin C analogues represent novel lead compounds with therapeutic potential for diabetes.
1. Introduction
Diabetes is a chronic metabolic disease characterized by elevated levels of blood glucose, which leads to serious damage to the heart, blood vessels, eyes, kidneys, and nerves [1]. The International Diabetes Foundation (IDF) confirms that diabetes is one of the fastest growing global health emergencies in the 21st century. In 2019, it was estimated that 463 million people had diabetes, which is predicted to increase to 578 million by 2030 and 700 million by 2045 [2]. The major contemporary conventional oral antihyperglycemic agents include biguanides (metformin), second- and third-generation sulfonylureas, α-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists [3]. These agents are either administered as monotherapy or given in combination with other agents to achieve hypoglycemic effects [4]. Along with solubility and permeability problems, lack of target specificity, low therapeutic efficacy, severe hypoglycemia, and weight gain are the major drawbacks associated with the above-mentioned conventional drugs [5]. Thus, there is still an urgent need for new and better antihyperglycemic agents or therapeutics to reduce both the morbidity and mortality caused by diabetes mellitus.
Glycogen synthase kinase 3β (GSK-3β) is an intermediate multifunctional serine/threonine kinase having diverse physiological pathways [6,7] and involved in numerous diseases, like diabetes, inflammation, cancer, Alzheimer’s disease, and bipolar disorder [8,9,10]. Furthermore, GSK-3β functionalized as the regulator of glycogen synthase (GCS), the rate-determining enzyme for glycogen synthesis, might regulate glycogen metabolism [11,12]. Glycogen homeostasis relies on a balance between glycogenesis and glycogenolysis. Phosphorylation of GCS by GSK-3β results in its deactivation, which causes an increase in blood glucose and then obviously reduces the glycogen storage of hepatocytes in type 2 diabetes [13]. The inhibition of GSK-3β activity leads to the activation of glycogen synthase activity and may enhance glucose uptake [14]. Therefore, the inhibition of GSK-3β might provide an important and alternative therapeutic strategy for the treatment of diabetes.
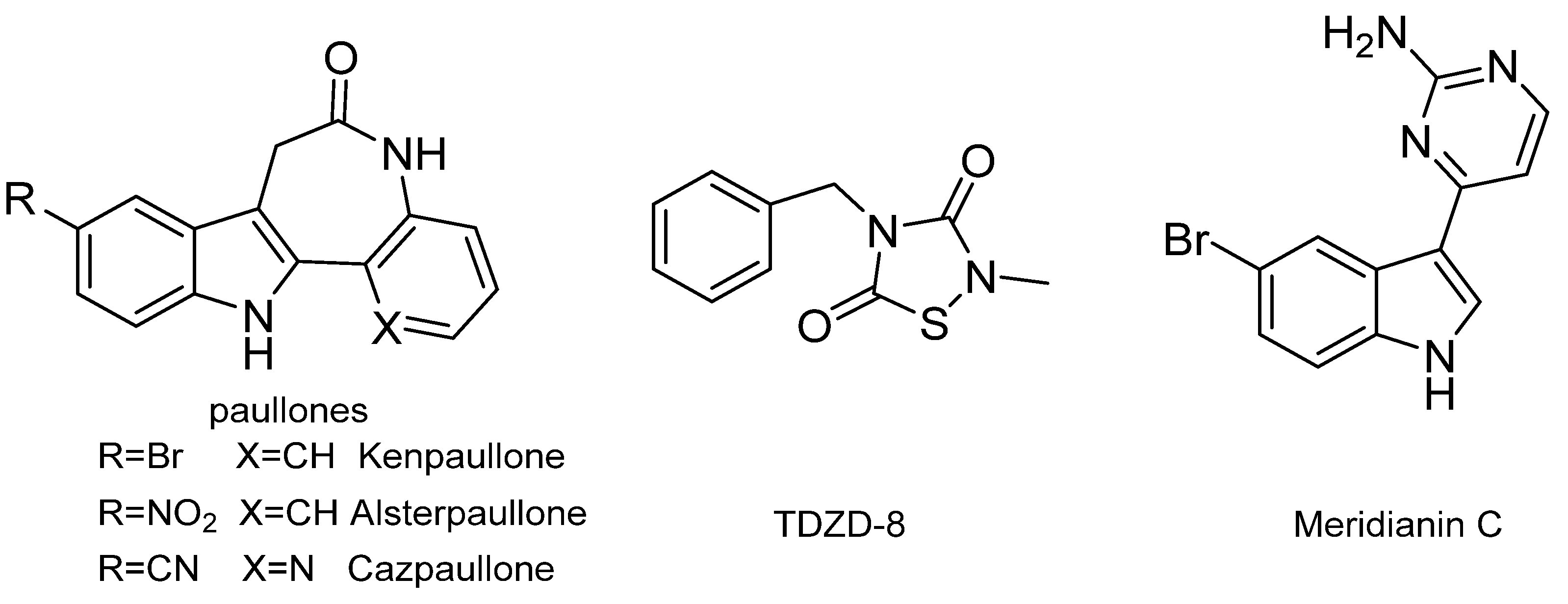
A number of GSK-3β inhibitors have been reported (Figure 1), including synthetic paullones, thiadiazolidinediones (TDZDs), and natural-origin-derived small molecules [15]. However, the majority of these compounds are considered to be toolkit compounds and do not have the necessary absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties to be advanced as drug candidates into the clinic [16]. Meridianin C is a brominated 3-(2-aminopyrimidine)-indole compound that was initially isolated from Aplidium meridianum, an ascidian from the South Georgia Islands, by Palermo and co-workers in 1998 [8,17,18]. Meridianin C was found to be a good inhibitor of GSK-3β and indicated a good antitumor activity [8,19]. However, the activity of meridianin C and its derivatives in glucose metabolism has not been disclosed yet. In the present study, we focused on structural optimizations based on meridianin C in order to obtain analogues with kinase inhibitory activity and antihyperglycemic activity.
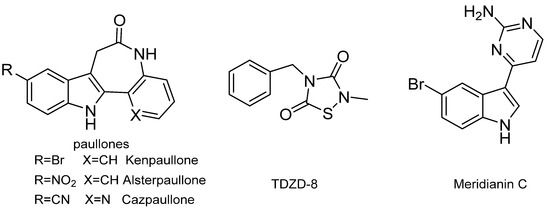
Figure 1.
Chemical structures of the representative small-molecule glycogen synthase kinase 3β (GSK-3β) inhibitors.
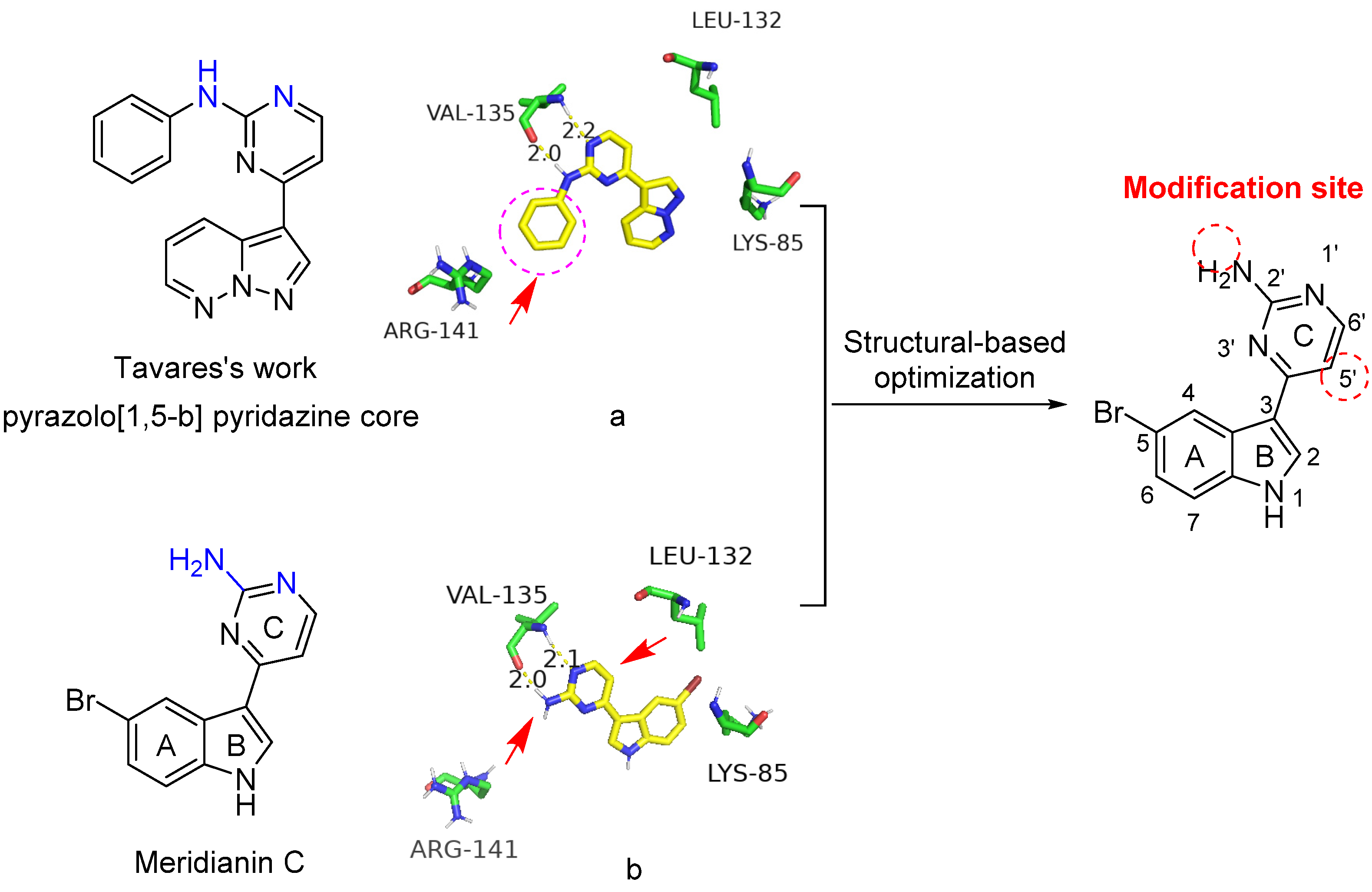
It has been reported that the 2-aminopyrimidine-substituted pyrazolo[1,5-b] pyridazine compound (Figure 2), developed by Tavares’s group, possesses excellent inhibitory activity against GSK-3β [11]. Consistent with the previous publication [11], the predicted binding mode of the compound with GSK-3β was reproduced as shown in Figure 2a. Similarly, we generated the docking model of meridianin C with the ATP-binding site of GSK-3β (Figure 2b). The NH and the adjacent nitrogen in the pyrimidine ring of these two compounds (highlighted in blue) have similar hydrogen-bonding interactions with the Val135 of GSK-3β. The aniline of the pyrazolo[1,5-b] pyridazine is located in the front solvent-exposed binding site, which has not been occupied in the meridianin C model. In addition, the back pocket formed by the residue Leu132 appears to be another pocket for optimization.
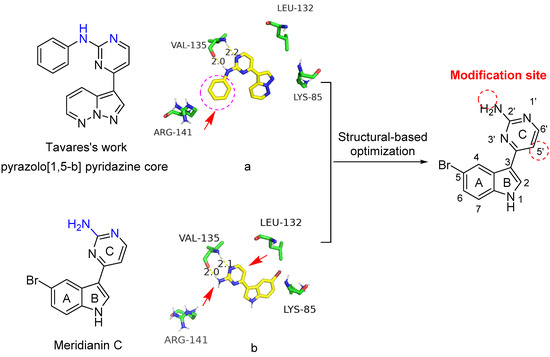
Figure 2.
Predicted binding modes of pyrazolo[1,5-b] pyridazine (a) and meridianin C (b) with GSK-3β and the structural-based optimization strategy of meridianin C (PDB code: 6B8J). The hydrogen bonds are shown by the yellow dashed lines.
Thus, based on the chemical structure of meridianin C and the predicted binding mode with GSK-3β, modifications (highlighted arrows in red) at the amino group (2′-position and 5′-position) are proposed according to important substitutions of the pyrazolo[1,5-b] pyridazine scaffold.
2. Results and Discussion
2.1. Chemistry
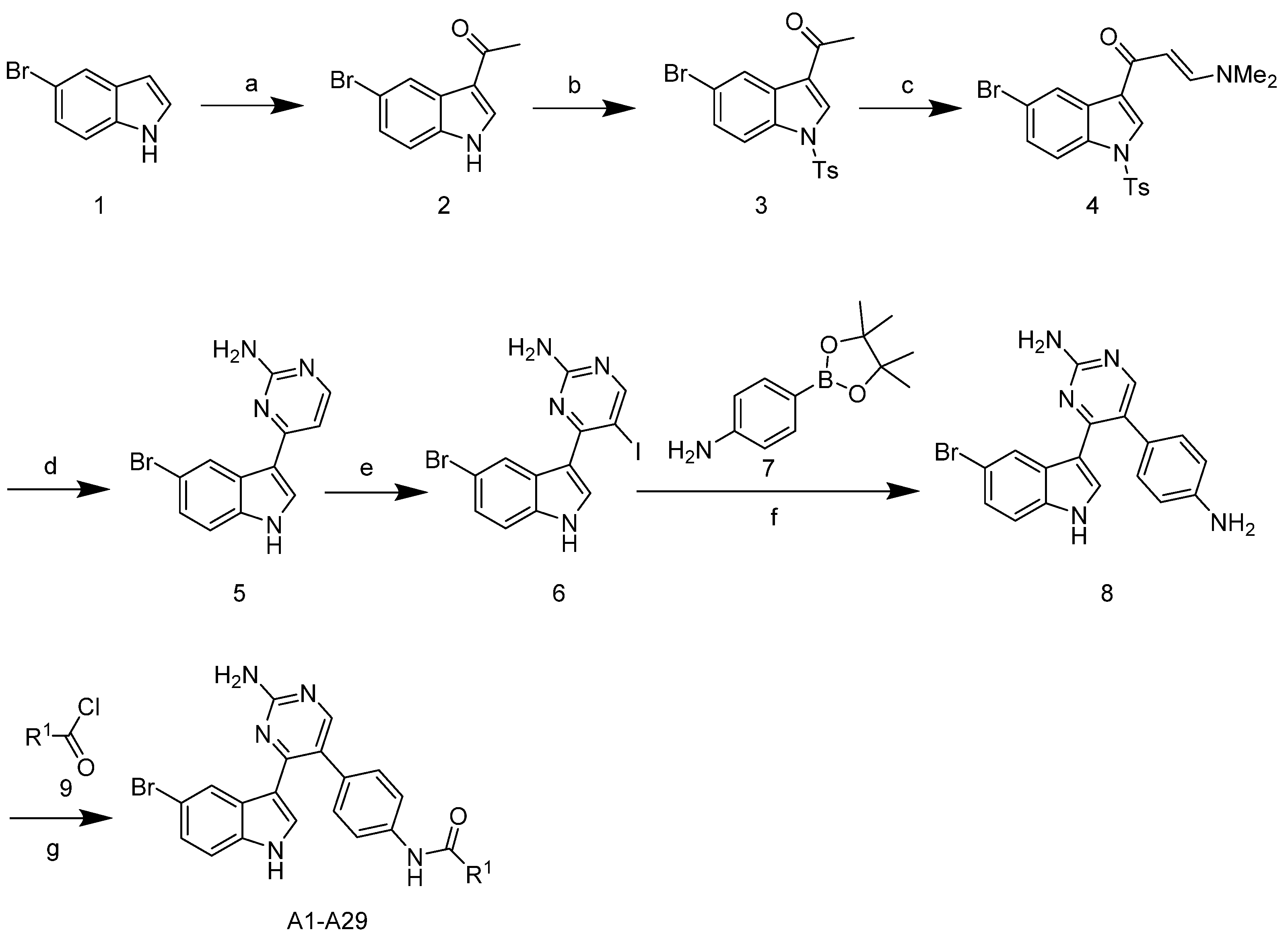
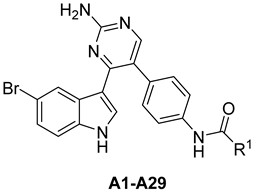
The synthetic route of the target compounds A1–29 is shown in Scheme 1. The commercially available starting material 5-bromoindole 1 was acetylated using acetyl chloride and SnCl4 in toluene to give the intermediate 2. Then, protection of indole was performed by reaction with tosyl chloride (TsCl) in the presence of 4-(dimethylamino)-pyridine (DMAP) and N,N-diisopropylethylamine (DIPEA) in methylene chloride, leading to the formation of compound 3. The reaction of compound 3 with N,N-dimethylformamide diethyl acetal (DMF-DMA) in N,N-dimethylformamide (DMF) provided enaminone 4 in a 52% yield. Treatment of enaminone 4 with guanidine hydrochloride in 2-methoxyethanol and potassium carbonate led to the formation of meridianin C 5 in a 51% yield. Meridianin C 5 was then iodinated with N-iodosuccinimide (NIS) in methylene chloride to give the corresponding compound 6 in an 87% yield. Suzuki cross coupling was then performed using boronate, a (1,1′-bis (diphenylphosphino) ferrocene) dichloropalladium (II) complex with dichloromethane, and sodium carbonate in an H2O/DME medium, which led to compound 8 in a 43% isolated yield. The target compounds A1–29 were obtained from the reaction of compound 8 with commercially available substituted acyl halides.
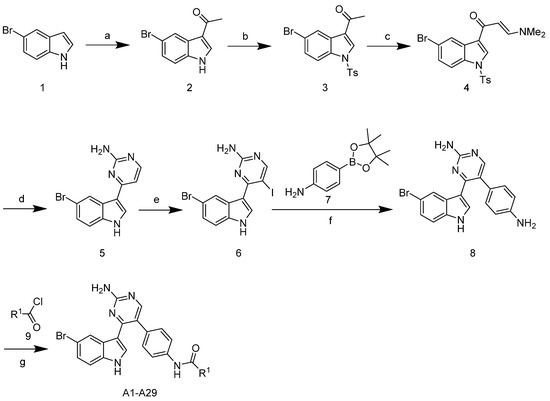
Scheme 1.
Synthetic route of the compounds A1–29. Reagents and conditions: (a) AcCl, SnCl4, toluene, 0 °C, 2 h, 87%; (b) TsCl, N,N-diisopropylethylamine (DIPEA), 4-(dimethylamino)-pyridine (DMAP), DCM, room temperature (rt), 20 h, 70%; (c) N,N-dimethylformamide diethyl acetal (DMF-DMA), DMF, 110 °C, 4 h, 52%; (d) guanidine-HCl, 2-methoxyethanol, K2CO3, reflux, 24 h, 51%; (e) N-iodosuccinimide (NIS), DMF, rt, 2 h, 87%; (f) Pd(dppf)Cl2CH2Cl2, Na2CO3, DME, H2O, 85 °C, 43%; (g) RCOCl, pyridine, 0 °C–rt, 15–51%.
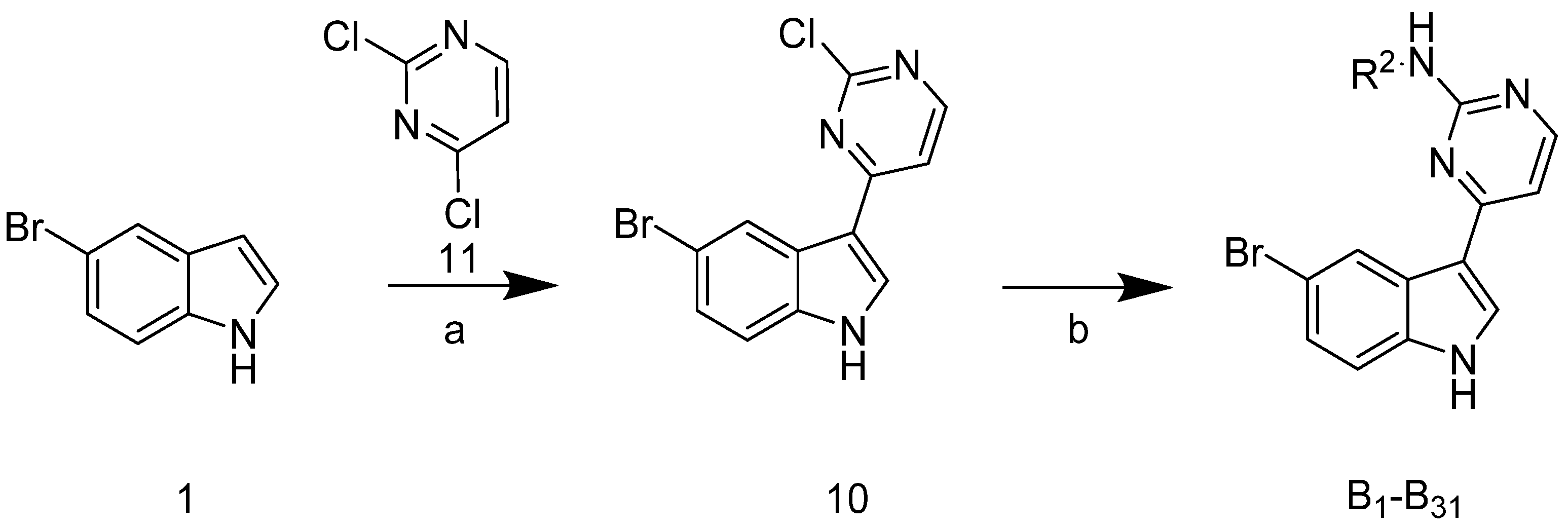
The synthetic route of the target compounds B1–31 is shown in Scheme 2. Compound 10 was obtained in a 63% yield using 5-bromoindole 1 and 2,4-dichloropyrimidine 11. Target compounds B1–31 were obtained from the reaction of 10 with commercially available substituted aromatic amines.
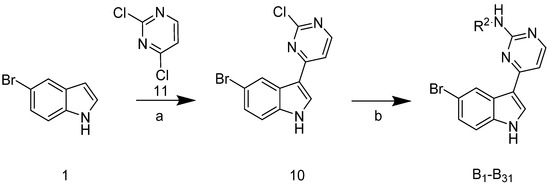
Scheme 2.
Synthetic route of the compounds B1–31. Reagents and conditions: (a) AlCl3, DCE, 85 °C, 3 h, 63%; (b) substituted aromatic amine, tosic acid, 2-pentanol, 125 °C, 25–63%.
2.2. Biological Activity
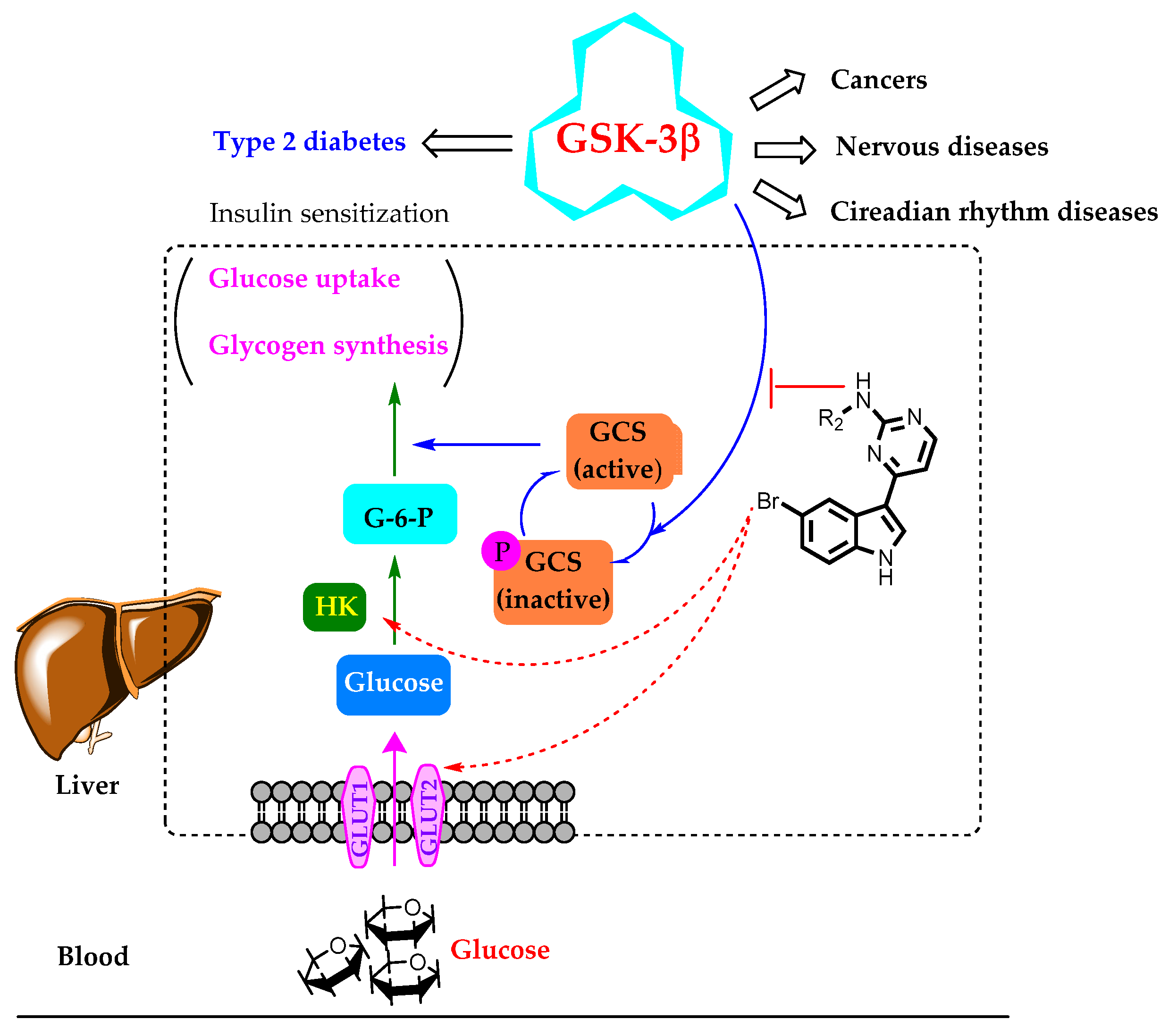
Regulatory control of glycogen synthase (GCS), the final enzyme in glycogen biosynthesis, is an obvious target for antihyperglycemic control. Overexpression/overactivation of GSK-3β can inhibit the activation of GCS effectively, and pharmacological inhibitors of GSK-3β have been demonstrated to activate glycogen synthase and improve glycogen synthesis, suppress gluconeogenesis, and increase glucose uptake (Figure 3) [12,14,15].
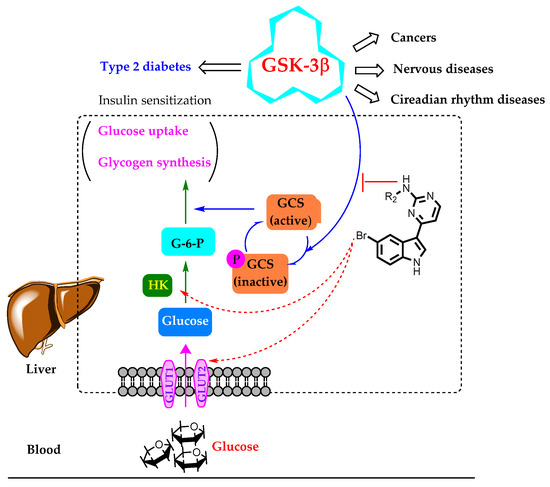
Figure 3.
GSK-3β-mediated multicellular responses and the hypothetic mechanism of the compounds in this study for glucose uptake improvement. G-6-P, glucose 6-phosphate; GCS, glycogen synthase; HK, hexokinase.
Meridianin C is a marine alkaloid characterized by an indole ring connected to a pyrimidine at the three position and has extensive biological activity in GSK-3β inhibition. Anticancer [19] and antimalarial [20] GSK-3β-inhibition-related activities of meridianin C were also investigated. However, to the best of our knowledge, antihyperglycemic activity of meridianin C and its derivatives has not been disclosed yet. Thus, we hypothesized that meridianin C might influence other upstream proteins at the same time, which leads to insipid activity in glucose uptake. Nevertheless, GSK-3β has a close association with glucose uptake, and meridianin C was confirmed to be a good inhibitor of GSK-3β. Thus, we selected meridianin C as the lead compound for modification to obtain better derivatives for diabetic therapy.
Considering that there are still several key upstream signaling proteins that would influence glucose uptake in the body, such as glucose transporters (GLUT), glycogen synthase (GCS), and glycogen phosphorylase (GP) (Figure 3), in this study, we set up a cell model of glucose uptake in HepG2 cells, instead of a GSK-3β assay, to screen active compounds and investigate the structure–activity relationship in order to evaluate the glucose uptake improving activity more accurately.
Herein, viabilities of the target-compound-treated HepG2 cells were first detected to exclude the influence of cell toxicity on the determination of glucose uptake using an MTT assay. As illustrated in Table 1 and Table 2, most of the tested compounds showed concentration dependence after 48 h of incubation and CC50 values were shown to be higher than 5 µM, as seen in Table 1 and Table 2. Comparatively, compounds with 5′-substitutions (series A), overall, had higher cytotoxicity than those with 2′-substitutions (series B). According to previous reports [21], compounds without apparent cytotoxicity at 5 µM were selected for investigation of their cell glucose uptake effects in the following study.

Table 1.
Chemical structures of compounds A1–29 and their relative glucose uptake and cytotoxicity in a HepG2 cell model.

Table 2.
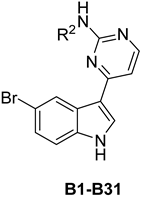
Chemical structures of compounds B1–31 and their relative glucose uptake and cytotoxicity in a HepG2 cell model.
Next, the synthesized meridianin derivatives were evaluated on the basis of their ability to increase glucose transport using HepG2 as the tool cell model to test their possible anti-diabetes effect. As shown in Table 1, most of the derivatives with 5′-substitutions exhibited no obvious effect on the cell glucose uptake at 5 µM, which is similar to or better than that of meridianin C (CC50 = 13 µM). However, compound A13 with an ortho-bromo-phenyl substitution exhibited a glucose uptake ability of 27%, which was different from that of the parent meridianin C and better than that of the reference compound TDZD-8 (16%). Compound A21 with para-methylphenyl and compound A26 with 3,5-dichlorophenyl showed glucose uptake of 24% and 22%, respectively.
As shown in Table 2, the derivatives with substitutions on 2′-position of the pyrimidine ring had significant improvement in terms of the relative hepatocyte glucose uptake. Overall, in this series, introducing substituents at the ortho-position of the phenyl ring improved the glucose uptake more than the presence of substituents at the para-position and the meta-position. For instance, compound B1 with 2-fluorophenyl showed a 20% glucose uptake, while compound B3 with 4-fluorophenyl showed a 7.4% uptake and compound B2 with 3-fluorophenyl showed no change at the same concentration. This phenomenon was also observed in the halogen and alkyl mono-substituted analogues (B1–22). At the ortho-position, the activity was along the following sequence: bromo > chloro > trifluoromethyl > fluorine > methoxy > methyl > isopropyl. Among the halogen-substituted compounds, compound B7 with a 2-bromo group showed the best glucose uptake, of 26% at 5 µM. For the di-substituted analogues, they all showed no apparent improvement in glucose uptake, with three exceptions. The 2,6-difluorophenyl compound B29 and the 2,6-dimethyl compound B30 showed excellent hepatocyte glucose uptake activity (29% and 38%, respectively), with no significant toxicity against HepG2 cells. Compound B31 with 2,6-dimethoxyl showed a 16% uptake.
To further confirm the inhibitory activities against GSK-3β of the synthesized meridianin derivatives, compounds with the best activity on cell glucose uptake were selected for a GSK-3β Kinase-Glo™ luminescent assay. As shown in Table 1 and Table 3, compounds with 5′-substitutions (A13 and A26) exhibited the ability of glucose uptake but showed no apparent inhibitory activity against GSK-3β at 30 µM. On the other hand, compounds B29 and B30 showed much better potencies, with inhibitory activity (IC50) values of 5.85 and 24.4 µM, respectively, which were slightly lower than that of the lead compound meridianin C (IC50 = 2 µM; [18]).

Table 3.
Inhibitory activity (IC50) against human GSK-3β.
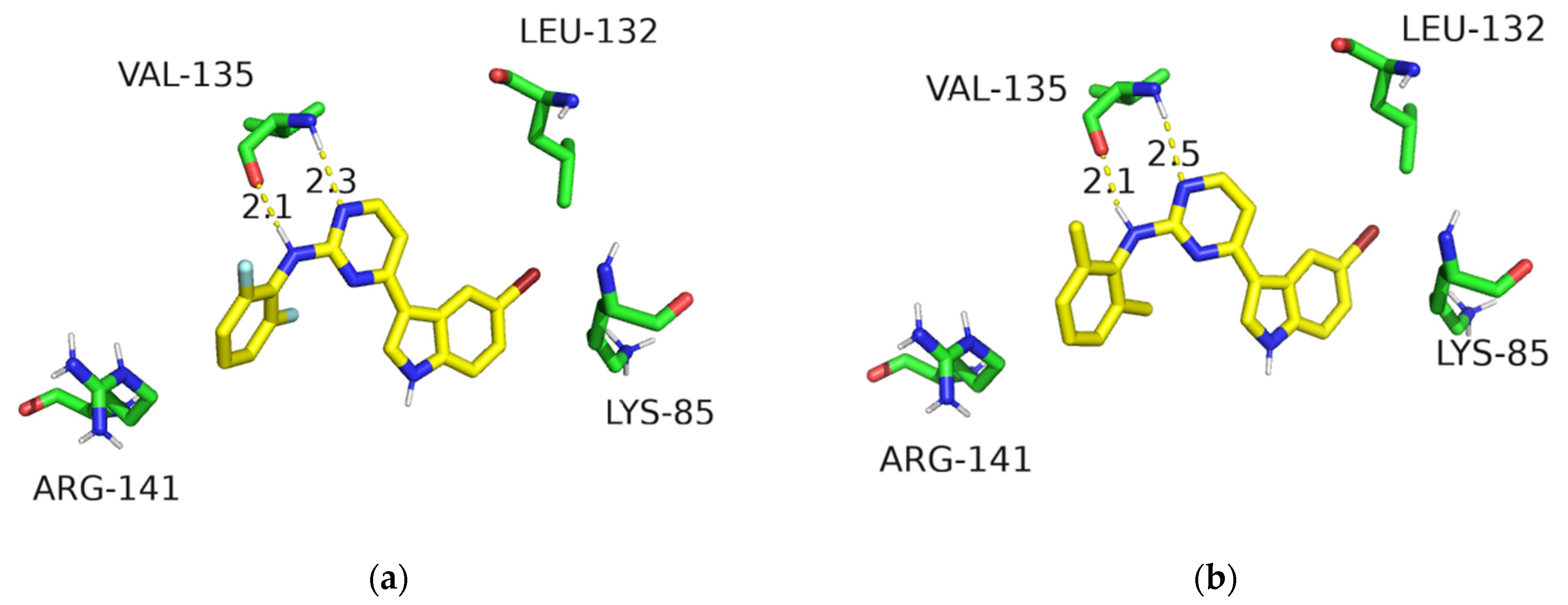
Finally, the structural binding mode of the potent 2-aminopyrimidine-modified analogues were predicted to show compounds B29 and B30 adopting similar poses within the ATP-binding site of GSK-3β (Figure 4). They maintained the key interactions of meridianin C as shown in Figure 2. Additionally, the aniline group well occupied the front solvent-exposed binding site. However, no new hydrogen-bonding interactions were formed, signifying that there was no improvement in the GSK-3β but a significant increase in the cell glucose uptake.
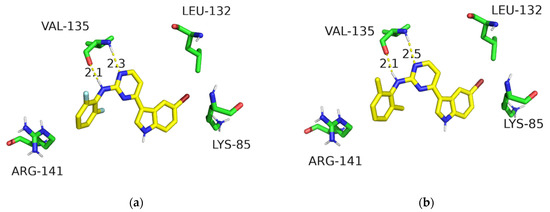
Figure 4.
Binding modes of compounds B29 (a) and B30 (b) in the ATP site of GSK-3β (PDB code: 6B8J). The hydrogen bonds are shown by the yellow dashed lines.
Conclusively, meridianin C had better GSK-3β inhibition but had no glucose uptake activity, A13 and A26 showed better glucose uptake activity but without GSK-3β inhibition, and B29 and B30 displayed the best glucose uptake ability with moderate GSK-3β inhibitory activities. Therefore, from our results, there seems no close association between GSK-3β inhibitory activity and glucose uptake efficiency for meridianin C and its analogues, which is consistent with our hypothesis that these novel compounds might interfere in the other signaling proteins (such as GLUT, HK, and GCS) at the same time to influence glucose uptake (Figure 3). The mechanisms of these active compounds need to be clarified in more detail. Anyhow, in this study, two novel hit compounds of B29 and B30 were screened and found to have proper glucose uptake improving ability, as well as a definite GSK-3β binding and inhibitory activity. Thus, in further studies, based on the current structures, we will optimize the structures continuously, along with the activities with other involved proteins, to obtain much better anti-diabetes drugs.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure
Chemical reagents and solvents, purchased from commercial sources, were of analytical grade and were used without further purification. All air-sensitive reactions were run under a nitrogen atmosphere. All the reactions were monitored by TLC on recoated silica gel G plates at 254 nm under a UV lamp using ethyl acetate/n-hexane as the eluent. Column chromatography was performed on a glass column packed with silica gel (200–300 mesh) using ethyl acetate/n-hexane as the eluent. Melting points were measured on an SGW X-1 microscopic melting point apparatus. Proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) spectra were recorded in DMSO-d6, CD3OD, or CDCl3 on a Bruker AV 400 MHz spectrometer. Chemical shifts were reported in δ (ppm) units relative to the internal standard tetramethylsilane (TMS). Mass spectra and HRMS were obtained on a Waters Quattro Micromass instrument and a Bruker Compact instrument, respectively, using electrospray ionization (ESI) techniques. The purities of the target compounds were ≥95%, measured by HPLC, performed on an Agilent 1260 HPLC system with a UV detector and an Agilent Eclipse Plus C18 column (150 × 4.6 mm, 5 μm), eluting with a mixture of solvents acetonitrile and water. (a) 0–12 min, 50–95% ACN; (b) 12–16 min, 95% ACN; (c) 16–16.01 min, 95–50% ACN; (d) 16.01–20 min, 50% ACN. Peaks were detected at λ 254 nm with a flow rate of 1.0 mL/min. The final structures were fully characterized by 1H NMR, 13C NMR and HRMS (see Supplementary Materials).
3.1.2. Synthesis of 1-(5-Bromo-1H-indol-3-yl) Ethanone 2
To a solution of 5-bromoindole 1 (5.00 g, 25.5 mmol, 1.00 eq) in 25 mL of anhydrous toluene was added (4.00 g, 51.0 mmol, 2.00 eq) acetyl chloride at 0 °C. The resulting mixture was stirred for 15 min at 0 °C, and a solution of SnCl4 (13.3 g, 51.0 mmol, 2.00 eq) in 24 mL anhydrous toluene was added. The resulting solution was stirred for 2 h at 0 °C, and 75 mL of 8% NaHCO3 was added dropwise. The resulting slurry was diluted with 150 mL of ethyl acetate, dried (Na2SO4), and filtered. The solvent was removed with a rotary evaporator. The crude product was purified by column chromatography to afford the compound 2. Yield: 87%, brick red solid, mp 250.4–251.3 °C. 1H NMR (400 MHz, CD3OD) δ 8.38 (d, J = 2.0 Hz, 1H), 8.17 (s, 1H), 7.45–7.23 (m, 2H), 2.51 (s, 3H).
3.1.3. Synthesis of 1-(5-Bromo-1-tosyl-1H-indol-3-yl) Ethanone 3
To the solution of 5-bromo-3-acetyl indole 2 (5.00 g, 21.0 mmol, 1.00 eq) in dichloromethane was added DMAP (0.128 g, 1.05 mmol, 0.0500 eq), p-toluenesulfonyl choride (TsCl, 4.40 g, 23.1 mmol, 1.10 eq), and N,N-diisopropylethylamine (DIPEA, 4.07 g, 31.5 mmol, 1.50 eq). The mixture was stirred at room temperature for 20 h. The reaction was then quenched by the addition of 10% HCl. Dichloromethane was added to the reaction mixture. The organic layer was separated, dried over anhydrous sodium sulphate, and concentrated to get a crude compound. The crude product was purified by column chromatography to afford the compound 3. Yield: 70%, white solid, mp 140.6–141.5 °C. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 2.0 Hz, 1H), 8.15 (s, 1H), 7.79 (s, 1H), 7.76 (d, J = 7.3 Hz, 2H), 7.44 (d, J = 8.9 Hz, 1H), 7.27 (d, J = 8.1 Hz, 2H), 2.53 (s, 3H), 2.36 (s, 3H).
3.1.4. Synthesis of 1-(5-Bromo-1-tosyl-1H-indol-3-yl)-3-(dimethylamino) prop-2-en-1-one 4
1-(5-bromo-1-tosyl-1H-indol-3-yl) ethanone 3 (4.00 g, 10.2 mmol, 1.00 eq) was taken in anhydrous N,N-dimethylformamide (DMF, 40 mL), and a solution of dimethyl formamide-dimethylacetal (DMF-DMA, 1.82 g, 15.3 mmol, 1.50 eq) was added to the same solvent (4 mL). The resultant solution was heated at 110 °C for 4 h under a N2 atmosphere. After cooling, the solution was poured into water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the compound 4. Yield: 52%, pale yellow solid, mp 176.7–178.5 °C. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.96 (s, 1H), 7.73–7.64 (m, 4H), 7.37–7.29 (m, 1H), 7.16 (d, J = 8.1 Hz, 2H), 5.46 (d, J = 12.3 Hz, 1H), 3.07 (s, 3H), 2.87 (s, 3H), 2.27 (s, 3H).
3.1.5. Synthesis of Meridianin C 5
A mixture of 1-(5-bromo-1-tosyl-1H-indol-3-yl)-3-(dimethylamino) prop-2-en-1-one 4 (2.00 g, 4.47 mmol, 1.00 eq), guanidine hydrochloride (0.64 g, 6.71 mmol, 1.50 eq), anhydrous K2CO3 (1.24 g, 8.94 mmol, 2.00 eq), and 2-methoxyethanol (20 mL) was heated at reflux temperature for 24 h under a N2 atmosphere. After cooling, the solution was poured into water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the compound 5. Yield: 51%, pale yellow solid, mp 105.4–106.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.75 (s, 1H), 8.25 (s, 1H), 8.11 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.00 (s, 1H), 6.49 (s, 2H).
3.1.6. Synthesis of 4-(5-bromo-1H-indol-3-yl)-5-iodopyrimidin-2-amine 6
N-Iodosuccinimide (NIS, 0.490 g, 2.17 mmol, 1.10 eq) was added to a solution of meridianin C 5 (0.570 g, 1.97 mmol, 1.00 eq) in anhydrous N,N-dimethylformamide (DMF, 6 mL). The reaction was stirred at room temperature for 1.5 h before concentrating to remove the solvent. The residue was partitioned between ethyl acetate (100 mL) and Na2S2O3 (100 mL). The organics were washed with NaHCO3 (80 mL) and brine (10 mL), dried over anhydrous sodium sulphate, and concentrated under reduced pressure. The crude product was purified by column chromatography to afford the compound 6. Yield: 87%, pale yellow solid, mp 203.5–204.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.51 (s, 1H), 8.46 (s, 1H), 8.39 (d, J = 2.0 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.30 (dd, J = 8.7, 2.1 Hz, 1H), 6.79 (s, 2H).
3.1.7. Synthesis of 5-(4-aminophenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine 8
A mixture of compound 6 (0.500 g, 1.20 mmol, 1.00 eq), 4-aminophenylboronic acid pinacol ester 7 (0.317 g, 1.45 mmol, 1.20 eq), and Na2CO3 (0.319 g, 3.01 mmol, 2.50 eq) under a nitrogen atmosphere was treated with DME (9 mL), water (3 mL), and Pd (dppf) Cl2CH2Cl2 (0.0500 g, 0.0600 mmol, 0.0500 eq). The mixture was purged with bubbling nitrogen for 2 min and then stirred at 85 °C for 16 h, cooled to room temperature, and partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate twice, and the combined organic layers were washed with brine, dried over anhydrous sodium sulphate, filtered, and concentrated. The crude product was purified by column chromatography to afford the compound 8. Yield: 43%, pale yellow solid, mp 217.7–218.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 8.65 (s, 1H), 7.94 (s, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.24 (d, J = 8.5 Hz, 1H), 6.89 (d, J = 7.9 Hz, 2H), 6.74 (s, 1H), 6.61 (d, J = 8.0 Hz, 2H), 6.55 (s, 2H), 5.33 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 161.55, 160.62, 156.92, 148.17, 134.70, 130.90, 130.22, 128.23, 125.40, 125.19, 124.80, 121.82, 114.52, 113.68, 113.45, 112.81. HRMS calcd for C18H15BrN5+ [M + H] + 380.0505, found 380.0504.
3.1.8. Synthesis of 5-bromo-3-(2-chloropyrimidin-4-yl)-1H-indole 10
To the solution of 2,4-dichloropyrimidine (3.04 g, 20.4 mmol, 1.00 eq) in dichloroethane (20 mL) was added aluminum chloride (AlCl3, 3.26 g, 24.5 mmol, 1.20 eq) under a N2 atmosphere, and the mixture was stirred for 5 min. 5-Bromoindole 1 (4.00 g, 20.4 mmol, 1.00 eq) was added, and the mixture was heated at 85 °C for 3 h. After completion of the reaction, the reaction mixture was cooled at room temperature and poured into ice-cold water (30 mL) with continuous stirring for 20 min. The crude products were isolated by extracting with ethyl acetate. The organic phase was separated and washed with brine, dried over sodium sulphate, and evaporated under vacuum. The crude products obtained were further purified by silica gel column chromatography using an ethyl acetate-hexane mixture. Yield: 63%, pale yellow solid, mp 260.5–261.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 8.57 (d, J = 2.6 Hz, 2H), 8.55 (d, J = 5.6 Hz, 1H), 7.92 (d, J = 5.5 Hz, 1H), 7.47 (d, J = 8.7 Hz, 1H), 7.36 (dd, J = 8.6, 1.9 Hz, 1H).
General Procedure for the Preparation of the Final Compounds A1–29
To a solution of 5-(4-aminophenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine 8 (200 mg, 0.530 mmol, 1.00 eq) in pyridine (5 mL), the appropriate substituted benzoyl chloride derivative (0.680 mmol, 1.30 eq) was added and the mixture stirred for 1 h. The solution was poured into ice-cold water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the target compounds.
General Procedure for the Preparation of the Final Compounds B1–31
p-Toluene sulfonic acid hydrate (203 mg, 1.07 mmol, 1.10 eq) was added in one portion to a mixture of substituted aromatic amine (0.972 mmol, 1.00 eq) and compound 10 (300 mg, 0.972 mmol, 1.00 eq) in 2-pentanol (10 mL). The resulting mixture was then stirred at 125 °C for 18 h. the solution was poured into ice-cold water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the target compounds.
3.1.9. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Propionamide A1
Yield: 30%, pale yellow solid, mp 193.6–194.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (s, 1H), 9.86 (s, 1H), 8.44 (s, 1H), 7.87 (s, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 8.6 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 7.05 (d, J = 8.1 Hz, 2H), 6.53 (d, J = 17.5 Hz, 3H), 2.21 (q, J = 7.5 Hz, 2H), 0.96 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 172.51, 162.91, 159.91, 158.66, 139.03, 134.97, 133.45, 130.61, 130.24, 128.37, 125.20, 124.99, 121.22, 119.67, 113.98, 113.55, 112.93, 30.01, 10.16. HRMS calcd for C21H19BrN5O+ [M + H] + 436.0767, found 437.0765. HPLC purity 98.27% (tR = 10.3 min).
3.1.10. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Butyramide A2
Yield: 33%, pale yellow solid, mp 187.6–188.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 9.94 (s, 1H), 8.55 (s, 1H), 7.99 (s, 1H), 7.63 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 8.6 Hz, 1H), 7.23 (d, J = 8.1 Hz, 1H), 7.17 (d, J = 8.1 Hz, 2H), 6.69 (s, 1H), 6.60 (s, 2H), 2.30 (t, J = 7.3 Hz, 2H), 1.62 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.76, 162.02, 159.01, 157.80, 138.08, 134.09, 132.63, 129.72, 129.34, 127.49, 124.30, 124.10, 120.38, 118.84, 113.06, 112.66, 112.09, 37.95, 18.15, 13.23. HRMS calcd for C22H21BrN5O+ [M + H] + 450.0924, found 450.0920. HPLC purity 95.59% (tR = 11.0 min).
3.1.11. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Isobutyramide A3
Yield: 33%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 9.90 (s, 1H), 8.57 (s, 1H), 8.00 (s, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.25 (d, J = 6.6 Hz, 1H), 7.18 (d, J = 8.2 Hz, 2H), 6.71 (s, 1H), 6.61 (s, 2H), 2.61 (m, 1H), 1.12 (d, J = 6.8 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 174.83, 162.03, 159.01, 157.81, 138.19, 134.09, 132.64, 129.74, 129.32, 127.50, 124.31, 124.11, 120.38, 118.94, 113.08, 112.67, 112.08, 34.54, 19.11. HRMS calcd for C22H21BrN5O+ [M + H] + 450.0924, found 450.0917. HPLC purity 95.64% (tR = 11.0 min).
3.1.12. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Pivalamide A4
Yield: 35%, pale yellow solid, mp 139.6–140.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (s, 1H), 9.26 (s, 1H), 8.57 (s, 1H), 7.97 (s, 1H), 7.67 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.6 Hz, 1H), 7.25–7.20 (m, 1H), 7.15 (d, J = 8.2 Hz, 2H), 6.67 (s, 1H), 6.61 (s, 2H), 1.21 (s, 9H). 13C NMR (100 MHz, DMSO-d6) δ 176.73, 162.69, 159.66, 158.48, 138.81, 134.75, 133.48, 130.41, 129.76, 128.17, 125.00, 124.79, 121.01, 120.57, 113.75, 113.35, 112.72, 31.39, 27.46. HRMS calcd for C23H23BrN5O+ [M + H] + 464.1080, found 464.1079. HPLC purity 96.11% (tR = 11.1 min).
3.1.13. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-phenylacetamide A5
Yield: 35%, pale yellow solid, mp 207.6–208.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.42 (s, 1H), 10.26 (s, 1H), 8.57 (s, 1H), 8.00 (s, 1H), 7.64 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 2.0 Hz, 2H), 7.33 (d, J = 3.6 Hz, 2H), 7.31 (d, J = 4.5 Hz, 1H), 7.30–7.20 (m, 2H), 7.20 (d, J = 8.2 Hz, 2H), 6.68 (s, 1H), 6.62 (s, 2H), 3.66 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 169.13, 162.46, 159.44, 158.19, 138.38, 135.98, 134.49, 133.36, 130.16, 129.84, 129.11, 128.32, 127.90, 126.56, 124.72, 124.53, 120.73, 119.35, 113.49, 113.09, 112.47, 43.40. HRMS calcd for C26H21BrN5O+ [M + H] + 498.0924, found 498.0924. HPLC purity 95.64% (tR = 11.8 min).
3.1.14. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Benzamide A6
Yield: 43%, pale yellow solid, mp 187.9–188.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1H), 10.13 (s, 1H), 9.21 (d, J = 2.0 Hz, 1H), 8.52 (s, 1H), 8.47 (d, J = 7.5 Hz, 2H), 8.37 (d, J = 8.5 Hz, 2H), 8.03 (d, J = 7.3 Hz, 1H), 7.97 (t, J = 7.5 Hz, 2H), 7.82 (d, J = 8.6 Hz, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.71 (dd, J = 8.6, 2.0 Hz, 1H), 7.32 (s, 1H), 6.55 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 175.94, 173.23, 170.46, 168.93, 149.17, 145.75, 145.40, 144.73, 141.90, 140.83, 140.34, 138.92, 138.81, 137.84, 135.79, 135.21, 132.19, 130.81. HRMS calcd for C25H19BrN5O+ [M + H] + 484.0767, found 484.0770. HPLC purity 95.56% (tR = 12.6 min).
3.1.15. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-fluorobenzamide A7
Yield: 37%, pale yellow solid, mp 159.6–160.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.51 (s, 1H), 8.57 (s, 1H), 8.01 (s, 1H), 7.77 (d, J = 8.1 Hz, 2H), 7.69 (s, 2H), 7.59 (d, J = 6.5 Hz, 1H), 7.36 (q, J = 8.3, 7.2 Hz, 3H), 7.24 (t, J = 8.1 Hz, 3H), 6.77 (s, 1H), 6.61 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 175.41, 163.29, 162.95, 160.57, 159.97, 158.71, 158.10, 138.52, 135.13, 134.52, 132.97 (d, J = 9.2 Hz), 130.81, 130.34, 128.41, 125.51 (d, J = 15.3 Hz), 125.10 (d, J = 8.8 Hz), 124.97 (d, J = 11.1 Hz), 121.11, 120.43, 116.63 (d, J = 21.7 Hz), 114.13, 113.53, 112.81. HRMS calcd for C25H18BrFN5O+, [M + H] + 502.0673, found 502.0668. HPLC purity 95.43% (tR = 12.0 min).
3.1.16. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidiN-5-yl) phenyl)-3-fluorobenzamide A8
Yield: 41%, pale yellow solid, mp 183.6–184.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.40 (s, 1H), 8.57 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.85–7.76 (m, 4H), 7.64–7.56 (m, 1H), 7.49–7.43 (m, 1H), 7.33 (d, J = 8.6 Hz, 1H), 7.29–7.24 (m, 3H), 6.75 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.23, 163.18, 162.51, 160.74, 159.46, 158.31, 138.07, 134.57, 134.10, 130.65 (d, J = 7.9 Hz), 130.16, 129.79, 127.93, 124.73, 124.59, 123.92 (d, J = 2.6 Hz), 120.75, 120.64 (d, J = 3.1 Hz), 114.63, 114.40, 113.55, 113.14, 112.54. HRMS calcd for C25H18BrFN5O+, [M + H] + 502.0673, found 502.0673. HPLC purity 98.18% (tR = 12.2 min).
3.1.17. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-fluorobenzamide A9
Yield: 38%, pale yellow solid, mp 178.6–179.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.35 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.07–8.03 (m, 3H), 7.82 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 8.8 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.5 Hz, 3H), 6.74 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.76, 164.94, 163.29, 162.92, 159.87, 158.72, 138.68, 134.98, 134.32, 131.81 (d, J = 3.0 Hz), 130.85 (d, J = 9.2 Hz), 130.57, 130.18, 128.35, 125.07 (d, J = 14.9 Hz), 121.20, 120.98, 115.80 (d, J = 22.0 Hz), 113.96, 113.55, 112.96. HRMS calcd for C25H18BrFN5O+ [M + H] + 502.0673, found 502.0669. HPLC purity 95.97% (tR = 12.1 min).
3.1.18. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-chlorobenzamide A10
Yield: 43%, pale yellow solid, mp 211.5–212.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.59 (s, 1H), 8.57 (s, 1H), 8.03 (s, 1H), 7.76 (d, J = 8.2 Hz, 2H), 7.62–7.57 (m, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.49 (d, J = 5.8 Hz, 1H), 7.46 (d, J = 7.3 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.2 Hz, 2H), 6.77 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.55, 162.09, 159.02, 157.94, 137.64, 136.55, 134.15, 133.64, 130.73, 129.80, 129.52, 129.49, 129.28, 128.54, 127.52, 126.89, 124.30, 124.17, 120.32, 119.45, 113.14, 112.72, 112.09. HRMS calcd for C25H18BrFN5O+ [M + H] + 518.0378, found 518.0381. HPLC purity 96.64% (tR = 12.1 min).
3.1.19. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-chlorobenzamide A11
Yield: 36%, pale yellow solid, mp 204.8–205.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 10.42 (s, 1H), 8.55 (s, 1H), 8.01 (d, J = 7.0 Hz, 2H), 7.92 (d, J = 7.7 Hz, 1H), 7.80 (d, J = 8.1 Hz, 2H), 7.66 (d, J = 7.3 Hz, 1H), 7.57 (t, J = 7.8 Hz, 1H), 7.32 (d, J = 8.6 Hz, 1H), 7.28–7.23 (m, 3H), 6.73 (d, J = 2.8 Hz, 1H), 6.62 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.13, 162.51, 159.44, 158.35, 158.31, 138.06, 136.91, 134.57, 134.11, 133.25, 131.44, 130.46, 130.15, 129.78, 127.43, 126.52, 124.72, 124.58, 120.74, 120.60, 113.54, 113.13, 112.53. HRMS calcd for C₂₅H₁₈BrClN₅O+, [M + H] + 518.0378, found 518.0371. HPLC purity 95.15% (tR = 13.1 min).
3.1.20. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-chlorobenzamide A12
Yield: 42%, pale yellow solid, mp 261.7–262.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.40 (s, 1H), 8.58 (d, J = 2.2 Hz, 1H), 8.04 (s, 1H), 8.01 (d, J = 8.6 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.27 (d, J = 8.4 Hz, 3H), 6.74 (s, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.70, 162.71, 159.65, 158.49, 138.36, 136.65, 134.76, 134.21, 133.85, 130.35, 129.97, 129.86, 128.70, 128.13, 124.93, 124.77, 120.96, 120.78, 113.73, 113.33, 112.74. HRMS calcd for C₂₅H₁₈BrClN₅O+ [M + H] + 518.0378, found 518.0385. HPLC purity 96.20% (tR = 12.9 min).
3.1.21. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-bromobenzamide A13
Yield: 46%, pale yellow solid, mp 147.4–148.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.58 (s, 1H), 8.59 (s, 1H), 8.04 (s, 1H), 7.80–7.71 (m, 3H), 7.61–7.57 (m, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.44 (td, J = 7.7, 1.9 Hz, 1H), 7.35 (d, J = 8.7 Hz, 1H), 7.29–7.25 (m, 3H), 6.78 (d, J = 2.8 Hz, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.38, 162.07, 158.96, 157.92, 138.68, 137.64, 134.11, 133.60, 132.31, 130.78, 129.75, 129.45, 128.44, 127.50, 127.32, 124.28, 124.13, 120.28, 119.42, 118.57, 113.09, 112.68, 112.06. HRMS calcd for C₂₅H₁₈Br₂N₅O+, [M + H] + 561.9873, found 561.9870. HPLC purity 96.11% (tR = 12.1min).
3.1.22. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-bromobenzamide A14
Yield: 38%, pale yellow solid, mp 176.6–177.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.44 (s, 1H), 8.58 (d, J = 2.1 Hz, 1H), 8.20–8.13 (m, 1H), 8.04 (s, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.86–7.77 (m, 3H), 7.52 (t, J = 7.8 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.31–7.23 (m, 3H), 6.75 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.40, 162.91, 159.82, 158.70, 138.45, 137.50, 134.95, 134.73, 134.51, 131.08, 130.66, 130.54, 130.17, 128.32, 127.29, 125.12, 124.96, 122.11, 121.12, 120.98, 113.92, 113.51, 112.93. HRMS calcd for C₂₅H₁₈Br₂N₅O+, [M + H] + 561.9873, found 561.9870. HPLC purity 97.54% (tR = 13.3 min).
3.1.23. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-bromobenzamide A15
Yield: 48%, pale yellow solid, mp 276.4–277.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.40 (s, 1H), 8.57 (d, J = 2.0 Hz, 1H), 8.03 (s, 1H), 7.93 (d, J = 8.5 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.76 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.5 Hz, 1H), 7.26 (d, J = 8.5 Hz, 3H), 6.74 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.02, 162.91, 159.84, 158.69, 138.55, 134.96, 134.41, 132.53, 131.85, 130.55, 130.24, 130.17, 128.33, 125.78, 125.13, 124.97, 121.15, 120.97, 113.93, 113.52, 112.94. HRMS calcd for C₂₅H₁₈Br₂N₅O+ [M + H] + 561.9873, found 561.9878. HPLC purity 95.23% (tR = 13.1 min).
3.1.24. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-(trifluoromethyl) Benzamide A16
Yield: 20%, pale yellow solid, mp 163.6–164.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 10.70 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.81 (t, J = 7.5 Hz, 1H), 7.77–7.69 (m, 4H), 7.35 (d, J = 8.6 Hz, 1H), 7.29–7.25 (m, 3H), 6.79 (s, 1H), 6.68 (s, 2H).13C NMR (100 MHz, DMSO-d6) δ 165.69, 162.57, 159.50, 158.45, 138.10, 136.24, 134.65, 134.19, 132.74, 130.30, 130.17, 129.98, 128.62, 126.45 (q, J = 4.9 Hz), 125.24(q, J = 274.0 Hz),126.05, 125.74, 124.78, 124.64, 120.75, 119.99, 113.65, 113.21, 112.53. HRMS calcd for C₂₆H₁₈BrF₃N₅O+ [M + H] +552.0641, found 552.0628. HPLC purity 96.07% (tR = 12.1 min).
3.1.25. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-(trifluoromethyl) Benzamide A17
Yield: 43%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.57 (s, 1H), 8.58 (s, 1H), 8.37–8.23 (m, 2H), 8.06 (s, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 8.5 Hz, 2H), 7.79 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.29 (d, J = 8.3 Hz, 2H), 7.26 (d, J = 9.0 Hz, 1H), 6.77 (s, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.09, 162.53, 159.46, 158.32, 137.98, 135.81, 134.58, 134.23, 131.86, 130.15, 129.78 (d, J = 4.8 Hz), 129.39, 129.07, 128.75, 128.18 (d, J = 3.6 Hz), 127.93, 126.49, 125.35(q, J = 273.7 Hz),124.65 (d, J = 14.9 Hz), 124.28 (q, J = 3.6 Hz), 120.73, 113.53, 113.13, 112.56. HRMS calcd for C₂₆H₁₈BrF₃N₅O+ [M + H] + 552.0641, found 552.0640. HPLC purity 97.48% (tR = 13.2min).
3.1.26. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-(trifluoromethyl) Benzamide A18
Yield: 43%, pale yellow solid, mp 312.7–313.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.56 (s, 1H), 8.56 (s, 1H), 8.17 (d, J = 8.1 Hz, 2H), 8.04 (s, 1H), 7.93 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.29 (s, 1H), 7.27–7.24 (m, 2H), 6.75 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.89, 162.93, 159.87, 158.72, 139.20, 138.42, 134.99, 134.65, 132.35, 131.97, 131.66, 130.40 (d, J = 32.8 Hz), 129.05, 128.34, 125.86 (q, J = 3.5 Hz), 125.80 (d, J = 279.0 Hz), 123.01(q, J = 3.5 Hz), 121.15, 121.04, 113.96, 113.55, 112.96. HRMS calcd for C₂₆H₁₈BrF₃N₅O+, [M + H] + 552.0641, found 552.0636. HPLC purity 97.91% (tR = 12.9 min).
3.1.27. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-methylbenzamide A19
Yield: 33%, pale yellow solid, mp 170.7–171.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.48 (s, 1 H), 7.93 (s, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 7.1 Hz, 1H), 7.31 (d, J = 7.8 Hz, 1H), 7.28 (d, J = 5.3 Hz, 1H), 7.24 (d, J = 7.2 Hz, 2H), 7.20 (d, J = 7.1 Hz, 1H), 7.18–7.13 (m, 3H), 6.69 (s, 1H), 6.56 (s, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 167.57, 162.12, 159.12, 157.98, 138.08, 136.86, 134.87, 134.31, 133.38, 130.20, 130.00, 129.44, 129.32, 127.59, 126.88, 125.34, 124.35, 124.16, 120.40, 119.54, 113.29, 112.75, 112.09, 19.00. HRMS calcd for C₂₆H₂₁BrN₅O+ [M + H] +498.0924, found 498.0921. HPLC purity 96.94% (tR = 12.2 min).
3.1.28. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-methylbenzamide A20
Yield: 42%, pale yellow solid, mp 211.8–212.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.45 (s, 1H), 10.23 (s, 1H), 8.49 (s, 1H), 7.93 (s, 1H), 7.74 (d, J = 8.2 Hz, 2H), 7.67 (d, J = 12.5 Hz, 2H), 7.32 (d, J = 5.8 Hz, 2H), 7.24 (d, J = 8.6 Hz, 1H), 7.15 (d, J = 8.3 Hz, 3H), 6.64 (s, 1H), 6.56 (s, 2H), 2.30 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.44, 163.17, 160.15, 158.97, 139.10, 138.43, 135.65, 135.24, 134.44, 132.88, 130.88, 130.41, 129.02, 128.85, 128.61, 125.54, 125.42, 125.26, 121.45, 121.16, 114.25, 113.82, 113.18, 21.68. HRMS calcd for C₂₆H₂₁BrN₅O+ [M + H] +498.0924, found 498.0923. HPLC purity 95.11% (tR = 12.4 min).
3.1.29. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-methylbenzamide A21
Yield: 42%, pale yellow solid, mp 291.7–292.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.24 (s, 1H), 8.58 (s, 1H), 8.04 (s, 1H), 7.89 (d, J = 7.9 Hz, 2H), 7.83 (d, J = 8.6 Hz, 2H), 7.37–7.31 (m, 3H), 7.25 (d, J = 8.5 Hz, 3H), 6.74 (s, 1H), 6.63 (s, 2H), 2.39 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.82, 162.90, 159.86, 158.67, 142.03, 138.85, 134.95, 134.09, 132.48, 130.56, 130.11, 129.34, 128.34, 128.12, 125.15, 124.97, 121.21, 120.89, 113.93, 113.52, 112.94, 21.43. HRMS calcd for C₂₆H₂₁BrN₅O+, [M + H] + 498.0924, found 498.0930. HPLC purity 96.26% (tR = 12.9 min).
3.1.30. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-methoxybenzamide A22
Yield: 22%, pale yellow solid, mp 154.9–155.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.21 (s, 1H), 8.60 (s, 1H), 8.03 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.65 (d, J = 5.8 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.27–7.23 (m, 3H), 7.19 (d, J = 8.4 Hz, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.75 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H), 3.91 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 164.37, 162.24, 159.30, 158.01, 156.28, 138.04, 134.35, 133.47, 131.85, 130.03, 129.63, 129.46, 127.75, 124.79, 124.54, 124.39, 120.59, 120.33, 119.75, 113.34, 112.95, 112.31, 111.82, 55.72. HRMS calcd for C₂₆H₂₁BrN₅O₂+ [M + H] + 514.0873, found 514.0869. HPLC purity 97.46% (tR = 13.1 min).
3.1.31. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-methoxybenzamide A23
Yield: 43%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 10.36 (s, 1H), 8.57 (s, 1H), 8.03 (s, 1H), 7.83 (d, J = 8.2 Hz, 2H), 7.56 (d, J = 7.7 Hz, 1H), 7.50 (s, 1H), 7.45 (t, J = 7.9 Hz, 1H), 7.34 (d, J = 8.5 Hz, 1H), 7.25 (d, J = 8.3 Hz, 3H), 7.19–7.13 (m, 1H), 6.75 (s, 1H), 6.65 (s, 2H), 3.84 (s, 3H).13C NMR (100 MHz, DMSO-d6) δ 165.05, 162.15, 159.16, 158.86, 157.95, 137.96, 136.01, 134.26, 133.54, 129.89, 129.40, 129.30, 127.59, 124.39, 124.24, 120.44, 120.30, 119.58, 117.04, 113.27, 112.81, 112.59, 112.15, 55.03. HRMS calcd for C₂₆H₂₁BrN₅O₂+ [M + H] + 514.0873, found 514.0871. HPLC purity 98.12% (tR = 12.1 min).
3.1.32. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-methoxybenzamide A24
Yield: 51%, pale yellow solid, mp 184.6–185.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.18 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.98 (d, J = 8.8 Hz, 2H), 7.82 (d, J = 8.6 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.24 (d, J = 8.5 Hz, 3H), 7.07 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 2.9 Hz, 1H), 6.62 (s, 2H), 3.84 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 164.77, 162.26, 161.73, 159.27, 158.03, 138.35, 134.34, 133.34, 129.96, 129.47, 129.42, 127.73, 126.76, 124.54, 124.36, 120.63, 120.27, 113.43, 113.32, 112.92, 112.33, 55.25. HRMS calcd for C₂₆H₂₁BrN₅O₂+, [M + H] + 514.0873, found 514.0871. HPLC purity 96.66% (tR = 12.1 min).
3.1.33. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2, 5-difluorobenzamide A25
Yield: 36%, pale yellow solid, mp 163.5–164.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.59 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.03 (s, 1H), 7.76 (d, J = 8.2 Hz, 2H), 7.58–7.53 (m, 1H), 7.47–7.40 (m, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.29–7.24 (m, 3H), 6.75 (d, J = 2.9 Hz,1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.94, 161.91, 159.86, 159.44, 158.72, 157.03, 156.71, 154.27, 138.21, 134.97, 134.71, 130.48 (d, J = 24.3 Hz), 128.35, 126.68 (dd, J = 18.6, 7.4 Hz), 125.07 (d, J = 12.5 Hz), 121.10, 120.46, 119.41 (dd, J = 24.2, 9.0 Hz), 118.47 (dd, J = 25.0, 8.5 Hz), 116.65 (dd, J = 25.8, 3.6 Hz), 113.97, 113.56, 112.91. HRMS calcd for C₂₅H₁₇BrF₂N₅O+, [M + H] + 520.0579, found 520.0586. HPLC purity 95.42% (tR = 12.4 min).
3.1.34. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3, 5-dichlorobenzamide A26
Yield: 33%, pale yellow solid, mp 177.7–178.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.51 (s, 1H), 8.57 (s, 1H), 8.04 (s, 1H), 8.00 (d, J = 2.0 Hz, 2H), 7.87 (s, 1H), 7.81 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.27 (t, J = 10.2 Hz, 3H), 6.75 (d, J = 3.0 Hz, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.28, 162.09, 158.99, 157.86, 137.66, 137.35, 134.13, 133.90, 130.53, 129.68, 129.38, 127.48, 126.59, 126.09, 124.27, 124.13, 120.25, 120.20, 113.09, 112.68, 112.09. HRMS calcd for C₂₅H₁₇BrCl₂N₅O+, [M + H] + 551.9988, found 551.9988. HPLC purity 97.16% (tR = 13.2 min).
3.1.35. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) thiophene-2-carboxamide A27
Yield: 15%, pale yellow solid, mp 303.8–304.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 10.39 (s, 1H), 8.57 (s, 1H), 8.06 (d, J = 15.0 Hz, 2H), 7.87 (d, J = 5.0 Hz, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.4 Hz, 4H), 6.70 (d, J = 27.3 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.93, 160.37, 159.90, 158.72, 140.49, 138.38, 135.03, 134.33, 132.40, 130.66, 130.23, 129.67, 128.57, 128.36, 125.15, 124.97, 121.13, 120.96, 114.03, 113.54, 112.92. HRMS calcd for C₂₃H₁₇BrN₅OS+ [M + H] +490.0332, found 490.0332. HPLC purity 95.22 % (tR = 11.7 min).
3.1.36. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) isoquinoline-3-carboxamide A28
Yield: 43%, pale yellow solid, mp 189.3–190.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 10.81 (s, 1H), 8.59 (d, J = 8.5 Hz, 1H), 8.52 (d, J = 2.0 Hz, 1H), 8.25–8.20 (m, 2H), 8.10–8.05 (m, 1H), 8.02 (s, 1H), 7.99–7.95 (m, 2H), 7.90–7.85 (m, 1H), 7.74–7.69 (m, 1H), 7.30–7.27 (m, 2H), 7.25 (d, J = 2.0 Hz, 1H), 7.22–7.18 (m, 1H), 6.71 (d, J = 2.8 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.46, 162.14, 159.10, 157.92, 149.73, 145.54, 137.88, 137.12, 134.19, 133.83, 130.35, 129.81, 129.51, 129.01, 128.58, 128.05, 127.79, 127.53, 124.34, 124.20, 120.34, 120.13, 118.45, 113.19, 112.75, 112.16. HRMS calcd for C₂₈H₂₀BrN₆O+ [M + H] +535.0876, found 535.0873. HPLC purity 95.84% (tR = 14.6 min).
3.1.37. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-naphthamide A29
Yield: 43%, pale yellow solid, mp 189.3–190.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (s, 1H), 10.59 (s, 1H), 8.60 (d, J = 7.1 Hz, 2H), 8.04 (q, J = 11.1, 7.4 Hz, 5H), 7.90 (d, J = 8.0 Hz, 2H), 7.75–7.58 (m, 2H), 7.35 (d, J = 8.7 Hz, 1H), 7.28 (d, J = 9.0 Hz, 3H), 6.72 (d, J = 43.7 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.10, 162.82, 159.89, 158.64, 138.78, 134.95, 134.65, 134.19, 132.57, 132.44, 130.58, 130.14, 129.36, 128.44, 128.39, 128.27, 128.05, 127.27, 125.07, 124.96, 124.84, 121.17, 120.95, 113.97, 113.52, 112.86. HRMS calcd for C₂₉H₂₁BrN₅O+ [M + H] +534.0924, found 534.0922. HPLC purity 98.46% (tR = 13.4 min).
3.1.38. 4-(5-Bromo-1H-indol-3-yl)-N-(2-fluorophenyl) pyrimidin-2-amine B1
Yield: 25%, pale yellow solid, mp 206.6–207.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 8.96 (s, 1H), 8.43 (s, 1H), 8.29 (s, 1H), 8.20 (d, J = 5.3 Hz, 1H), 7.70–7.63 (m, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.25–7.08 (m, 5H). 13C NMR (100 MHz, DMSO-d6) δ 162.71, 161.10, 157.53, 157.22, 154.78, 136.21, 130.77, 128.25 (d, J = 11.4 Hz), 127.32, 126.46, 125.47 (d, J = 7.3 Hz), 125.25, 124.87, 124.78 (d, J = 4.0 Hz), 116.29 (d, J = 19.8 Hz), 114.13 (d, J = 29.7 Hz), 113.58, 107.68. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0301. HPLC purity 99.29% (tR = 14.1 min).
3.1.39. 4-(5-Bromo-1H-indol-3-yl)-N-(3-fluorophenyl) pyrimidin-2-amine B2
Yield: 48%, pale yellow solid, mp 211.8–212.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.93 (s, 1H), 9.64 (s, 1H), 8.67 (s, 1H), 8.29 (s, 2H), 7.72 (d, J = 12.4 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.29–7.19 (m, 3H), 6.66 (t, J = 8.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 164.12, 162.67, 161.73, 160.28, 157.42, 143.20 (d, J = 11.4 Hz), 136.35, 130.99, 130.44 (d, J = 9.7 Hz), 127.26, 125.39, 124.75, 115.27, 114.33 (d, J = 29.6 Hz), 113.62, 108.46, 107.85 (d, J = 21.1 Hz), 106.01 (d, J = 26.4 Hz). HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0295. HPLC purity 98.07% (tR = 14.2 min).
3.1.40. 4-(5-Bromo-1H-indol-3-yl)-N-(4-fluorophenyl) pyrimidin-2-amine B3
Yield: 41%, pale yellow solid, mp 246.8–247.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.44 (s, 1H), 8.65 (s, 1H), 8.34 (s, 1H), 8.27 (d, J = 5.3 Hz, 1H), 7.75–7.70 (m, 2H), 7.39 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 9.0 Hz, 1H), 7.23 (d, J = 5.3 Hz, 1H), 7.13 (t, J = 8.7 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.53, 161.51, 159.55, 157.77, 156.42, 155.41, 136.45, 135.25, 129.84, 126.20, 123.96 (d, J = 46.4 Hz), 120.75 (d, J = 7.5 Hz), 114.47 (d, J = 22.2 Hz), 113.39, 112.76 (d, J = 37.2 Hz), 106.71. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0296 HPLC purity 96.24% (tR = 14.1 min).
3.1.41. 4-(5-Bromo-1H-indol-3-yl)-N-(2-chlorophenyl) pyrimidin-2-amine B4
Yield: 41%, pale yellow solid, mp 173.8–174.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.94 (s, 1H), 8.74 (s, 1H), 8.41 (s, 1H), 8.36 (d, J = 2.7 Hz, 1H), 8.29 (d, J = 5.4 Hz, 1H), 7.83 (d, J = 8.3 Hz, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.3 Hz, 1H), 7.26 (t, J = 6.8 Hz, 2H), 7.21 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.65, 161.04, 157.64, 137.31, 136.19, 130.82, 130.15, 128.30, 128.06, 127.30, 126.98, 125.99, 125.24, 124.85, 114.29, 113.96, 113.52, 107.76. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +399.0007, found 399. 0002.HPLC purity 98.97% (tR = 15.7 min).
3.1.42. 4-(5-Bromo-1H-indol-3-yl)-N-(3-chlorophenyl) pyrimidin-2-amine B5
Yield: 38%, pale yellow solid, mp 210.3–211.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 9.69 (s, 1H), 8.73 (s, 1H), 8.40 (s, 1H), 8.38 (d, J = 5.4 Hz, 1H), 8.10 (s, 1H), 7.83 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.35–7.30 (m, 2H), 7.28 (d, J = 8.1 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 161.83, 159.32, 156.51, 142.18, 135.47, 130.14, 130.03, 126.40, 124.53, 123.87, 123.14, 121.21, 120.66, 117.35, 113.59, 113.33, 112.70, 107.64. HRMS calcd for C₁₈H₁₃BrClN₄+ [M + H] +399.0007, found 399.0000. HPLC purity 96.15% (tR = 14.4 min).
3.1.43. 4-(5-Bromo-1H-indol-3-yl)-N-(4-chlorophenyl) pyrimidin-2-amine B6
Yield: 43%, pale yellow solid, mp 273.5–274.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H), 9.58 (s, 1H), 8.66 (s, 1H), 8.36 (s, 1H), 8.28 (d, J = 5.3 Hz, 1H), 7.77 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 8.6 Hz, 1H), 7.31 (d, J = 8.8 Hz, 2H), 7.28–7.23 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.15, 159.93, 157.03, 139.74, 135.88, 130.59, 128.42, 126.81, 124.87, 124.80, 124.33, 120.94, 114.03, 113.62, 113.15, 107.69. HRMS calcd for C₁₈H₁₃BrClN₄+, [M + H] +399.0007, found 399.0005. HPLC purity 95.20% (tR = 15.4 min).
3.1.44. 4-(5-Bromo-1H-indol-3-yl)-N-(2-bromophenyl) pyrimidin-2-amine B7
Yield: 37%, pale yellow solid, mp 177.8–178.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 8.71 (s, 1H), 8.39–8.36 (m, 2H), 8.28 (d, J = 5.3 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.29–7.23 (m, 2H), 7.15 (t, J = 7.7 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.63, 161.06, 157.65, 138.64, 136.18, 133.31, 130.88, 130.80, 128.71, 127.30, 126.53, 125.22, 124.86, 119.55, 114.27, 113.94, 113.52, 107.70. HRMS calcd for C₁₈H₁₃Br₂N₄+ [M + H] +442.9501, found 442.9504. HPLC purity 98.68% (tR = 15.9 min).
3.1.45. 4-(5-Bromo-1H-indol-3-yl)-N-(3-bromophenyl) pyrimidin-2-amine B8
Yield: 48%, pale yellow solid, mp 214.8–215.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.04 (s, 1H), 9.71 (s, 1H), 8.73 (d, J = 2.0 Hz, 1H), 8.40 (d, J = 3.9 Hz, 1H), 8.38 (s, 1H), 7.99 (s, 1H), 7.79–7.71 (m, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.34–7.30 (m, 2H), 7.02–6.96 (m, 1H).13C NMR (100 MHz, DMSO-d6) δ 161.82, 159.34, 156.53, 142.01, 135.46, 132.64, 130.15, 129.71, 126.38, 124.52, 123.85, 120.24, 117.80, 116.97, 113.59, 113.30, 112.70, 107.64. HRMS calcd for C₁₈H₁₃Br₂N₄+ [M + H] +442.9501, found 442.9498. HPLC purity 98.57% (tR = 15.1 min).
3.1.46. 4-(5-Bromo-1H-indol-3-yl)-N-(4-bromophenyl) pyrimidin-2-amine B9
Yield: 36%, pale yellow solid, mp 276.7–277.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.22 (s, 1H), 9.55 (s, 1H), 8.63 (s, 1H), 8.32 (s, 1H), 8.25 (d, J = 5.4 Hz, 1H), 7.69 (d, J = 8.5 Hz, 2H), 7.44–7.33 (m, 3H), 7.24–7.20 (m, 2H).13C NMR (100 MHz, DMSO-d6) δ 161.57, 159.28, 156.39, 139.56, 135.31, 130.68, 129.99, 126.18, 124.18, 123.70, 120.78, 113.48, 112.98, 112.42, 112.07, 107.12. HRMS calcd for C₁₈H₁₃Br₂N₄+, [M + H] + 442.9501, found 442.9502. HPLC purity 95.11% (tR = 15.7 min).
3.1.47. 4-(5-Bromo-1H-indol-3-yl)-N-(2-(trifluoromethyl) phenyl) pyrimidin-2-amine B10
Yield: 40%, pale yellow solid, mp 150.5–151.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.10 (s, 1H), 8.68 (s, 1H), 8.34 (s, 1H), 8.26 (d, J = 5.3 Hz, 1H), 8.21 (s, 1H), 7.78 (d, J = 8.2 Hz, 3H), 7.47 (d, J = 7.6 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.28–7.21 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.51, 161.89, 157.49, 138.26, 136.06, 133.55, 130.58, 130.00, 127.18, 126.75 (q, J = 5.3 Hz), 126.07, 125.74 (q, J = 272 Hz), 125.04, 124.70 (d, J = 5.7 Hz), 124.44, 114.15, 113.76, 113.29, 107.41. HRMS calcd for C₁₉H₁₃BrF₃N₄+, [M + H] +433.0270, found 433.0271. HPLC purity 95.40% (tR = 14.7 min).
3.1.48. 4-(5-Bromo-1H-indol-3-yl)-N-(3-(trifluoromethyl) phenyl) pyrimidin-2-amine B11
Yield: 45%, pale yellow solid, mp 171.8–172.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.82 (s, 1H), 8.72 (s, 1H), 8.41 (d, J = 5.2 Hz, 2H), 8.22 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 8.9 Hz, 1H), 7.35 (t, J = 4.9 Hz, 2H), 7.32-7.27 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.31, 159.80, 156.97, 141.74, 135.93, 130.58, 129.93 (q, J = 29.3 Hz), 129.00, 126.83, 125.79 (q, J = 272.7 Hz) 124.97, 124.27, 122.45, 117.22, 114.90, 114.04, 113.76, 113.12, 108.24. HRMS calcd for C₁₉H₁₃BrF₃N₄+, [M + H] +433.0270, found 433.0278. HPLC purity 99.39% (tR = 15.1 min).
3.1.49. 4-(5-Bromo-1H-indol-3-yl)-N-(4-(trifluoromethyl) phenyl) pyrimidin-2-amine B12
Yield: 34%, pale yellow solid, mp 279.5–280.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.91 (s, 1H), 8.76 (s, 1H), 8.43 (d, J = 3.0 Hz, 1H), 8.41 (d, J = 5.4 Hz, 1H), 8.02 (d, J = 8.5 Hz, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.45 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 5.4 Hz, 1H), 7.35–7.31 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.25, 159.72, 157.06, 144.51, 135.94, 130.76, 126.81, 125.87 (d, J = 4.1 Hz), 124.92, 124.32, 123.47 (q, J = 272.7 Hz), 120.95 (q, J = 31.1 Hz), 118.75, 114.08, 113.66, 113.09, 108.36. HRMS calcd for C₁₉H₁₃BrF₃N₄+ [M + H] +433.0270, found 433.0273. HPLC purity 97.41% (tR = 15.3 min).
3.1.50. 4-(5-Bromo-1H-indol-3-yl)-N-(o-tolyl) pyrimidin-2-amine B13
Yield: 39%, pale yellow solid, mp 187.6–188.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.65 (s, 1H), 8.39–8.06 (m, 3H), 7.38 (d, J = 57.8 Hz, 2H), 7.12 (d, J = 59.2 Hz, 5H), 2.20 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.58, 161.66, 157.61, 138.73, 136.16, 134.54, 132.91, 131.01, 130.56, 127.34, 126.65, 125.85, 125.21, 124.93, 114.22, 113.94, 113.72, 106.80, 18.80. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] +379.0553, found 379.0561. HPLC purity 95.08% (tR = 14.4 min).
3.1.51. 4-(5-Bromo-1H-indol-3-yl)-N-(m-tolyl) pyrimidin-2-amine B14
Yield: 52%, pale yellow solid, mp 141.5–142.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.00 (s, 1H), 9.42 (s, 1H), 8.76 (s, 1H), 8.39 (s, 1H), 8.34 (d, J = 5.3 Hz, 1H), 7.63 (d, J = 7.1 Hz, 2H), 7.44 (d, J = 8.6 Hz, 1H), 7.34–7.30 (m, 1H), 7.27 (d, J = 5.4 Hz, 1H), 7.25–7.19 (m, 1H), 6.80 (d, J = 7.5 Hz, 1H), 2.31 (s, 3H).13C NMR (100 MHz, DMSO-d6) δ 162.47, 160.58, 157.42, 141.10, 138.02, 136.28, 130.76, 128.84, 127.27, 125.28, 124.77, 122.49, 120.16, 116.97, 114.38, 114.00, 113.76, 107.69, 21.90. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] + 379.0553, found 379.0547. HPLC purity 97.81% (tR = 14.7 min).
3.1.52. 4-(5-Bromo-1H-indol-3-yl)-N-(p-tolyl) pyrimidin-2-amine B15
Yield: 41%, pale yellow solid, mp 227.8–228.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.36 (s, 1H), 8.74 (s, 1H), 8.34 (d, J = 30.0 Hz, 2H), 7.71–7.59 (m, 2H), 7.42 (s, 1H), 7.28 (d, J = 29.2 Hz, 2H), 7.21–7.11 (m, 2H), 2.28 (s, 3H) 13C NMR (100 MHz, DMSO-d6) δ 162.47, 160.73, 157.43, 138.52, 136.28, 130.78, 130.63, 129.46, 127.24, 125.22, 124.84, 120.24, 114.38, 113.96, 113.71, 107.41, 20.97. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] +379.0553, found 379.0552. HPLC purity 96.65% (tR = 14.8 min).
3.1.53. 4-(5-Bromo-1H-indol-3-yl)-N-(2-methoxyphenyl) pyrimidin-2-amine B16
Yield: 36%, pale yellow solid, mp 207.9–208.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.97 (s, 1H), 8.58 (s, 1H), 8.36 (s, 1H), 8.29 (d, J = 5.4 Hz, 1H), 8.10–8.05 (m, 2H), 7.40 (d, J = 8.5 Hz, 1H), 7.31–7.24 (m, 2H), 7.12–6.98 (m, 3H), 3.84 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.77, 160.89, 157.80, 150.46, 136.45, 131.11, 129.35, 127.42, 125.41, 124.83, 123.74, 122.18, 121.13, 114.61, 114.15, 113.79, 111.72, 107.96, 56.31. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0502. HPLC purity 98.24% (tR = 15.0 min).
3.1.54. 4-(5-Bromo-1H-indol-3-yl)-N-(3-methoxyphenyl) pyrimidin-2-amine B17
Yield: 49%, pale yellow solid, mp 208.7–209.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.44 (s, 1H), 8.73 (s, 1H), 8.34 (s, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.39 (d, J = 8.7 Hz, 3H), 7.27 (d, J = 8.6 Hz, 1H), 7.23 (d, J = 5.4 Hz, 1H), 7.19 (t, J = 8.1 Hz, 1H), 6.51 (d, J = 8.0 Hz, 1H), 3.68 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 161.69, 159.67, 159.22, 156.56, 141.57, 135.45, 129.97, 128.87, 126.40, 124.47, 124.01, 113.54, 113.26, 112.88, 111.32, 107.07, 106.16, 104.64, 54.45. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0498. HPLC purity 97.43% (tR = 13.6 min).
3.1.55. 4-(5-Bromo-1H-indol-3-yl)-N-(4-methoxyphenyl) pyrimidin-2-amine B18
Yield: 47%, pale yellow solid, mp 245.7–246.3 °C.1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.24 (s, 1H), 8.72 (s, 1H), 8.38 (d, J = 2.8 Hz, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.44 (d, J = 8.5 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H), 7.23 (d, J = 5.3 Hz, 1H), 6.96 (d, J = 8.6 Hz, 2H), 3.77 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.41, 160.86, 157.46, 154.73, 136.24, 134.11, 130.68, 127.25, 125.18, 124.84, 122.00, 114.32, 114.26, 113.91, 113.77, 107.09, 55.60. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0497. HPLC purity 95.61% (tR = 13.4 min).
3.1.56. 4-(5-Bromo-1H-indol-3-yl)-N-(2-isopropylphenyl) pyrimidin-2-amine B19
Yield: 41%, pale yellow solid, mp 110.5–111.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 8.70 (d, J = 3.8 Hz, 1H), 8.29 (s, 1H), 8.21 (d, J = 17.1 Hz, 2H), 7.41–7.30 (m, 3H), 7.29–7.20 (m, 3H), 7.13 (t, J = 4.6 Hz, 1H), 3.26 (q, J = 6.4, 5.7 Hz, 1H), 1.12 (d, J = 3.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.08, 162.02, 157.01, 144.29, 136.77, 135.53, 129.74, 127.55, 126.76, 125.92, 125.88, 125.61, 124.62, 124.55, 113.53, 113.27, 113.15, 105.81, 27.28, 23.10. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0863. HPLC purity 98.51% (tR = 15.1 min).
3.1.57. 4-(5-Bromo-1H-indol-3-yl)-N-(3-isopropylphenyl) pyrimidin-2-amine B20
Yield: 60%, pale yellow solid, mp 152.6–153.4 °C.1H NMR (400 MHz, DMSO-d6) δ 11.92 (s, 1H), 9.33 (s, 1H), 8.68 (d, J = 4.6 Hz, 1H), 8.32–8.23 (m, 2H), 7.70 (t, J = 6.1 Hz, 1H), 7.45 (d, J = 4.6 Hz, 1H), 7.39–7.34 (m, 1H), 7.25–7.15 (m, 3H), 6.77 (t, J = 6.2 Hz, 1H), 2.77 (q, J = 6.8 Hz, 1H), 1.13 (d, J = 6.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.33, 160.45, 157.22, 148.95, 140.94, 136.11, 130.56, 128.71, 127.07, 125.11, 124.58, 119.58, 117.56, 117.19, 114.21, 113.86, 113.58, 107.54, 33.85, 24.25. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0866. HPLC purity 98.50% (tR = 15.8 min).
3.1.58. 4-(5-Bromo-1H-indol-3-yl)-N-(4-isopropylphenyl) pyrimidin-2-amine B21
Yield: 41%, pale yellow solid, mp 218.7–219.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 9.26 (s, 1H), 8.67 (s, 1H), 8.27 (s, 1H), 8.19 (d, J = 5.3 Hz, 1H), 7.56 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.15–7.10 (m, 3H), 2.78–2.72 (m, 1H), 1.10 (d, J = 6.9 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 161.88, 160.12, 156.93, 141.25, 138.17, 135.72, 130.22, 126.67, 126.22, 124.66, 124.28, 119.63, 113.83, 113.38, 113.22, 106.85, 32.78, 23.99. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0870. HPLC purity 98.97% (tR = 16.3 min).
3.1.59. 4-(5-Bromo-1H-indol-3-yl)-N-phenylpyrimidin-2-amine B22
Yield: 46%, pale yellow solid, mp 197.6–198.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.46 (s, 1H), 8.77 (s, 1H), 8.37 (s, 1H), 8.33 (d, J = 5.3 Hz, 1H), 7.79 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.6 Hz, 1H), 7.33 (q, J = 9.4, 8.5 Hz, 3H), 7.27 (d, J = 5.3 Hz, 1H), 6.98 (t, J = 7.3 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.36, 160.38, 157.23, 140.98, 136.21, 130.81, 128.82, 127.06, 124.97, 124.55, 121.53, 119.60, 114.31, 113.78, 113.42, 107.56. HRMS calcd for C₁₈H₁₄BrN₄+ [M + H] +365.0396, found 365.0397. HPLC purity 98.81% (tR = 10.5 min).
3.1.60. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-difluorophenyl) pyrimidin-2-amine B23
Yield: 52%, pale yellow solid, mp 251.3–252.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.92 (s, 1H), 8.73 (d, J = 2.0 Hz, 1H), 8.52–8.24 (m, 2H), 7.63–7.58 (m, 2H), 7.46 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 5.4 Hz, 1H), 7.35–7.32 (m, 1H), 6.74 (t, J = 9.3 Hz, 1H).13C NMR (100 MHz, DMSO-d6) δ 163.86 (d, J = 16.1 Hz), 162.34, 161.47 (d, J = 16.0 Hz), 159.54, 156.97, 143.53 (d, J = 14.6 Hz), 135.92, 130.73, 126.78, 125.01, 124.17, 114.10, 113.79, 113.02, 108.62, 101.42 (d, J = 8.1 Hz). HRMS calcd for C₁₈H₁₂BrF₂N₄+ [M + H] + 401.0208, found 401.0201 HPLC purity 96.39% (tR = 14.7 min).
3.1.61. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-dichlorophenyl) pyrimidin-2-amine B24
Yield: 63%, pale yellow solid, mp 223.6–224.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.84 (s, 1H), 8.66 (d, J = 2.0 Hz, 1H), 8.39 (s, 1H), 8.37 (d, J = 3.0 Hz, 1H), 7.91 (d, J = 1.9 Hz, 2H), 7.41 (d, J = 8.6 Hz, 1H), 7.34 (d, J = 5.4 Hz, 1H), 7.31–7.27 (m, 1H), 7.06 (t, J = 1.8 Hz, 1H).13C NMR (100 MHz, DMSO-d6) δ 162.82, 159.84, 157.34, 143.86, 136.33, 134.40, 131.13, 127.23, 125.49, 124.60, 120.30, 117.01, 114.50, 114.27, 113.39, 109.14. HRMS calcd for C₁₈H₁₂BrCl₂N₄+ [M + H] +432.9617, found 432. 9611. HPLC purity 97.08% (tR = 16.7 min).
3.1.62. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-dibromophenyl) pyrimidin-2-amine B25
Yield: 48%, pale yellow solid, mp 193.4–193.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.91 (s, 1H), 9.68 (s, 1H), 8.54 (s, 1H), 8.29–8.23 (m, 2H), 7.98–7.94 (m, 2H), 7.30 (d, J = 8.5 Hz, 1H), 7.21 (d, J = 5.4 Hz, 1H), 7.18 (d, J = 4.9 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.28, 159.25, 156.82, 143.68, 135.80, 130.53, 126.69, 125.08, 125.00, 124.09, 122.23, 119.67, 113.99, 113.79, 112.85, 108.61. HRMS calcd for C₁₈H₁₄Br₃N₄+ [M + H] +522.8763, found 522.8587. HPLC purity 97.05% (tR = 17.3 min).
3.1.63. N-(3,5-bis(trifluoromethyl)phenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine B26
Yield: 59%, pale yellow solid, mp 220.9–221.3 °C.1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 10.17 (s, 1H), 8.68 (s, 1H), 8.55 (s, 2H), 8.45 (d, J = 5.4 Hz, 1H), 8.40 (s, 1H), 7.56 (s, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.31 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.54, 159.37, 156.90, 142.96, 135.93, 130.93 (d, J = 34.2 Hz), 130.30 (d, J = 32.3 Hz), 126.79, 125.08, 124.94 (q, J = 273.7 Hz),124.12, 118.04, 114.09, 113.84, 113.17, 112.88, 109.08. HRMS calcd for C₂₀H₁₂BrF₆N₄+, [M + H] +501.0144, found 501.0138. HPLC purity 97.89% (tR = 16.1 min).
3.1.64. 4-(5-Bromo-1H-indol-3-yl)-N-(3, 5-dimethylphenyl) pyrimidin-2-amine B27
Yield: 42%, pale yellow solid, mp 184.6–185.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.94 (s, 1H), 9.29 (s, 1H), 8.66 (s, 1H), 8.30 (d, J = 16.5 Hz, 2H), 7.38 (s, 3H), 7.23 (d, J = 30.4 Hz, 2H), 6.55 (s, 1H), 2.21 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 161.84, 159.96, 156.81, 140.44, 137.19, 135.67, 130.06, 126.70, 124.71, 124.15, 122.78, 116.73, 113.77, 113.37, 113.20, 107.03, 21.21. HRMS calcd for C₂₀H₁₈BrN₄+ [M + H] +393.0709, found 393.0712 HPLC purity 97.05% (tR = 15.3 min).
3.1.65. 4-(5-Bromo-1H-indol-3-yl)-N-(3, 5-dimethoxyphenyl) pyrimidin-2-amine B28
Yield: 48%, pale yellow solid, mp 255.6–256.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.40 (s, 1H), 8.71 (s, 1H), 8.33 (d, J = 2.5 Hz, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.29–7.25 (m, 1H), 7.23 (d, J = 5.4 Hz, 1H), 7.04 (d, J = 2.2 Hz, 2H), 6.09 (s, 1H), 3.67 (s, 6H).13C NMR (100 MHz, DMSO-d6) δ 162.53, 160.96, 160.46, 157.37, 142.89, 136.29, 130.76, 127.25, 125.37, 124.87, 114.39, 114.16, 113.70, 108.04, 97.92, 93.74, 55.40. HRMS calcd for C₂₀H₁₈BrN₄O₂+ [M + H] +425.0608, found 425.0601. HPLC purity 96.76% (tR = 13.3 min).
3.1.66. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-difluorophenyl) pyrimidin-2-amine B29
Yield: 40%, pale yellow solid, mp 263.3–264.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.87 (s, 1H), 8.91 (s, 1H), 8.23 (d, J = 40.8 Hz, 3H), 7.53–7.01 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 162.85, 161.48, 160.29 (d, J = 5.2 Hz), 157.83 (d, J = 5.1 Hz), 157.57, 136.15, 130.69, 127.36, 125.23, 124.72, 114.23, 113.91, 113.41, 112.50 (d, J = 5.9 Hz), 112.32 (d, J = 5.2 Hz), 107.43. HRMS calcd for C₁₈H₁₂BrF₂N₄+, [M + H] +401.0208, found 401.0201. HPLC purity 97.49% (tR = 12.4 min).
3.1.67. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-dimethylphenyl) pyrimidin-2-amine B30
Yield: 34%, pale yellow solid, mp 228.5–229.4 °C.1H NMR (400 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.54 (s, 1H), 8.24 (d, J = 38.1 Hz, 2H), 7.82 (s, 1H), 7.33 (s, 1H), 7.25–6.98 (m, 5H), 2.20 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.27, 161.60, 157.27, 148.03, 137.14, 135.62, 129.80, 128.05, 126.99, 126.08, 124.73, 124.44, 113.64, 113.40, 113.32, 105.52, 18.58. HRMS calcd for C₂₀H₁₈BrN₄+ [M + H] +393.0709, found 393.0715. HPLC purity 96.56% (tR = 14.2 min).
3.1.68. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-dimethoxyphenyl) pyrimidin-2-amine B31
Yield: 62%, pale yellow solid, mp 244.3–245.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 8.15 (s, 1H), 8.01 (d, J = 5.3 Hz, 1H), 7.92 (s, 1H), 7.25 (d, J = 8.5 Hz, 1H), 7.15–7.09 (m, 2H), 6.95 (d, J = 5.3 Hz, 1H), 6.65 (d, J = 8.4 Hz, 2H), 3.60 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.50, 162.37, 157.25, 156.70, 136.12, 130.09, 127.49, 127.38, 125.20, 125.04, 117.15, 114.14, 113.93, 113.82, 106.08, 105.16, 56.00. HRMS calcd for C₂₀H₁₈BrN₄O₂+ [M + H] +425.0608, found 425.0600. HPLC purity 97.85% (tR = 12.2 min).
3.2. Biological Evaluation
3.2.1. General Procedure
The hepatocyte cell line HepG2 cells obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) were maintained in high-glucose DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in an atmosphere of 5% CO2 at 37 °C.
3.2.2. MTT Assay for Cell Viability
Into a 96-well plate, 1 × 103 HepG2 cells per well were seeded. After 24 h, stock solutions of test compounds in DMSO were diluted in DMEM to ≤0.1% final DMSO concentration. Cells were treated with various concentrations (2.5–25 µM) of tested compounds for 48 h, and the MTT solution (0.5 mg/mL) was added. After 4 h of incubation at 37 °C for MTT-formazan formation, the supernatant was discarded and 100 µL of DMSO was applied into each well to dissolve formazan crystals. Absorbance at 490 nm was determined spectrophotometrically by using a microplate reader (Epoch, BioTek Instruments, Inc., Winooski, VT, USA).
3.2.3. Inhibition of GSK-3β
The activity of recombinant GSK-3β (Invitrogen) was measured in a Z-lyte assay of Invitrogen. In this assay, 100 nL of the compound solution in DMSO was transferred to a 384-well assay plate (the compound got threefold serial dilution, and the top concentration of the compound was 30 μM in the final reaction mixture). Then 5 μL of an enzyme (2 nM) and Ser/Thr 9 peptide (4 μM) mixture was added. After the enzyme and peptide pre-incubation with the compound for 30 min at 23 °C, 5 μL of ATP (12 μM) solution was added. The plate was incubated for 60 min at 23 °C. The reaction was stopped with 5 μL of development reagent. Fluorescence signals were detected with Envision (PerkinElmer, America) after 30 min of incubation, and the emission ratio (RFU 460 nm/RFU 535 nm) was used to analyze the data. The relative IC50 was reported using four-parameter logistic fit of IDBS Xlfit software. The IC50 values were calculated using the following equation:
where
Emission ratio = Coumarin/Fluorescein ratio of sample wells
C100% = Average coumarin emission signal of the 100% phos. control
C0% = Average coumarin emission signal of the 0% phos. control
F100% = Average fluorescein emission signal of the 100% phos. control
F0% = Average fluorescein emission signal of the 0% phos. control
3.2.4. Cell Glucose Uptake Assay
Into a 96-well plate, 5 × 103 HepG2 cells per well were seeded. After 48 h, phenol red-free DMEM (Gibco) with 1% FBS was transferred to deprive the cells overnight, and then the tested compounds dissolved in a glucose-HEPES buffer (glucose concentration was 100 µg/mL) at a concentration of 5 µM were added to the culture system. Three hours later, the culture supernatants were collected and the glucose concentrations were detected using the Glucose (GO) Assay Kit (Sigma-Aldrich Corp.). Compared with the original glucose concentration of the buffer, the relative glucose uptake was calculated.
3.2.5. Assay Molecular Modeling
The modeling study was carried out using Schrödinger Maestro 11.4. GSK-3β protein models (PDB entry 6B8J) were minimized by Schrödinger BioLuminate. The docking study was performed using Glide. The parameters chosen were the default ones, without any constraints. The molecular docking result was generated using PyMol (http://pymol.sourceforge.net/ accessed on 10 January 2021).
4. Conclusions
In summary, a series of 2-aminopyrimidine- and 5-aminophenyl-modified marine-originated meridianin C analogues were designed and synthesized by a structural-based optimization strategy. Among the analogues, B29 and B30 with 2-aminopyrimidine substitutions could significantly increase hepatocyte glucose uptake in HepG2 cells, with good inhibitory activity against GSK-3β, showing potential for the treatment of diabetes. They represent potential lead compounds for further development of GSK-3β and anti-diabetic drugs.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/19/3/149/s1: NMR, HRMS, HPLC purity.
Author Contributions
F.C. and W.Z. conceived and designed the experiments; S.H. and W.Z. performed the experiments. S.H. and W.Z. collected and analyzed the data; S.H., C.Z., and F.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (22077018) and Fudan University (JIH1615060).
Acknowledgments
We thank Ningxia Medical University for providing us the computational sources. This work was supported by National Key R&D Program of China (2017YFA0506000).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
GSK-3β, glycogen synthase kinase 3β; GCS, glycogen synthase; HepG2, human hepatocellular carcinoma; FBS, fetal bovine serum; PBS, phosphate buffered saline; HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance; DMF, dimethylformamide; DMAP, 4-(dimethylamino)-pyridine; DIPEA, N,N-diisopropylethylamine; NIS, N-iodosuccinimide; TsCl, tosyl chloride; ADMET, (absorption, distribution, metabolism, excretion, and toxicity); TDZD-8, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione.
References
- Disbetes. Available online: https://www.who.int/health-topics/diabetes (accessed on 18 January 2021).
- Karuranga, S.; Malanda, B.; Saeedi, P.; Salpea, P. IDF Diabetes Atlas, 9th ed.; International Diabetes Foundation: Brussels, Belgium, 2019; pp. 1–168. [Google Scholar]
- Gangopadhyay, K.; Singh, P. Consensus statement on dose modifications of antidiabetic agents in patients with hepatic impairment. Indian J. Endocr. Metab. 2017, 21, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708–111100. [Google Scholar] [CrossRef]
- Feingold, K.R.; Anawalt, B.; Boyce, A. (Eds.) Oral and Injectable (Non-Insulin) Pharmacological Agents for Type 2 Diabetes; MDText. com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Palomo, V.; Perez, D.I.; Roca, C.; Anderson, C.; Rodriguez-Muela, N.; Perez, C.; Morales-Garcia, J.A.; Reyes, J.A.; Campillo, N.E.; Perez-Castillo, A.M.; et al. Subtly Modulating Glycogen Synthase Kinase 3 beta: Allosteric Inhibitor Development and Their Potential for the Treatment of Chronic Diseases. J. Med. Chem. 2017, 60, 4983–5001. [Google Scholar] [CrossRef]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef]
- Bharate, S.B.; Sawant, S.D.; Singh, P.P.; Vishwakarma, R.A. Kinase inhibitors of marine origin. Chem. Rev. 2013, 113, 6761–6815. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, S.; Sinha, B.N.; Tajne, S.; Jagarlapudi, S.A. Fragment and knowledge-based design of selective GSK-3beta inhibitors using virtual screening models. Eur. J. Med. Chem. 2009, 44, 2361–2371. [Google Scholar] [CrossRef]
- Park, H.; Shin, Y.; Kim, J.; Hong, S. Application of Fragment-Based de Novo Design to the Discovery of Selective Picomolar Inhibitors of Glycogen Synthase Kinase-3 Beta. J. Med. Chem. 2016, 59, 9018–9034. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.X.; Boucheron, J.A.; Dickerson, S.H.; Griffin, R.J.; Preugschat, F.; Thomson, S.A.; Wang, T.Y.; Zhou, H.Q. N-Phenyl-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amines as potent and selective inhibitors of glycogen synthase kinase 3 with good cellular efficacy. J.Med. Chem. 2004, 47, 4716–4730. [Google Scholar] [CrossRef] [PubMed]
- Wagman, A.S.; Boyce, R.S.; Brown, S.P.; Fang, E.; Goff, D.; Jansen, J.M.; Le, V.P.; Levine, B.H.; Ng, S.C.; Ni, Z.J.; et al. Synthesis, Binding Mode, and Antihyperglycemic Activity of Potent and Selective (5-Imidazol-2-yl-4-phenylpyrimidin-2-yl)[2-(2-pyridylamino)ethyl]amine Inhibitors of Glycogen Synthase Kinase 3. J. Med. Chem. 2017, 60, 8482–8514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Zhao, T.T.; Gui, D.K.; Gao, C.L.; Gu, J.L.; Gan, W.J.; Huang, W.; Xu, Y.; Zhou, H.; Chen, W.N.; et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food. Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Cline, G.W.; Johnson, K.; Regittnig, W.; Perret, P.; Tozzo, E.; Xiao, L.; Damico, C.; Shulman, G.I. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes 2002, 51, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tantray, M.A.; Alam, M.S.; Hamid, H. Natural and synthetic bioactive inhibitors of glycogen synthase kinase. Eur. J. Med. Chem. 2017, 125, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Walz, A.; Ugolkov, A.; Chandra, S.; Kozikowski, A.; Carneiro, B.A.; Halloran, T.V.; Giles, F.J.; Billadeau, D.D.; Mazar, A.P. Molecular Pathways: Revisiting Glycogen Synthase Kinase-3β as a Target for the Treatment of Cancer. Clin. Cancer Res. 2017, 23, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Seldes, A.M.; Rodriguez Brasco, M.F.; Hernandez Franco, L.; Palermo, J.A. Identification of two meridianins from the crude extract of the tunicate Aplidium meridianum by tandem mass spectrometry. Nat. Prod. Res. 2007, 21, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Gompel, M.; Leost, M.; De Kier Joffe, E.B.; Puricelli, L.; Franco, L.H.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Yadav, R.R.; Battula, S.; Vishwakarma, R.A. Meridianins: Marine-derived potent kinase inhibitors. Mini Rev. Med. Chem. 2012, 12, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Yadav, R.R.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Khan, I.A.; Vishwakarma, R.A. Meridianin G and its analogs as antimalarial agents. Med. Chem. Commun. 2013, 4, 1042–1048. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Gao, Y.; Lu, W.; Huang, K.; Ye, D.; Li, X.; Chu, Y. Novel benzothiazinones (BTOs) as allosteric modulator or substrate competitive inhibitor of glycogen synthase kinase 3β (GSK-3β) with cellular activity of promoting glucose uptake. Bioorg. Med. Chem. Lett. 2014, 24, 5639–5643. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).