Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. CPPs as Drug Delivery Carriers
2.2. Identification of CPPs and Analysis of Their Safety and Physiochemical Properties
2.3. Blood-brain Barrier Penetrating Peptides
2.4. CPPs Containing Nuclear Localization Signals
2.5. CPPs as Mitochondrial Penetrating Peptides
2.6. Cytokine Inducing Peptides
2.6.1. IL-2 Inducing Peptides
2.6.2. IL-4 Inducing Peptides
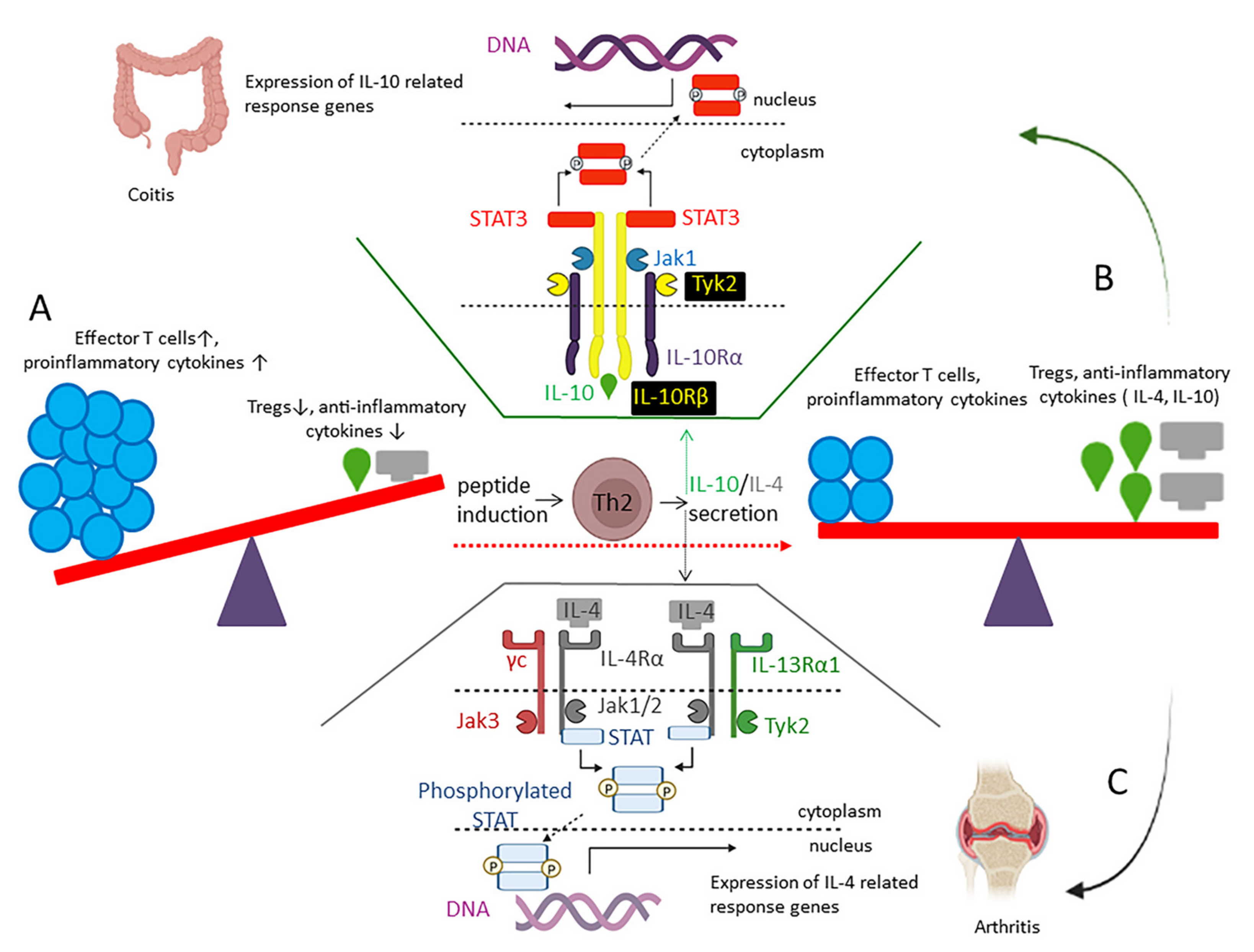
2.6.3. IL-10 Inducing Peptides
2.6.4. IFN-γ Inducing Peptides
2.6.5. TNF-α Inducing Peptides
2.7. Immunoadjuvant Peptides
2.8. Antimicrobial Peptides
2.8.1. Immunomodulatory AMPs
2.8.2. Antibacterial Peptides
2.8.3. Antitubercular Peptides
2.8.4. Antifungal Peptides
2.8.5. Antiviral Peptides
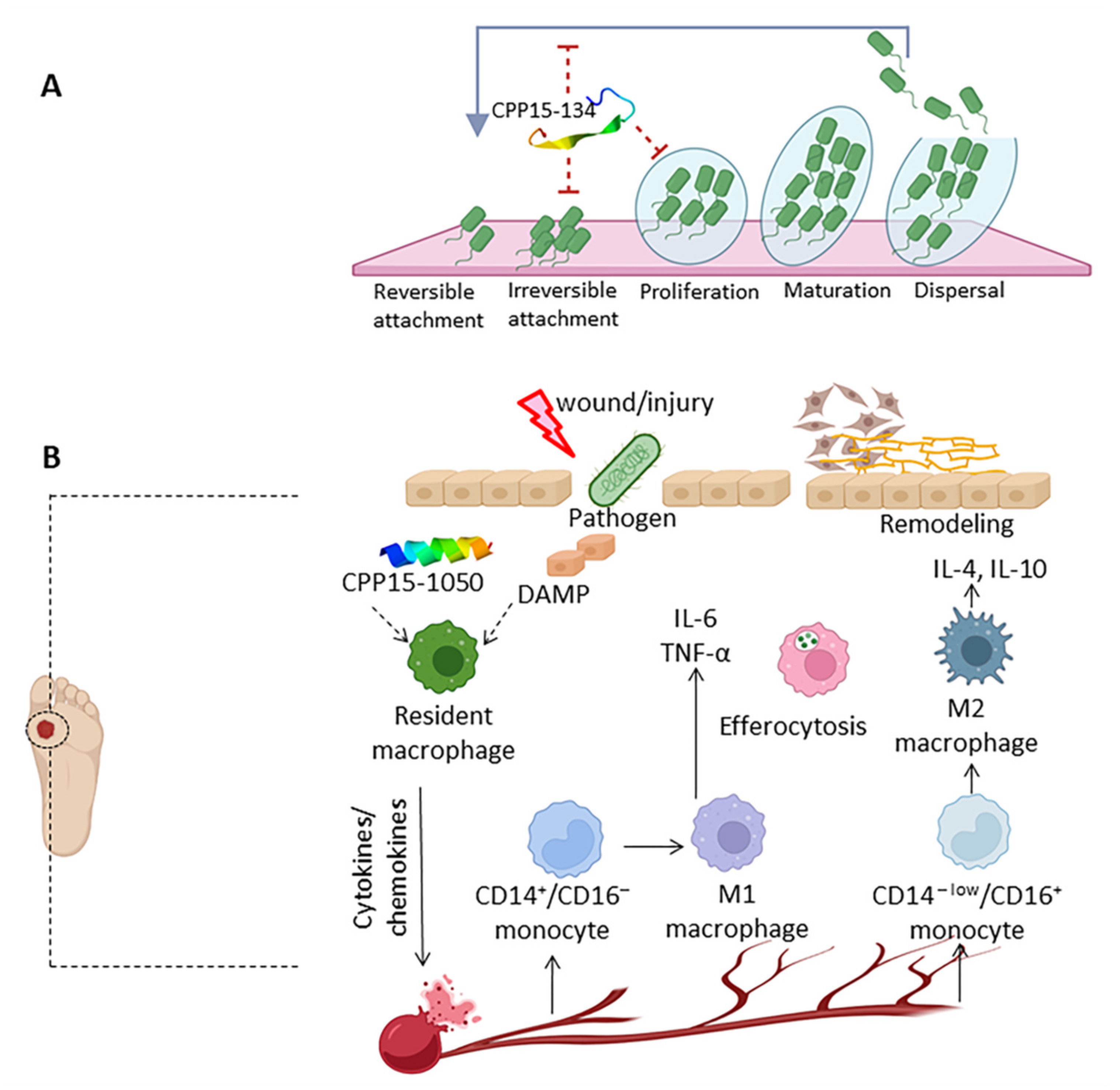
2.8.6. Antibiofilm Peptides
2.8.7. Wound Healing Peptides
2.9. Anticancer Peptides
2.9.1. Apoptosis and Necrosis Inducing Peptides
2.9.2. Antiangiogenic Peptides
2.9.3. Immunomodulatory ACPs
2.9.4. Tumor Targeting Peptides
2.9.5. RGD-Containing Peptides
2.9.6. NRP-Targeting Peptides
2.10. Antioxidant Peptides
3. Materials and Methods
3.1. Retrieval of Cryptic CPPs from Toxins and Analysis of Safety and Physiochemical Characteristics
3.2. Identification of Cytokine-Inducing Peptides
3.3. Identification of Antimicrobial Peptides
3.4. Identification of Anticancer and Tumor-Homing Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trinidad-Calderón, P.A.; Varela-Chinchilla, C.D.; García-Lara, S. Natural Peptides Inducing Cancer Cell Death: Mechanisms and Properties of Specific Candidates for Cancer Therapeutics. Molecules 2021, 26, 7453. [Google Scholar] [PubMed]
- Sadeghian, I.; Khalvati, B.; Ghasemi, Y.; Hemmati, S. TAT-Mediated Intracellular Delivery of Carboxypeptidase G2 Protects against Methotrexate-Induced Cell Death in HepG2 Cells. Toxicol. Appl. Pharmacol. 2018, 346, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Behzadipour, Y.; Hemmati, S. Considerations on the Rational Design of Covalently Conjugated Cell-Penetrating Peptides (CPPs) for Intracellular Delivery of Proteins: A Guide to CPP Selection Using Glucarpidase as the Model Cargo Molecule. Molecules 2019, 24, 4318. [Google Scholar]
- Zorko, M.; Langel, Ü. Cell-Penetrating Peptides. In Cell Penetrating Peptides. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2022; Volume 2383, pp. 3–32. [Google Scholar]
- Grau, M.; Walker, P.R.; Derouazi, M. Mechanistic Insights into the Efficacy of Cell Penetrating Peptide-Based Cancer Vaccines. Cell. Mol. Life Sci. 2018, 75, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.; Ambite, I.; Wan, M.L.Y.; Tran, T.H.; Wullt, B.; Svanborg, C. Immunomodulation Therapy Offers New Molecular Strategies to Treat UTI. Nat. Rev. Urol. 2022, 19, 419–437. [Google Scholar]
- Van Harten, R.M.; Van Woudenbergh, E.; Van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63. [Google Scholar]
- Howl, J.; Jones, S. A New Biology of Cell Penetrating Peptides. Pept. Sci. 2021, 113, e24154. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, F.S.; Choudhary, I.M. Snake Venom: From Deadly Toxins to Life-Saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion Venom Components as Potential Candidates for Drug Development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef]
- Radis-Baptista, G. Cell-Penetrating Peptides Derived from Animal Venoms and Toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef]
- De Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar]
- Ponnappan, N.; Budagavi, D.P.; Yadav, B.K.; Chugh, A. Membrane-Active Peptides from Marine Organisms—Antimicrobials, Cell-Penetrating Peptides and Peptide Toxins: Applications and Prospects. Probiotics Antimicrob. Proteins 2015, 7, 75–89. [Google Scholar] [PubMed]
- Yu, S.; Li, Y.; Chen, J.; Zhang, Y.; Tao, X.; Dai, Q.; Wang, Y.; Li, S.; Dong, M. TAT-Modified ω-Conotoxin MVIIA for Crossing the Blood-Brain Barrier. Mar. Drugs 2019, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Manda, P.; Kushwaha, A.S.; Kundu, S.; Shivakumar, H.; Jo, S.B.; Murthy, S.N. Delivery of Ziconotide to Cerebrospinal Fluid via Intranasal Pathway for the Treatment of Chronic Pain. J. Control. Release 2016, 224, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Muth, A. Polypharmacology: The Science of Multi-Targeting Molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef] [PubMed]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Devel. Ther. 2020, 14, 3235. [Google Scholar] [CrossRef]
- Chaudhary, K.; Kumar, R.; Singh, S.; Tuknait, A.; Gautam, A.; Mathur, D.; Anand, P.; Varshney, G.C.; Raghava, G.P.S. A Web Server and Mobile App for Computing Hemolytic Potency of Peptides. Sci. Rep. 2016, 6, 22843. [Google Scholar] [CrossRef]
- Behzadipour, Y.; Sadeghian, I.; Ghaffarian Bahraman, A.; Hemmati, S. Introducing a Delivery System for Melanogenesis Inhibition in Melanoma B16F10 Cells Mediated by the Conjugation of Tyrosine Ammonia-lyase and a TAT-penetrating Peptide. Biotechnol. Prog. 2021, 37, e3071. [Google Scholar] [CrossRef]
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [PubMed]
- Zapadka, K.L.; Becher, F.J.; Gomes dos Santos, A.; Jackson, S.E. Factors Affecting the Physical Stability (Aggregation) of Peptide Therapeutics. Interface Focus 2017, 7, 20170030. [Google Scholar] [CrossRef]
- Nabipour, I. The Venomous Animals of the Persian Gulf; Bushehr University of Medical Sciences Press: Bushehr, Iran, 2012; Volume 32. [Google Scholar]
- Mirshamsi, M.R.; Omranipour, R.; Vazirizadeh, A.; Fakhri, A.; Zangeneh, F.; Mohebbi, G.H.; Seyedian, R.; Pourahmad, J. Persian Gulf Jellyfish (Cassiopea Andromeda) Venom Fractions Induce Selective Injury and Cytochrome c Release in Mitochondria Obtained from Breast Adenocarcinoma Patients. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 277. [Google Scholar] [PubMed]
- Jafari, H.; Honari, H.; Zargan, J.; Jahromi, S.T. Identification and Hemolytic Activity of Jellyfish (Rhopilema Sp., Scyphozoa: Rhizostomeae) Venom from the Persian Gulf and Oman Sea. Biodiversitas J. Biol. Divers. 2019, 20, 1228–1232. [Google Scholar] [CrossRef]
- Jafari, H.; Zargan, J.; Tamadoni Jahromi, S. Extraction the Venom of Rhopilema Nomadica from the Persian Gulf Coast and the Investigation of Its Hemolytical Activity. Yafteh 2019, 21, 86–95. [Google Scholar]
- Moghadasi, Z.; Jamili, S.; Shahbazadeh, D.; Bagheri, K.P. Toxicity and Potential Pharmacological Activities in the Persian Gulf Venomous Sea Anemone, Stichodactyla Haddoni. Iran. J. Pharm. Res. IJPR 2018, 17, 940. [Google Scholar]
- Dabbagh, A.-R.; Keshavarz, M.; Mohammadikia, D.; Afkhami, M.; Nateghi, S.A. Holothuria Scabra (Holothuroidea: Aspidochirotida): First Record of a Highly Valued Sea Cucumber, in the Persian Gulf, Iran. Mar. Biodivers. Rec. 2012, 5. [Google Scholar] [CrossRef]
- Memar, B.; Jamili, S.; Shahbazzadeh, D.; Bagheri, K.P. The First Report on Coagulation and Phospholipase A2 Activities of Persian Gulf Lionfish, Pterois Russelli, an Iranian Venomous Fish. Toxicon 2016, 113, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Eagderi, S.; Fricke, R.; Esmaeili, H.R.; Jalili, P. Annotated Checklist of the Fishes of the Persian Gulf: Diversity and Conservation Status. Iran. J. Ichthyol. 2019, 6, 1–171. [Google Scholar]
- Aghvami, M.; Zarei, M.H.; Mirshamsi, M.R.; Pourahmad, J. Selective Toxicity of Persian Gulf Stonefish (Pseudosynanceia Melanostigma) Venom on Human Acute Lymphocytic Leukemia B Lymphocytes. Trends Pept. Protein Sci. 2017, 1, 56–60. [Google Scholar]
- Ghafari, S.M.; Jamili, S.; Bagheri, K.P.; Ardakani, E.M.; Fatemi, M.R.; Shahbazzadeh, F.; Shahbazzadeh, D. The First Report on Some Toxic Effects of Green Scat, Scatophagus Argus an Iranian Persian Gulf Venomous Fish. Toxicon 2013, 66, 82–87. [Google Scholar] [CrossRef]
- Tu, A.T.; Hong, B.-S. Purification and Chemical Studies of a Toxin from the Venom of Lapemis Hardwickii (Hardwick’s Sea Snake). J. Biol. Chem. 1971, 246, 2772–2779. [Google Scholar] [CrossRef]
- Tamiya, N.; Maeda, N.; Cogger, H. Neurotoxins from the Venoms of the Sea Snakes Hydrophis Ornatus and Hydrophis Lapemoides. Biochem. J. 1983, 213, 31–38. [Google Scholar] [PubMed]
- Mosafer Khorjestan, S.; Abtahi, B.; Ranei Siadat, S.O.; Motevalli, M.; Rezadoost, H.; Ghezellou, P.; Ghassempour, A. Analysis of Annulated Sea Snake Venom, Hydrophis Cyanocinctus, Using Liquid Chromatography and MALDI-TOF/TOF. Curr. Proteom. 2015, 12, 45–55. [Google Scholar]
- Calvete, J.J.; Ghezellou, P.; Paiva, O.; Matainaho, T.; Ghassempour, A.; Goudarzi, H.; Kraus, F.; Sanz, L.; Williams, D.J. Snake Venomics of Two Poorly Known Hydrophiinae: Comparative Proteomics of the Venoms of Terrestrial Toxicocalamus Longissimus and Marine Hydrophis Cyanocinctus. J. Proteom. 2012, 75, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Sajjadi, M.M.; Rajaian, H.; Sajedianfard, J.; Parto, P. Study of Patient’s Injuries by Stingrays, Lethal Activity Determination and Cardiac Effects Induced by Himantura Gerrardi Venom. Toxicon 2009, 54, 881–886. [Google Scholar]
- Dehghan, H.; Sajjadi, M.M.; Parto, P.; Rajaian, H.; Jalaei, J. Histological Characterization of Venom Secretory Cells in the Stinger of 3 Stingrays (Dasyatidae) Species: Dasyatis Bennetti, Himantura Walga, Himantura Gerrardi, in Northern Water of Persian Gulf and Oman Sea. JMST 2014, 12, 33–41. [Google Scholar]
- Maleki, L.; Malek, M.; Palm, H.W. Two New Species of Acanthobothrium (Tetraphyllidea: Onchobothriidae) from Pastinachus Cf. Sephen (Myliobatiformes: Dasyatidae) from the Persian Gulf and Gulf of Oman. Folia Parasitol. (Praha) 2013, 60, 448. [Google Scholar] [CrossRef]
- Vossoughi, G.; Vosoughi, A. Study of Batoid Fishes in Northern Part of Hormoz Strait, with Emphasis on Some Species New to the Persian Gulf and Sea of Oman. Indian J. Fish. 1999, 46, 301–306. [Google Scholar]
- Kouchaksaraee, R.M.; Li, F.; Nazemi, M.; Farimani, M.M.; Tasdemir, D. Molecular Networking-Guided Isolation of New Etzionin-Type Diketopiperazine Hydroxamates from the Persian Gulf Sponge Cliona Celata. Mar. Drugs 2021, 19, 439. [Google Scholar] [CrossRef]
- Nazemi, M.; Gilkolai, F.R.; Lakzaei, F.; Pishvarzad, F.; Ahmadzadeh, O. First Record on the Distribution and Abundance of Three Sponge Species from Hormoz Island, Persian Gulf-Iran. Biol. Forum Int. J. 2015, 7, 72–78. [Google Scholar]
- Tabaraki, N.; Shahbazzadeh, D.; Moradi, A.M.; Vosughi, G.; Mostafavi, P.G. Analgesic Effect of Persian Gulf Conus Textile Venom. Iran. J. Basic Med. Sci. 2014, 17, 793. [Google Scholar]
- Rajabi, H.; Zolgharnein, H.; Ronagh, M.T.; Savari, A.; Amuzandeh, A.; Jomehzadeh, N. Pharmacological Studies (Analgesic and Hemolytic) on the Cone Snail Venom Conus Coronatus Gmelin, 1791. Int J Aquat. Sci. 2018, 9, 106–111. [Google Scholar]
- Bosch, D.T.; Dance, S.P.; Moolenbeek, R.G.; Oliver, P.G. Seashells of Eastern Arabia; Motivate Publishing: Abu Dhabi, United Arab Emirates, 1995; ISBN 1-873544-64-2. [Google Scholar]
- Dehghani, H.; Fazeli, M.; Rajaeian, H.; Hosseinzadeh, S.; Ashrafi, M. Identification of “Alpha-Conotoxin-like” Peptide in Conus Pennaceus Born, 1778, Venom. J. Aquat. Ecol. 2018, 8, 105–114. [Google Scholar]
- Khobdel, M.; Ataghlipour, M.R.; Dakhteh, S.M.; Hassani, M.A. Identification of Poisonous and Venomous Marine Animals in the Inter-Tidal Zone and Near-Coastal Waters of the Persian Gulf. J. Mar. Med. 2020, 2, 99–107. [Google Scholar]
- Gao, X.; Hong, S.; Liu, Z.; Yue, T.; Dobnikar, J.; Zhang, X. Membrane Potential Drives Direct Translocation of Cell-Penetrating Peptides. Nanoscale 2019, 11, 1949–1958. [Google Scholar] [PubMed]
- Robison, A.D.; Sun, S.; Poyton, M.F.; Johnson, G.A.; Pellois, J.-P.; Jungwirth, P.; Vazdar, M.; Cremer, P.S. Polyarginine Interacts More Strongly and Cooperatively than Polylysine with Phospholipid Bilayers. J. Phys. Chem. B 2016, 120, 9287–9296. [Google Scholar] [PubMed]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A Web Server to Screen Sequences with Specific α-Helical Properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Behzadipour, Y.; Haddad, M. Decoding the Proteome of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Cell-Penetrating Peptides Involved in Pathogenesis or Applicable as Drug Delivery Vectors. Infect. Genet. Evol. 2020, 85, 104474. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Nadal-Bufí, F.; Henriques, S.T. How to Overcome Endosomal Entrapment of Cell-Penetrating Peptides to Release the Therapeutic Potential of Peptides? Pept. Sci. 2020, 112, e24168. [Google Scholar]
- Kanjilal, P.; Dutta, K.; Thayumanavan, S. Thiol-Disulfide Exchange as a Route for Endosomal Escape of Polymeric Nanoparticles. Angew. Chem. Int. Ed. 2022, 61, e202209227. [Google Scholar] [CrossRef]
- Li, T.; Gao, W.; Liang, J.; Zha, M.; Chen, Y.; Zhao, Y.; Wu, C. Biscysteine-Bearing Peptide Probes to Reveal Extracellular Thiol–Disulfide Exchange Reactions Promoting Cellular Uptake. Anal. Chem. 2017, 89, 8501–8508. [Google Scholar] [PubMed]
- Mathur, D.; Singh, S.; Mehta, A.; Agrawal, P.; Raghava, G.P. In Silico Approaches for Predicting the Half-Life of Natural and Modified Peptides in Blood. PLoS ONE 2018, 13, e0196829. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.C.; Cheon, D.H.; Lee, Y. Challenge to Overcome Current Limitations of Cell-Penetrating Peptides. Biochim. Biophys. Acta BBA-Proteins Proteom. 2021, 1869, 140604. [Google Scholar] [CrossRef] [PubMed]
- Frøslev, P.; Franzyk, H.; Ozgür, B.; Brodin, B.; Kristensen, M. Highly Cationic Cell-Penetrating Peptides Affect the Barrier Integrity and Facilitates Mannitol Permeation in a Human Stem Cell-Based Blood-Brain Barrier Model. Eur. J. Pharm. Sci. 2022, 168, 106054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain Penetrating Peptides and Peptide–Drug Conjugates to Overcome the Blood–Brain Barrier and Target CNS Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef]
- Beck, M.; Hurt, E. The Nuclear Pore Complex: Understanding Its Function through Structural Insight. Nat. Rev. Mol. Cell Biol. 2017, 18, 73–89. [Google Scholar] [CrossRef]
- Langel, Ü. Targeting Strategies. In CPP, Cell-Penetrating Peptides; Langel, Ü., Ed.; Springer: Singapore, 2019; pp. 195–263. ISBN 9789811387470. [Google Scholar]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of Nuclear Localization Signals and Mechanisms of Protein Import into the Nucleus. Cell Commun. Signal. 2021, 19, 1–10. [Google Scholar]
- Cerrato, C.P.; Langel, Ü. An Update on Cell-Penetrating Peptides with Intracellular Organelle Targeting. Expert Opin. Drug Deliv. 2022, 19, 133–146. [Google Scholar] [CrossRef]
- Bhunia, D.; Mondal, P.; Das, G.; Saha, A.; Sengupta, P.; Jana, J.; Mohapatra, S.; Chatterjee, S.; Ghosh, S. Spatial Position Regulates Power of Tryptophan: Discovery of a Major-Groove-Specific Nuclear-Localizing, Cell-Penetrating Tetrapeptide. J. Am. Chem. Soc. 2018, 140, 1697–1714. [Google Scholar]
- Anoar, S.; Woodling, N.S.; Niccoli, T. Mitochondria Dysfunction in Frontotemporal Dementia/Amyotrophic Lateral Sclerosis: Lessons From Drosophila Models. Front. Neurosci. 2021, 15, 786076. [Google Scholar]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [PubMed]
- Chatterjee, A.; Ansar, S.; Gopal, D.; Vetrivel, U.; George, R.; Narayanan, J. Elucidating the Therapeutic Potential of Cell-Penetrating Peptides in Human Tenon Fibroblast Cells. ACS Omega 2022, 7, 16536–16546. [Google Scholar] [PubMed]
- Koo, J.-H.; Kim, G.-R.; Nam, K.-H.; Choi, J.-M. Unleashing Cell-Penetrating Peptide Applications for Immunotherapy. Trends Mol. Med. 2022, 28, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-J.; Liao, E.-C.; She, M.-L.; Chang, D.-T.M.; Tsai, J.-J. Cell-Penetrating Peptide Derived from Human Eosinophil Cationic Protein Inhibits Mite Allergen Der p 2 Induced Inflammasome Activation. PLoS ONE 2015, 10, e0121393. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Interleukin-2 Therapy of Cancer-Clinical Perspectives. Int. Immunopharmacol. 2021, 98, 107836. [Google Scholar] [CrossRef]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. BioMed Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the Crossroads of Effector Responses, Tolerance, and Immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Yuan, Y.; Kolios, A.G.; Liu, Y.; Zhang, B.; Li, H.; Tsokos, G.C.; Zhang, X. Therapeutic Potential of Interleukin-2 in Autoimmune Diseases. Trends Mol. Med. 2022, 28, 596–612. [Google Scholar] [CrossRef]
- Klatzmann, D.; Abbas, A.K. The Promise of Low-Dose Interleukin-2 Therapy for Autoimmune and Inflammatory Diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar]
- Abbas, A.K.; Trotta, E.; Simeonov, R.D.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and Therapeutic Prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Alva, A.; Daniels, G.A.; Wong, M.K.; Kaufman, H.L.; Morse, M.A.; McDermott, D.F.; Clark, J.I.; Agarwala, S.S.; Miletello, G.; Logan, T.F. Contemporary Experience with High-Dose Interleukin-2 Therapy and Impact on Survival in Patients with Metastatic Melanoma and Metastatic Renal Cell Carcinoma. Cancer Immunol. Immunother. 2016, 65, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Põder, J.; LaPorte, K.M.; Malek, T.R. Engineering IL-2 for Immunotherapy of Autoimmunity and Cancer. Nat. Rev. Immunol. 2022, 22, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Huinen, Z.R.; Huijbers, E.J.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-Angiogenic Agents—Overcoming Tumour Endothelial Cell Anergy and Improving Immunotherapy Outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [PubMed]
- Tarhini, A.A.; Frankel, P.; Ruel, C.; Ernstoff, M.S.; Kuzel, T.M.; Logan, T.F.; Khushalani, N.I.; Tawbi, H.A.; Margolin, K.A.; Awasthi, S. NCI 8628-A Randomized Phase II Study of Ziv-Aflibercept and High Dose Interleukin-2 (HD IL-2) or HD IL-2 Alone for Inoperable Stage III or IV Melanoma. Cancer 2018, 124, 4332. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.P.; Schwartzentruber, D.J.; Kaufman, H.L.; Agarwala, S.S.; Tarhini, A.A.; Lowder, J.N.; Atkins, M.B. High Dose Interleukin-2 (Aldesleukin)-Expert Consensus on Best Management Practices-2014. J. Immunother. Cancer 2014, 2, 1–23. [Google Scholar] [CrossRef]
- Heeb, L.E.; Egholm, C.; Boyman, O. Evolution and Function of Interleukin-4 Receptor Signaling in Adaptive Immunity and Neutrophils. Genes Immun. 2020, 21, 143–149. [Google Scholar] [CrossRef]
- Anovazzi, G.; Medeiros, M.C.; Pigossi, S.C.; Finoti, L.S.; Souza Moreira, T.M.; Mayer, M.P.A.; Zanelli, C.F.; Valentini, S.R.; Rossa-Junior, C.; Scarel-Caminaga, R.M. Functionality and Opposite Roles of Two Interleukin 4 Haplotypes in Immune Cells. Genes Immun. 2017, 18, 33–41. [Google Scholar] [CrossRef]
- Spieler, V.; Ludwig, M.-G.; Dawson, J.; Tigani, B.; Littlewood-Evans, A.; Safina, C.; Ebersbach, H.; Seuwen, K.; Raschig, M.; Ter Mors, B. Targeting Interleukin-4 to the Arthritic Joint. J. Control. Release 2020, 326, 172–180. [Google Scholar]
- Song, S.Y.; Hong, J.; Go, S.; Lim, S.; Sohn, H.S.; Kang, M.; Jung, G.; Yoon, J.; Kang, M.L.; Im, G. Interleukin-4 Gene Transfection and Spheroid Formation Potentiate Therapeutic Efficacy of Mesenchymal Stem Cells for Osteoarthritis. Adv. Healthc. Mater. 2020, 9, 1901612. [Google Scholar]
- Chang, Y.-H.; Tsai, J.-N.; Chen, T.-L.; Ho, K.-T.; Cheng, H.-Y.; Hsiao, C.-W.; Shiau, M.-Y. Interleukin-4 Promotes Myogenesis and Boosts Myocyte Insulin Efficacy. Mediators Inflamm. 2019, 2019, 4182015. [Google Scholar] [CrossRef]
- Shiau, M.-Y.; Chuang, P.-H.; Yang, C.-P.; Hsiao, C.-W.; Chang, S.-W.; Chang, K.-Y.; Liu, T.-M.; Chen, H.-W.; Chuang, C.-C.; Yuan, S.-Y. Mechanism of Interleukin-4 Reducing Lipid Deposit by Regulating Hormone-Sensitive Lipase. Sci. Rep. 2019, 9, 11974. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, C.; Celikkaya, H.; Cosacak, M.I.; Mashkaryan, V.; Bray, L.; Bhattarai, P.; Brandt, K.; Hollak, H.; Chen, X.; He, S. 3D Culture Method for Alzheimer’s Disease Modeling Reveals Interleukin-4 Rescues Aβ42-Induced Loss of Human Neural Stem Cell Plasticity. Dev. Cell 2018, 46, 85–101. [Google Scholar] [CrossRef] [PubMed]
- May, R.D.; Fung, M. Strategies Targeting the IL-4/IL-13 Axes in Disease. Cytokine 2015, 75, 89–116. [Google Scholar] [PubMed]
- Braddock, M.; Hanania, N.A.; Sharafkhaneh, A.; Colice, G.; Carlsson, M. Potential Risks Related to Modulating Interleukin-13 and Interleukin-4 Signalling: A Systematic Review. Drug Saf. 2018, 41, 489–509. [Google Scholar] [PubMed]
- Dhiman, N.; Ovsyannikova, I.G.; Howe, R.C.; Ryan, J.E.; Jacobson, R.M.; Poland, G.A. Interleukin-4 Induced by Measles Virus and Measles-Derived Peptides as Measured by IL-4 Receptor-Blocking ELISA. J. Immunol. Methods 2004, 287, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Steen-Louws, C.; Hartgring, S.; Popov-Celeketic, J.; Lopes, A.; de Smet, M.; Eijkelkamp, N.; Lafeber, F.; Hack, C.; van Roon, J. IL4-10 Fusion Protein: A Novel Immunoregulatory Drug Combining Activities of Interleukin 4 and Interleukin 10. Clin. Exp. Immunol. 2019, 195, 1–9. [Google Scholar]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar]
- Saraiva, M.; Vieira, P.; O’garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Verma, R.; Balakrishnan, L.; Sharma, K.; Khan, A.A.; Advani, J.; Gowda, H.; Tripathy, S.P.; Suar, M.; Pandey, A.; Gandotra, S. A Network Map of Interleukin-10 Signaling Pathway. J. Cell Commun. Signal. 2016, 10, 61–67. [Google Scholar] [CrossRef]
- Krawiec, P.; Pawłowska-Kamieniak, A.; Pac-Kożuchowska, E. Interleukin 10 and Interleukin 10 Receptor in Paediatric Inflammatory Bowel Disease: From Bench to Bedside Lesson. J. Inflamm. 2021, 18, 1–5. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Xu, L.; Yu, T.; Zhao, X.; Yao, S.; Zhao, Q.; Barnes, S.; Cohn, S.M.; Dann, S.M. GPR120 Inhibits Colitis through Regulation of CD4+ T Cell Interleukin 10 Production. Gastroenterology 2022, 162, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barrera, R.; Rivas-González, M.; García-Sánchez, J.; Mojica-Torres, D.; Ibarra, A. Neurogenesis after Spinal Cord Injury: State of the Art. Cells 2021, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.L.; Jiang, S.; Chisholm, D.; Jantzie, L.L.; Bhaskar, K. Interleukin-10 Deficiency Exacerbates Inflammation-Induced Tau Pathology. J. Neuroinflammation 2021, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, N.; Dickenson, R.E.; Odendall, C. Interferons: Tug of War between Bacteria and Their Host. Front. Cell. Infect. Microbiol. 2021, 11, 624094. [Google Scholar] [CrossRef]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A. Interferon-γ: Teammate or Opponent in the Tumour Microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar]
- Skariah, S.; Sultan, A.A.; Mordue, D.G. IFN-Induced Cell-Autonomous Immune Mechanisms in the Control of Intracellular Protozoa. Parasitol. Res. 2022, 121, 1559–1571. [Google Scholar] [CrossRef]
- Xiang, W.; Yu, N.; Lei, A.; Li, X.; Tan, S.; Huang, L.; Zhou, Z. Insights into Host Cell Cytokines in Chlamydia Infection. Front. Immunol. 2021, 12, 639834. [Google Scholar] [CrossRef]
- Razaghi, A.; Owens, L.; Heimann, K. Review of the Recombinant Human Interferon Gamma as an Immunotherapeutic: Impacts of Production Platforms and Glycosylation. J. Biotechnol. 2016, 240, 48–60. [Google Scholar]
- Kar, T.; Narsaria, U.; Basak, S.; Deb, D.; Castiglione, F.; Mueller, D.M.; Srivastava, A.P. A Candidate Multi-Epitope Vaccine against SARS-CoV-2. Sci. Rep. 2020, 10, 10895. [Google Scholar] [CrossRef]
- Shey, R.A.; Ghogomu, S.M.; Esoh, K.K.; Nebangwa, N.D.; Shintouo, C.M.; Nongley, N.F.; Asa, B.F.; Ngale, F.N.; Vanhamme, L.; Souopgui, J. In-Silico Design of a Multi-Epitope Vaccine Candidate against Onchocerciasis and Related Filarial Diseases. Sci. Rep. 2019, 9, 4409. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-Receptors: From Mediators of Cell Death and Inflammation to Therapeutic Giants–Past, Present and Future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar]
- Hughes, J.T.; Long, M.D. Tumor Necrosis Factor-Alpha Inhibitors and Risks of Malignancy. In Treatment of Inflammatory Bowel Disease with Biologics; Springer: Berlin/Heidelberg, Germany, 2018; pp. 213–229. [Google Scholar]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [PubMed]
- Pogostin, B.H.; McHugh, K.J. Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness. Bioengineering 2021, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Tinto, H.; Otieno, W.; Gesase, S.; Sorgho, H.; Otieno, L.; Liheluka, E.; Valéa, I.; Sing’oei, V.; Malabeja, A.; Valia, D. Long-Term Incidence of Severe Malaria Following RTS, S/AS01 Vaccination in Children and Infants in Africa: An Open-Label 3-Year Extension Study of a Phase 3 Randomised Controlled Trial. Lancet Infect. Dis. 2019, 19, 821–832. [Google Scholar] [CrossRef]
- Nagpal, G.; Chaudhary, K.; Agrawal, P.; Raghava, G.P. Computer-Aided Prediction of Antigen Presenting Cell Modulators for Designing Peptide-Based Vaccine Adjuvants. J. Transl. Med. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides and Cell-Penetrating Peptides for Treating Intracellular Bacterial Infections. Front. Cell. Infect. Microbiol. 2021, 10, 612931. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Baeder, D.Y.; Johnston, P.; Regoes, R.R.; Rolff, J. Bacteria Primed by Antimicrobial Peptides Develop Tolerance and Persist. PLoS Pathog. 2021, 17, e1009443. [Google Scholar] [CrossRef] [PubMed]
- Ben Hur, D.; Kapach, G.; Wani, N.A.; Kiper, E.; Ashkenazi, M.; Smollan, G.; Keller, N.; Efrati, O.; Shai, Y. Antimicrobial Peptides against Multidrug-Resistant Pseudomonas Aeruginosa Biofilm from Cystic Fibrosis Patients. J. Med. Chem. 2022, 65, 9050–9062. [Google Scholar] [PubMed]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M.; Managadze, G. Physicochemical Features and Peculiarities of Interaction of AMP with the Membrane. Pharmaceuticals 2021, 14, 471. [Google Scholar] [PubMed]
- Parchebafi, A.; Tamanaee, F.; Ehteram, H.; Ahmad, E.; Nikzad, H.; Haddad Kashani, H. The Dual Interaction of Antimicrobial Peptides on Bacteria and Cancer Cells; Mechanism of Action and Therapeutic Strategies of Nanostructures. Microb. Cell Factories 2022, 21, 1–18. [Google Scholar]
- Gabere, M.N.; Noble, W.S. Empirical Comparison of Web-Based Antimicrobial Peptide Prediction Tools. Bioinformatics 2017, 33, 1921–1929. [Google Scholar] [CrossRef]
- Boman, H.G. Antibacterial Peptides: Basic Facts and Emerging Concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- Varkey, J.; Nagaraj, R. Antibacterial Activity of Human Neutrophil Defensin HNP-1 Analogs without Cysteines. Antimicrob. Agents Chemother. 2005, 49, 4561–4566. [Google Scholar]
- Kozic, M.; Vukicevic, D.; Simunic, J.; Roncevic, T.; Antcheva, N.; Tossi, A.; Juretic, D. Predicting the Minimal Inhibitory Concentration for Antimicrobial Peptides with Rana-Box Domain. J. Chem. Inf. Model. 2015, 55, 2275–2287. [Google Scholar] [CrossRef]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H.; Corriden, R.; Rohde, M.; et al. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef]
- Tuxpan-Pérez, A.; Ibarra-Valencia, M.A.; Estrada, B.E.; Clement, H.; Corrales-García, L.L.; Espino-Solis, G.P.; Corzo, G. Antimicrobial and Immunomodulatory Effects of Selected Chemokine and Antimicrobial Peptide on Cytokine Profile during Salmonella Typhimurium Infection in Mouse. Antibiotics 2022, 11, 607. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; Van Harten, R.M.; Veldhuizen, E.J.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [PubMed]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Chen, H.; Wan, P.; Luo, L.; Ye, Z.; Huang, J.; Chen, D.; Pan, J. Isolation and Identification of Immunomodulatory Peptides from the Protein Hydrolysate of Tuna Trimmings (Thunnas Albacares). LWT 2022, 164, 113614. [Google Scholar]
- Silva, O.; De La Fuente-Núñez, C.; Haney, E.; Fensterseifer, I.; Ribeiro, S.; Porto, W.; Brown, P.; Faria-Junior, C.; Rezende, T.; Moreno, S. An Anti-Infective Synthetic Peptide with Dual Antimicrobial and Immunomodulatory Activities. Sci. Rep. 2016, 6, 35465. [Google Scholar] [PubMed]
- Ching, C.B.; Gupta, S.; Li, B.; Cortado, H.; Mayne, N.; Jackson, A.R.; McHugh, K.M.; Becknell, B. Interleukin-6/Stat3 Signaling Has an Essential Role in the Host Antimicrobial Response to Urinary Tract Infection. Kidney Int. 2018, 93, 1320–1329. [Google Scholar] [CrossRef]
- Acharya, D.; Sullivan, M.J.; Duell, B.L.; Goh, K.G.; Katupitiya, L.; Gosling, D.; Chamoun, M.N.; Kakkanat, A.; Chattopadhyay, D.; Crowley, M. Rapid Bladder Interleukin-10 Synthesis in Response to Uropathogenic Escherichia Coli Is Part of a Defense Strategy Triggered by the Major Bacterial Flagellar Filament FliC and Contingent on TLR5. Msphere 2019, 4, e00545-19. [Google Scholar] [CrossRef]
- Yue, R.; Wei, X.; Zhao, J.; Zhou, Z.; Zhong, W. Essential Role of IFN-γ in Regulating Gut Antimicrobial Peptides and Microbiota to Protect against Alcohol-Induced Bacterial Translocation and Hepatic Inflammation in Mice. Front. Physiol. 2021, 11, 629141. [Google Scholar]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE Pathogens in the Environment: Antibiotic Resistance Status, Community-Acquired Infection and Risk to Human Health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar]
- Lin, Q.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Prevention of ESKAPE Pathogen Biofilm Formation by Antimicrobial Peptides WLBU2 and LL37. Int. J. Antimicrob. Agents 2018, 52, 667–672. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; De La Fuente-Núñez, C.; Baquir, B.; Faria-Junior, C.; Franco, O.L.; Hancock, R.E. Antibiofilm Peptides Increase the Susceptibility of Carbapenemase-Producing Klebsiella Pneumoniae Clinical Isolates to β-Lactam Antibiotics. Antimicrob. Agents Chemother. 2015, 59, 3906–3912. [Google Scholar]
- Walrant, A.; Bauzá, A.; Girardet, C.; Alves, I.D.; Lecomte, S.; Illien, F.; Cardon, S.; Chaianantakul, N.; Pallerla, M.; Burlina, F. Ionpair-π Interactions Favor Cell Penetration of Arginine/Tryptophan-Rich Cell-Penetrating Peptides. Biochim. Biophys. Acta BBA-Biomembr. 2020, 1862, 183098. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Bourzac, K. Infectious Disease: Beating the Big Three. Nature 2014, 507, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Matsa, R. “Big Three” Infectious Diseases: Tuberculosis, Malaria and HIV/AIDS. Curr. Top. Med. Chem. 2021, 21, 2779–2799. [Google Scholar]
- Santucci, P.; Greenwood, D.J.; Fearns, A.; Chen, K.; Jiang, H.; Gutierrez, M.G. Intracellular Localisation of Mycobacterium Tuberculosis Affects Efficacy of the Antibiotic Pyrazinamide. Nat. Commun. 2021, 12, 3816. [Google Scholar] [PubMed]
- Oliveira, G.S.; Costa, R.P.; Gomes, P.; Gomes, M.S.; Silva, T.; Teixeira, C. Antimicrobial Peptides as Potential Anti-Tubercular Leads: A Concise Review. Pharmaceuticals 2021, 14, 323. [Google Scholar] [CrossRef]
- Upadhyay, S.; Mittal, E.; Philips, J.A. Tuberculosis and the Art of Macrophage Manipulation. Pathog. Dis. 2018, 76, fty037. [Google Scholar] [CrossRef]
- Ramírez-Carreto, S.; Jiménez-Vargas, J.M.; Rivas-Santiago, B.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Peptides from the Scorpion Vaejovis Punctatus with Broad Antimicrobial Activity. Peptides 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Ma, W.; Peng, H.; Liu, K.; Wang, Y.; Wang, W.; Qu, S.; Li, Y.; Bi, L.; Zhang, X.; Zhang, L. Efficacy of Dual-Targeting Combined Anti-Tuberculosis Drug Delivery System in the Treatment of Tuberculous Meningitis. J. Biomed. Nanotechnol. 2021, 17, 2034–2042. [Google Scholar] [CrossRef]
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, E46. [Google Scholar] [CrossRef]
- Popp, C.; Ramírez-Zavala, B.; Schwanfelder, S.; Krüger, I.; Morschhäuser, J. Evolution of Fluconazole-Resistant Candida Albicans Strains by Drug-Induced Mating Competence and Parasexual Recombination. MBio 2019, 10, e02740-18. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Kini, S.G.; Wong, K.H.; Tan, W.L.; Xiao, T.; Tam, J.P. Morintides: Cargo-Free Chitin-Binding Peptides from Moringa Oleifera. BMC Plant Biol. 2017, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; O’Neil, D.A. Innate Inspiration: Antifungal Peptides and Other Immunotherapeutics From the Host Immune Response. Front. Immunol. 2020, 11, 2177. [Google Scholar] [CrossRef]
- Czechowicz, P.; Neubauer, D.; Nowicka, J.; Kamysz, W.; Gościniak, G. Antifungal Activity of Linear and Disulfide-Cyclized Ultrashort Cationic Lipopeptides Alone and in Combination with Fluconazole against Vulvovaginal Candida Spp. Pharmaceutics 2021, 13, 1589. [Google Scholar] [CrossRef]
- Behzadipour, Y.; Hemmati, S. Viral Prefusion Targeting Using Entry Inhibitor Peptides: The Case of SARS-CoV-2 and Influenza A Virus. Int. J. Pept. Res. Ther. 2022, 28, 1–19. [Google Scholar] [CrossRef]
- Behzadipour, Y.; Gholampour, M.; Pirhadi, S.; Seradj, H.; Khoshneviszadeh, M.; Hemmati, S. Viral 3CLpro as a Target for Antiviral Intervention Using Milk-Derived Bioactive Peptides. Int. J. Pept. Res. Ther. 2021, 27, 2703–2716. [Google Scholar]
- Ghanbarzadeh, Z.; Hemmati, S.; Mohagheghzadeh, A. Humanizing Plant-Derived Snakins and Their Encrypted Antimicrobial Peptides. Biochimie 2022, 199, 92–111. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Anuwongcharoen, N.; Nantasenamat, C.; Hasan, M.M.; Shoombuatong, W. In Silico Approaches for the Prediction and Analysis of Antiviral Peptides: A Review. Curr. Pharm. Des. 2021, 27, 2180–2188. [Google Scholar] [CrossRef]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Gomes, B.; Augusto, M.T.; Felício, M.R.; Hollmann, A.; Franco, O.L.; Gonçalves, S.; Santos, N.C. Designing Improved Active Peptides for Therapeutic Approaches against Infectious Diseases. Biotechnol. Adv. 2018, 36, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living Together in Biofilms: The Microbial Cell Factory and Its Biotechnological Implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef]
- Jo, J.; Price-Whelan, A.; Dietrich, L.E.P. Gradients and Consequences of Heterogeneity in Biofilms. Nat. Rev. Microbiol. 2022, 20, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.S.; Hanafiah, A.; Nachimuthu, R.; Muthupandian, S.; Md Nesran, Z.N.; Patil, S. The Role of Antimicrobial Peptides as Antimicrobial and Antibiofilm Agents in Tackling the Silent Pandemic of Antimicrobial Resistance. Molecules 2022, 27, 2995. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm Activity of Host Defence Peptides: Complexity Provides Opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, P.; Kumar, R.; Bhardwaj, A. DPABBs: A Novel in Silico Approach for Predicting and Designing Anti-Biofilm Peptides. Sci. Rep. 2016, 6, 21839. [Google Scholar] [CrossRef]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. Synergy between Conventional Antibiotics and Anti-Biofilm Peptides in a Murine, Sub-Cutaneous Abscess Model Caused by Recalcitrant ESKAPE Pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef]
- Mnif, S.; Jardak, M.; Graiet, I.; Abid, S.; Driss, D.; Kharrat, N. The Novel Cationic Cell-Penetrating Peptide PEP-NJSM Is Highly Active against Staphylococcus Epidermidis Biofilm. Int. J. Biol. Macromol. 2019, 125, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, D.; Lin, H. Antibiofilm Peptides as a Promising Strategy: Comparative Research. Appl. Microbiol. Biotechnol. 2021, 105, 1647–1656. [Google Scholar] [CrossRef]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and Assessment of Anti-Biofilm Peptides: Steps Toward Clinical Application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory Cytokines Regulate Epidermal Stem Cells in Wound Epithelialization. Stem Cell Res. Ther. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Nosenko, M.; Ambaryan, S.; Drutskaya, M. Proinflammatory Cytokines and Skin Wound Healing in Mice. Mol. Biol. 2019, 53, 653–664. [Google Scholar] [CrossRef]
- Peluzzo, A.M.; Autieri, M.V. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells 2022, 11, 587. [Google Scholar] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical Antimicrobial Peptide Formulations for Wound Healing: Current Developments and Future Prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Horn, M.; Neundorf, I. Design of a Novel Cell-Permeable Chimeric Peptide to Promote Wound Healing. Sci. Rep. 2018, 8, 16279. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Hwang, J.S.; Kim, S.G.; Shin, T.H.; Jang, Y.E.; Kwon, D.H.; Lee, G. Development of Anticancer Peptides Using Artificial Intelligence and Combinational Therapy for Cancer Therapeutics. Pharmaceutics 2022, 14, 997. [Google Scholar]
- Shoari, A.; Khodabakhsh, F.; Cohan, R.A.; Salimian, M.; Karami, E. Anti-Angiogenic Peptides Application in Cancer Therapy; a Review. Res. Pharm. Sci. 2021, 16, 559. [Google Scholar]
- De la Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0. Molecules 2020, 25, 2293. [Google Scholar]
- Wang, L.; Dong, C.; Li, X.; Han, W.; Su, X. Anticancer Potential of Bioactive Peptides from Animal Sources. Oncol. Rep. 2017, 38, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.T.; Mason, P.E.; Anderson, J.; Dempsey, C.E. Arginine Side Chain Interactions and the Role of Arginine as a Gating Charge Carrier in Voltage Sensitive Ion Channels. Sci. Rep. 2016, 6, 21759. [Google Scholar] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- De Castro, S.; Camarasa, M.-J. Polypharmacology in HIV Inhibition: Can a Drug with Simultaneous Action against Two Relevant Targets Be an Alternative to Combination Therapy? Eur. J. Med. Chem. 2018, 150, 206–227. [Google Scholar] [CrossRef]
- Ma, X.; Lv, X.; Zhang, J. Exploiting Polypharmacology for Improving Therapeutic Outcome of Kinase Inhibitors (KIs): An Update of Recent Medicinal Chemistry Efforts. Eur. J. Med. Chem. 2018, 143, 449–463. [Google Scholar]
- Min, K.A.; Maharjan, P.; Ham, S.; Shin, M.C. Pro-Apoptotic Peptides-Based Cancer Therapies: Challenges and Strategies to Enhance Therapeutic Efficacy. Arch. Pharm. Res. 2018, 41, 594–616. [Google Scholar] [CrossRef]
- Burdukiewicz, M.; Sidorczuk, K.; Rafacz, D.; Pietluch, F.; Bąkała, M.; Słowik, J.; Gagat, P. Cancergram: An Effective Classifier for Differentiating Anticancer from Antimicrobial Peptides. Pharmaceutics 2020, 12, 1045. [Google Scholar] [CrossRef]
- Garner, T.P.; Lopez, A.; Reyna, D.E.; Spitz, A.Z.; Gavathiotis, E. Progress in Targeting the BCL-2 Family of Proteins. Curr. Opin. Chem. Biol. 2017, 39, 133–142. [Google Scholar] [CrossRef]
- Ma, C.; Yin, G.; You, F.; Wei, Y.; Huang, Z.; Chen, X.; Yan, D. A Specific Cell-Penetrating Peptide Induces Apoptosis in SKOV3 Cells by down-Regulation of Bcl-2. Biotechnol. Lett. 2013, 35, 1791–1797. [Google Scholar]
- Park, J.; Han, J.-H.; Myung, S.-H.; Seo, Y.-W.; Kim, T.-H. MTD-like Motif of a BH3-Only Protein, BNIP1, Induces Necrosis Accompanied by an Intracellular Calcium Spike. Biochem. Biophys. Res. Commun. 2018, 495, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Shteinfer-Kuzmine, A.; Verma, A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomolecules 2020, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Farsinejad, S.; Gheisary, Z.; Ebrahimi Samani, S.; Alizadeh, A.M. Mitochondrial Targeted Peptides for Cancer Therapy. Tumor Biol. 2015, 36, 5715–5725. [Google Scholar]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [PubMed]
- Emelianova, A.A.; Kuzmin, D.V.; Panteleev, P.V.; Sorokin, M.; Buzdin, A.A.; Ovchinnikova, T.V. Anticancer Activity of the Goat Antimicrobial Peptide ChMAP-28. Front. Pharmacol. 2018, 9, 1501. [Google Scholar] [CrossRef]
- Chang, W.-T.; Pan, C.-Y.; Rajanbabu, V.; Cheng, C.-W.; Chen, J.-Y. Tilapia (Oreochromis Mossambicus) Antimicrobial Peptide, Hepcidin 1–5, Shows Antitumor Activity in Cancer Cells. Peptides 2011, 32, 342–352. [Google Scholar]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Katayama, Y.; Uchino, J.; Chihara, Y.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Takayama, K. Tumor Neovascularization and Developments in Therapeutics. Cancers 2019, 11, 316. [Google Scholar] [CrossRef]
- Qin, S.; Li, A.; Yi, M.; Yu, S.; Zhang, M.; Wu, K. Recent Advances on Anti-Angiogenesis Receptor Tyrosine Kinase Inhibitors in Cancer Therapy. J. Hematol. Oncol. 2019, 12, 1–11. [Google Scholar]
- Laengsri, V.; Nantasenamat, C.; Schaduangrat, N.; Nuchnoi, P.; Prachayasittikul, V.; Shoombuatong, W. TargetAntiAngio: A Sequence-Based Tool for the Prediction and Analysis of Anti-Angiogenic Peptides. Int. J. Mol. Sci. 2019, 20, 2950. [Google Scholar] [CrossRef]
- Chlenski, A.; Guerrero, L.J.; Peddinti, R.; Spitz, J.A.; Leonhardt, P.T.; Yang, Q.; Tian, Y.; Salwen, H.R.; Cohn, S.L. Anti-Angiogenic SPARC Peptides Inhibit Progression of Neuroblastoma Tumors. Mol. Cancer 2010, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [PubMed]
- Soriano, M.E.T.; Dimattia, J.; Gordon, M. Anti-Angiogenic Therapy for Retinal Diseases. Anti-Angiogenesis Drug Discov. Dev. Vol. 4 2019, 4, 108. [Google Scholar]
- Anderson, W.J.; da Cruz, N.F.S.; Lima, L.H.; Emerson, G.G.; Rodrigues, E.B.; Melo, G.B. Mechanisms of Sterile Inflammation after Intravitreal Injection of Antiangiogenic Drugs: A Narrative Review. Int. J. Retina Vitr. 2021, 7, 1–12. [Google Scholar]
- Nikoi, N.-D.; Berwick, M.; Bryant, J.A.; Riordan, L.; Slope, L.; Peacock, A.F.; de Cogan, F. Stability of Cell-Penetrating Peptide Anti-VEGF Formulations for the Treatment of Age-Related Macular Degeneration. Curr. Eye Res. 2021, 46, 751–757. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Ramachandra, R.K.; Adurthi, S.; Dhudashiya, A.A.; Vadlamani, S.; Vemula, K.; Vunnum, S.; Satyam, L.K.; Samiulla, D.S.; Subbarao, K. A Rationally Designed Peptide Antagonist of the PD-1 Signaling Pathway as an Immunomodulatory Agent for Cancer TherapyPeptide Antagonist of PD-1 Signaling Pathway. Mol. Cancer Ther. 2019, 18, 1081–1091. [Google Scholar]
- Pötzl, J.; Roser, D.; Bankel, L.; Hömberg, N.; Geishauser, A.; Brenner, C.D.; Weigand, M.; Röcken, M.; Mocikat, R. Reversal of Tumor Acidosis by Systemic Buffering Reactivates NK Cells to Express IFN-γ and Induces NK Cell-dependent Lymphoma Control without Other Immunotherapies. Int. J. Cancer 2017, 140, 2125–2133. [Google Scholar]
- Karachaliou, N.; Gonzalez-Cao, M.; Crespo, G.; Drozdowskyj, A.; Aldeguer, E.; Gimenez-Capitan, A.; Teixido, C.; Molina-Vila, M.A.; Viteri, S.; De Los Llanos Gil, M. Interferon Gamma, an Important Marker of Response to Immune Checkpoint Blockade in Non-Small Cell Lung Cancer and Melanoma Patients. Ther. Adv. Med. Oncol. 2018, 10, 1758834017749748. [Google Scholar] [CrossRef]
- He, B.; Li, B.; Chen, X.; Zhang, Q.; Lu, C.; Yang, S.; Long, J.; Ning, L.; Chen, H.; Huang, J. PDL1Binder: Identifying Programmed Cell Death Ligand 1 Binding Peptides by Incorporating next-Generation Phage Display Data and Different Peptide Descriptors. Front. Microbiol. 2022, 13, 928774. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T. Adaptive Plasticity of IL-10+ and IL-35+ Treg Cells Cooperatively Promotes Tumor T Cell Exhaustion. Nat. Immunol. 2019, 20, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Wang, K.; Zhong, J. Tumor-Penetrating Peptides. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing: Duxford, UK, 2018; pp. 371–386. [Google Scholar]
- Shoombuatong, W.; Schaduangrat, N.; Pratiwi, R.; Nantasenamat, C. THPep: A Machine Learning-Based Approach for Predicting Tumor Homing Peptides. Comput. Biol. Chem. 2019, 80, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Iioka, H.; Saito, K. Tumor-homing Peptide and Its Utility for Advanced Cancer Medicine. Cancer Sci. 2021, 112, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef] [PubMed]
- Ciobanasu, C.; Dragomir, I.; Apetrei, A. The Penetrating Properties of the Tumor Homing Peptide LyP-1 in Model Lipid Membranes. J. Pept. Sci. 2019, 25, e3145. [Google Scholar] [CrossRef]
- Kapoor, P.; Singh, H.; Gautam, A.; Chaudhary, K.; Kumar, R.; Raghava, G.P. TumorHoPe: A Database of Tumor Homing Peptides. PLoS ONE 2012, 7, e35187. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Chiangjong, W.; Nantasenamat, C.; Moni, M.A.; Lio’, P.; Manavalan, B.; Shoombuatong, W. SCMTHP: A New Approach for Identifying and Characterizing of Tumor-Homing Peptides Using Estimated Propensity Scores of Amino Acids. Pharmaceutics 2022, 14, 122. [Google Scholar] [CrossRef]
- Agüero-Chapin, G.; Galpert-Cañizares, D.; Domínguez-Pérez, D.; Marrero-Ponce, Y.; Pérez-Machado, G.; Teijeira, M.; Antunes, A. Emerging Computational Approaches for Antimicrobial Peptide Discovery. Antibiotics 2022, 11, 936. [Google Scholar]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-) Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.-J.; Mas-Moruno, C. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-Binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [PubMed]
- Hou, J.; Diao, Y.; Li, W.; Yang, Z.; Zhang, L.; Chen, Z.; Wu, Y. RGD Peptide Conjugation Results in Enhanced Antitumor Activity of PD0325901 against Glioblastoma by Both Tumor-Targeting Delivery and Combination Therapy. Int. J. Pharm. 2016, 505, 329–340. [Google Scholar] [PubMed]
- Sun, L.; Gai, Y.; Li, Z.; Li, H.; Li, J.; Muschler, J.; Kang, R.; Tang, D.; Zeng, D. Heterodimeric RGD-NGR PET Tracer for the Early Detection of Pancreatic Cancer. Mol. Imaging Biol. 2022, 24, 580–589. [Google Scholar] [PubMed]
- Mo, C.; Wang, Z.; Yang, J.; Ouyang, Y.; Mo, Q.; Li, S.; He, P.; Chen, L.; Li, X. Rational Assembly of RGD/MoS2/Doxorubicin Nanodrug for Targeted Drug Delivery, GSH-Stimulus Release and Chemo-Photothermal Synergistic Antitumor Activity. J. Photochem. Photobiol. B 2022, 233, 112487. [Google Scholar] [CrossRef]
- Alanazi, J.S.; Alqahtani, F.Y.; Aleanizy, F.S.; Radwan, A.A.; Bari, A.; Alqahtani, Q.H.; Abdelhady, H.G.; Alsarra, I. MicroRNA-539-5p-Loaded PLGA Nanoparticles Grafted with IRGD as a Targeting Treatment for Choroidal Neovascularization. Pharmaceutics 2022, 14, 243. [Google Scholar] [CrossRef]
- Migliorini, F.; Schenker, H.; Maffulli, N.; Hildebrand, F.; Eschweiler, J. Histomorphometry of Ossification in Functionalised Ceramics with Tripeptide Arg-Gly-Asp (RGD): An In Vivo Study. Life 2022, 12, 761. [Google Scholar] [CrossRef]
- Eikesdal, H.P.; Sugimoto, H.; Birrane, G.; Maeshima, Y.; Cooke, V.G.; Kieran, M.; Kalluri, R. Identification of Amino Acids Essential for the Antiangiogenic Activity of Tumstatin and Its Use in Combination Antitumor Activity. Proc. Natl. Acad. Sci. USA 2008, 105, 15040–15045. [Google Scholar] [PubMed]
- Garciarena, C.D.; McHale, T.M.; Martin-Loeches, I.; Kerrigan, S.W. Pre-Emptive and Therapeutic Value of Blocking Bacterial Attachment to the Endothelial AlphaVbeta3 Integrin with Cilengitide in Sepsis. Crit. Care 2017, 21, 1–2. [Google Scholar]
- Nader, D.; Curley, G.F.; Kerrigan, S.W. A New Perspective in Sepsis Treatment: Could RGD-Dependent Integrins Be Novel Targets? Drug Discov. Today 2020, 25, 2317–2325. [Google Scholar] [CrossRef]
- Peng, K.; Bai, Y.; Zhu, Q.; Hu, B.; Xu, Y. Targeting VEGF–Neuropilin Interactions: A Promising Antitumor Strategy. Drug Discov. Today 2019, 24, 656–664. [Google Scholar]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-End Rule Peptides Mediate Neuropilin-1-Dependent Cell, Vascular, and Tissue Penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, G.; Yamauchi, Y.; Teesalu, T. A Widespread Viral Entry Mechanism: The C-End Rule Motif–Neuropilin Receptor Interaction. Proc. Natl. Acad. Sci. USA 2021, 118, e2112457118. [Google Scholar] [CrossRef] [PubMed]
- Kadonosono, T.; Yamano, A.; Goto, T.; Tsubaki, T.; Niibori, M.; Kuchimaru, T.; Kizaka-Kondoh, S. Cell Penetrating Peptides Improve Tumor Delivery of Cargos through Neuropilin-1-Dependent Extravasation. J. Control. Release 2015, 201, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Liao, X.; Li, L.; Chen, Z.; Tian, T.; Yu, L.; Chen, Z. In Vitro Mitochondrial-Targeted Antioxidant Peptide Induces Apoptosis in Cancer Cells. Oncotargets Ther. 2019, 12, 7297–7306. [Google Scholar] [CrossRef]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated Hydroxyanisole: Carcinogenic Food Additive to Be Avoided or Harmless Antioxidant Important to Protect Food Supply? Regul. Toxicol. Pharmacol. 2021, 121, 104887. [Google Scholar] [PubMed]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B. AnOxPePred: Using Deep Learning for the Prediction of Antioxidative Properties of Peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar]
- Wu, J.; Li, J.; Wang, H.; Liu, C.-B. Mitochondrial-Targeted Penetrating Peptide Delivery for Cancer Therapy. Expert Opin. Drug Deliv. 2018, 15, 951–964. [Google Scholar] [CrossRef]
- Cho, S.; Szeto, H.H.; Kim, E.; Kim, H.; Tolhurst, A.T.; Pinto, J.T. A Novel Cell-Permeable Antioxidant Peptide, SS31, Attenuates Ischemic Brain Injury by down-Regulating CD36. J. Biol. Chem. 2007, 282, 4634–4642. [Google Scholar]
- Jia, Y.-L.; Sun, S.-J.; Chen, J.-H.; Jia, Q.; Huo, T.-T.; Chu, L.-F.; Bai, J.-T.; Yu, Y.-J.; Yan, X.-X.; Wang, J.-H. SS31, a Small Molecule Antioxidant Peptide, Attenuates β-Amyloid Elevation, Mitochondrial/Synaptic Deterioration and Cognitive Deficit in SAMP8 Mice. Curr. Alzheimer Res. 2016, 13, 297–306. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Choi, I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef]
- Gautam, A.; Chaudhary, K.; Kumar, R.; Sharma, A.; Kapoor, P.; Tyagi, A.; Raghava, G.P. In Silico Approaches for Designing Highly Effective Cell Penetrating Peptides. J. Transl. Med. 2013, 11, 1–12. [Google Scholar]
- Porosk, L.; Gaidutšik, I.; Langel, Ü. Approaches for the Discovery of New Cell-Penetrating Peptides. Expert Opin. Drug Discov. 2021, 16, 553–565. [Google Scholar] [PubMed]
- Manavalan, B.; Subramaniyam, S.; Shin, T.H.; Kim, M.O.; Lee, G. Machine-Learning-Based Prediction of Cell-Penetrating Peptides and Their Uptake Efficiency with Improved Accuracy. J. Proteome Res. 2018, 17, 2715–2726. [Google Scholar] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Consortium, O.S.D.D.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Dimitrov, I.; Naneva, L.; Doytchinova, I.; Bangov, I. AllergenFP: Allergenicity Prediction by Descriptor Fingerprints. Bioinformatics 2014, 30, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of Antimicrobial/Cytotoxic Activity and Structure of Peptides as a Resource for Development of New Therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Rahmatabadi, S.S.; Sadeghian, I.; Ghasemi, Y.; Sakhteman, A.; Hemmati, S. Identification and Characterization of a Sterically Robust Phenylalanine Ammonia-Lyase among 481 Natural Isoforms through Association of in Silico and in Vitro Studies. Enzyme Microb. Technol. 2019, 122, 36–54. [Google Scholar] [CrossRef]
- Song, J.; Tan, H.; Perry, A.J.; Akutsu, T.; Webb, G.I.; Whisstock, J.C.; Pike, R.N. PROSPER: An Integrated Feature-Based Tool for Predicting Protease Substrate Cleavage Sites. PLoS ONE 2012, 7, e50300. [Google Scholar] [CrossRef]
- Ferrè, F.; Clote, P. DiANNA: A Web Server for Disulfide Connectivity Prediction. Nucleic Acids Res. 2005, 33, W230–W232. [Google Scholar] [CrossRef]
- Lomize, A.L.; Lomize, M.A.; Krolicki, S.R.; Pogozheva, I.D. Membranome: A Database for Proteome-Wide Analysis of Single-Pass Membrane Proteins. Nucleic Acids Res. 2017, 45, D250–D255. [Google Scholar] [PubMed]
- Armenteros, J.J.A.; Salvatore, M.; Emanuelsson, O.; Winther, O.; Von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting Sequence Signals in Targeting Peptides Using Deep Learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A Web Server for Blind Peptide–Protein Docking Based on a Hierarchical Algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patiyal, S.; Dhall, A.; Sharma, N.; Raghava, G.P.S. B3pred: A Random-Forest-Based Method for Predicting and Designing Blood–Brain Barrier Penetrating Peptides. Pharmaceutics 2021, 13, 1237. [Google Scholar] [CrossRef]
- Lathwal, A.; Kumar, R.; Raghava, G.P. In Silico Model for Predicting IL-2 Inducing Peptides in Human. bioRxiv 2021. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Gupta, S.; Vir, P.; Raghava, G. Prediction of IL4 Inducing Peptides. Clin. Dev. Immunol. 2013, 2013, 263952. [Google Scholar]
- Nagpal, G.; Usmani, S.S.; Dhanda, S.K.; Kaur, H.; Singh, S.; Sharma, M.; Raghava, G.P. Computer-Aided Designing of Immunosuppressive Peptides Based on IL-10 Inducing Potential. Sci. Rep. 2017, 7, 42851. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Vir, P.; Raghava, G.P. Designing of Interferon-Gamma Inducing MHC Class-II Binders. Biol. Direct 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Dhall, A.; Patiyal, S.; Sharma, N.; Usmani, S.S.; Raghava, G.P.S. Computer-Aided Prediction and Design of IL-6 Inducing Peptides: IL-6 Plays a Crucial Role in COVID-19. Brief. Bioinform. 2021, 22, 936–945. [Google Scholar] [CrossRef]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A Database on Sequences, Structures and Signatures of Antimicrobial Peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef]
- Usmani, S.S.; Bhalla, S.; Raghava, G.P. Prediction of Antitubercular Peptides from Sequence Information Using Ensemble Classifier and Hybrid Features. Front. Pharmacol. 2018, 9, 954. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Chaudhary, K.; Kumar, R.; Sharma, M.; Raghava, G.P. In Silico Approach for Prediction of Antifungal Peptides. Front. Microbiol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. Meta-IAVP: A Sequence-Based Meta-Predictor for Improving the Prediction of Antiviral Peptides Using Effective Feature Representation. Int. J. Mol. Sci. 2019, 20, 5743. [Google Scholar] [CrossRef] [PubMed]
- Kamech, N.; Vukičević, D.; Ladram, A.; Piesse, C.; Vasseur, J.; Bojović, V.; Simunić, J.; Juretić, D. Improving the Selectivity of Antimicrobial Peptides from Anuran Skin. J. Chem. Inf. Model. 2012, 52, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
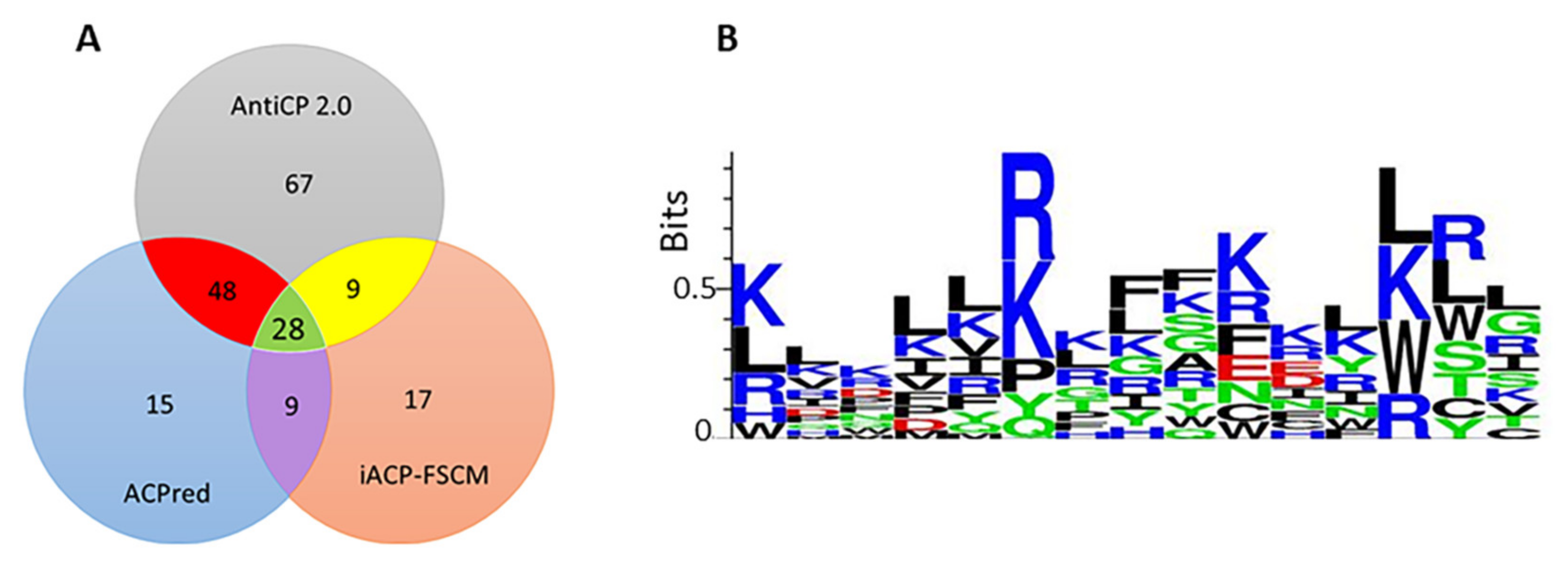
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P.S. AntiCP 2.0: An Updated Model for Predicting Anticancer Peptides. Brief. Bioinform. 2021, 22, bbaa153. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. ACPred: A Computational Tool for the Prediction and Analysis of Anticancer Peptides. Molecules 2019, 24, 1973. [Google Scholar]
- Charoenkwan, P.; Chiangjong, W.; Lee, V.S.; Nantasenamat, C.; Hasan, M.M.; Shoombuatong, W. Improved Prediction and Characterization of Anticancer Activities of Peptides Using a Novel Flexible Scoring Card Method. Sci. Rep. 2021, 11, 3017. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, P.; Gautam, A.; Chaudhary, K.; Kumar, R.; Chauhan, J.S.; Tyagi, A.; Raghava, G.P. Computational Approach for Designing Tumor Homing Peptides. Sci. Rep. 2013, 3, 1607. [Google Scholar] [CrossRef]






| Phylum | Class | Order | Family | Species | Reference |
|---|---|---|---|---|---|
Cnidaria | Cubozoa | Chirodropida | Chirodropidae | Chironex fleckeri | [22] |
| Carybdeida | Carukiidae | Carukia barnesi | |||
| Hydrozoa | Siphonophorae | Physaliidae | Physalia physalis Physalia utriculus | ||
| Anthoathecata | Milleporidae | Millepora alcicornis | |||
| Scyphozoa | Semaeostomeae | Cyaneidae | Cyanea capillata | ||
| Pelagiidae | Chrysaora quinquecirrha Pelagia noctiluca | ||||
| Coronatae | Linuchidae | Linuche unguiculata | |||
| Rhizostomeae | Cassiopeidae | Cassiopea Andromeda | [23] | ||
| Rhizostomatidae | Rhopilema esculentum Rhopilema nomadica | [24,25] | |||
| Anthozoa | Actiniaria | Stichodactylidae | Stichodactyla haddoni | [26] | |
Echinodermata | Ophiuroidae | Ophiacanthida | Ophiocomidae | Ophiomastix annulosa | [22] |
| Asteroidea | Valvatida | Acanthasteridae | Acanthaster planci | ||
| Spinulosida | Echinasteridae | Echinaster sp. | |||
| Velatida | Solasteridae | Solaster sp. | |||
| Holothuroidea | Aspidochirotida | Holothuriidae | Holothuria scabra | [27] | |
| Dendrochirotida | Sclerodactylidae | Ohshimella ehrenbergii | |||
| Echinoidea | Diadematoida | Diadematidae | Echinothrix sp. | [22] | |
| Echinothurioida | Phormosomatidae | Phormosoma sp. | |||
| Echinothuriidae | Araeosoma sp. Asthenosoma sp. | ||||
| Camarodonta | Toxopneustidae | Toxopneustes pileolus Tripneustes sp. | |||
 Chordata | Actinopterygii | Scorpaeniformes | Scorpaenidae | Pterois volitans Pterois lunulata Pterois antennata Pterois russelli Dendrochirus zebra Scorpaenopsis oxycephala | [22,28,29] |
| Synanceiidae | Pseudosynanceia melanostigma Synanceia trachyni Synanceia verrucosa Synanceia horrida | [22,30] | |||
| Scorpaenidae | Scorpaena plumieri Scorpaena spotted | ||||
| Perciformes | Scatophagidae | Scatophagus argus | [31] | ||
| Latidae | Lates calcarifer | [22,29] | |||
| Lutjanidae | Lutjanus lutjanus | ||||
| Serranidae | Epinephelus coioides | ||||
| Siganidae | Siganus canaliculatus | ||||
| Sparidae | Sparus aurata | ||||
| Pomacentridae | Amphiprion clarkii | ||||
| Gonorynchiformes | Chanidae | Chanos chanos | |||
| Blenniiformes | Blenniidae | Salarias fasciatus | |||
| Carangiformes | Carangidae | Seriola dumerili | |||
| Echeneidae | Echeneis naucrates | ||||
| Mugiliformes | Mugilidae | Mugil cephalus | |||
| Pleuronectiformes | soleidae | Pardachirus marmuratus | |||
| Siluriformes | Plotosidae | Plotosus lineatus | |||
| Tetraodontiformes | Molidae | Mola mola | |||
| Tetraodontidae | Lagocephalus lunaris Takifugu oblongus | ||||
 | Reptilia | Squamata | Elapidae | Hydrophis hardwickii Hydrophis ornatus Hydrophis cyanocinctus Enhydrina schistosa Toxicocalamus longissimus Laticauda sp. Pelamis platurus Hydrophis lapemoides | [22,32,33,34,35] |
| Chondrichthyes | Milyobatiformes | Dasyatidae | Himantura gerrardi Hemitrygon bennetti Dasyatis kuhlii Himantura walga Pastinachus sephen | [22,36,37,38] | |
| Myliobatidae | Aetobatus flagellum | [39] | |||
| Gymnuridae | Gymnura poecilura | ||||
Porifera | Demospongiae | Clionaida | Clionaidae | Cliona celata Cliona vastifica | [40,41] |
Mollusca | Gastropda | Neogastropoda | Conidae | Conus textile Conus coronatus Conus betulinus Conus elegans Conus flavidus Conus monile Conus striatus Conus tessulatus Conus abbreviates Conus pennaceus Conus frigidus | [42,43,44,45,46] |
| Peptide ID | Pattern of Disulfide Bond Formation | * Protease Sensitivity | * BE DOPC (Kcal/mol) | Toxin ID | Species |
|---|---|---|---|---|---|
| CPP10-352 |  | R | −2.4 | Q8UW25 (cys-rich venome) | Hydrophis hardwickii |
| CPP10-1032 |  | R | −1.2 | A0A068B6R0 (conotoxin) | Conus betulinus |
| CPP10-1344 |  | R | −1.4 | P0C195 (µ-conotoxin) | Conus kinoshitai |
| CPP10-1537 |  | S | −1.6 | Q9UA85 (conotoxin) | Conus abbreviatus |
| CPP10-1743 |  | S | −1.7 | S4UJD3 (conotoxin) | Conus coronatus |
| CPP15-3 |  | S | −1.3 | B1B5I9 (δ-stichotoxin) | Stichodactyla haddoni |
| CPP15-4 |  | S | −1.7 | B1B5I9 (δ-stichotoxin) | S. haddoni |
| CPP20-2 | 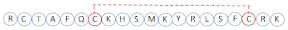 | S | −3.2 | E2S062 (κ-stichotoxin) | S. haddoni |
| CPP20-209 |  | S | −3.0 | P01437 (neurotoxin) | Hydrophis lapemoides |
| Target | Peptide ID | Peptide Sequence | Charge | Hydrophobicity (%) | µH | Toxin ID | Species |
|---|---|---|---|---|---|---|---|
| BBB-pp * | CPP10-946 | KKGRKNLWRR | 6 | 20 | 0.438 | A0A142C1C7 | Conus betulinus |
| CPP10-963 | KNFWKRNLYL | 3 | 40 | 0.371 | A0A142C1Q1 | C. betulinus | |
| CPP10-757 | VRWYRNGTCR | 3 | 20 | 0.241 | A0A6J2VWA1 | Chanos chanos | |
| NLS * | CPP15-29 | KPKKLRPSTKDYWYI | 4 | 33.33 | 0.267 | F2ZAF1 | Pterois volitans |
| CPP15-1019 | FLSKRKPSAERWRRD | 4 | 33.33 | 0.330 | P56711 | Conus pennaceus | |
| CPP15-377 | KRPRENIRFLSKRKS | 6 | 26.67 | 0.34 | Q9BHB7 | Conus textile | |
| mtCPP * | CPP15-81 | KLQATIAKKLFAIRS | 4 | 53.33 | 0.349 | Q91453 | Synanceia horrida |
| CPP15-144 | KLNLQRTIAKKLLSI | 4 | 46.67 | 0.361 | A0A068BD83 | Dendrochirus zebra | |
| CPP20-272 | LLTRRSLKNFWKRNLYLRDE | 4 | 35.00 | 0.215 | A0A142C1Q1 | C. betulinus | |
| THP * | CPP10-1537 | CSKICWRPRC | 3 | 30.00 | 0.244 | Q9UA85 | Conus abbreviatus |
| CPP10-1743 | SCWQRYPERC | 1 | 20 | 0.674 | S4UJD3 | Conus coronatus | |
| CPP10-1344 | SKWCRDHSRC | 2.5 | 10 | 0.621 | P0C195 | Conus kinoshitai |
| Peptide Name | Peptide Sequence | Charge | µH | Boman Index (Kcal/mol) | BBB | AMP Targets | Toxin Uniprot ID | Species |
|---|---|---|---|---|---|---|---|---|
| CPP10-11 | MKYRLSFCRK | 4 | 0.363 | 3.29 | √ | AFP (Ca), AVP, APCE | E2S062 | Stichodactyla haddoni |
| CPP10-757 | VRWYRNGTCR | 3 | 0.241 | 4.55 | √ | AVP, RSV, INFV, HSV, BA, TB | A0A6J2VWA1 | Chanos chanos |
| CPP10-858 | EKPLKRRVKQ | 4 | 0.472 | 4.99 | × | AFP, AVP, Kn, APCE | B0KZ78 | Conus betulinus |
| CPP15-81 | KLQATIAKKLFAIRS | 4 | 0.349 | 4.62 | × | BA, APCE, Sa, Ec, Pa, Kn | Q91453 | Synanceia horrida |
| CPP15-134 | KRQCRGVRVTRRSLR | 7 | 0.390 | 6.09 | √ | BA, Sa, Ec, Kn, TB, AFP, APCE | Q98993 | Synanceia verrucosa |
| CPP15-1029 | RDAINFRWRRSLIRR | 5 | 0.15 | 5.76 | √ | Sa, Ec, Kn, TB, APCE | Q9BP63 | Conus pennaceus |
| CPP15-1030 | DAINFRWRRSLIRRT | 4 | 0.226 | 4.94 | √ | Sa, Ec, Kn, TB, APCE | Q9BP63 | C. pennaceus |
| CPP15-1043 | AINVRRRRSITRRVS | 6 | 0.189 | 5.72 | √ | Sa, BA, APCE | Q9BP64 | C. pennaceus |
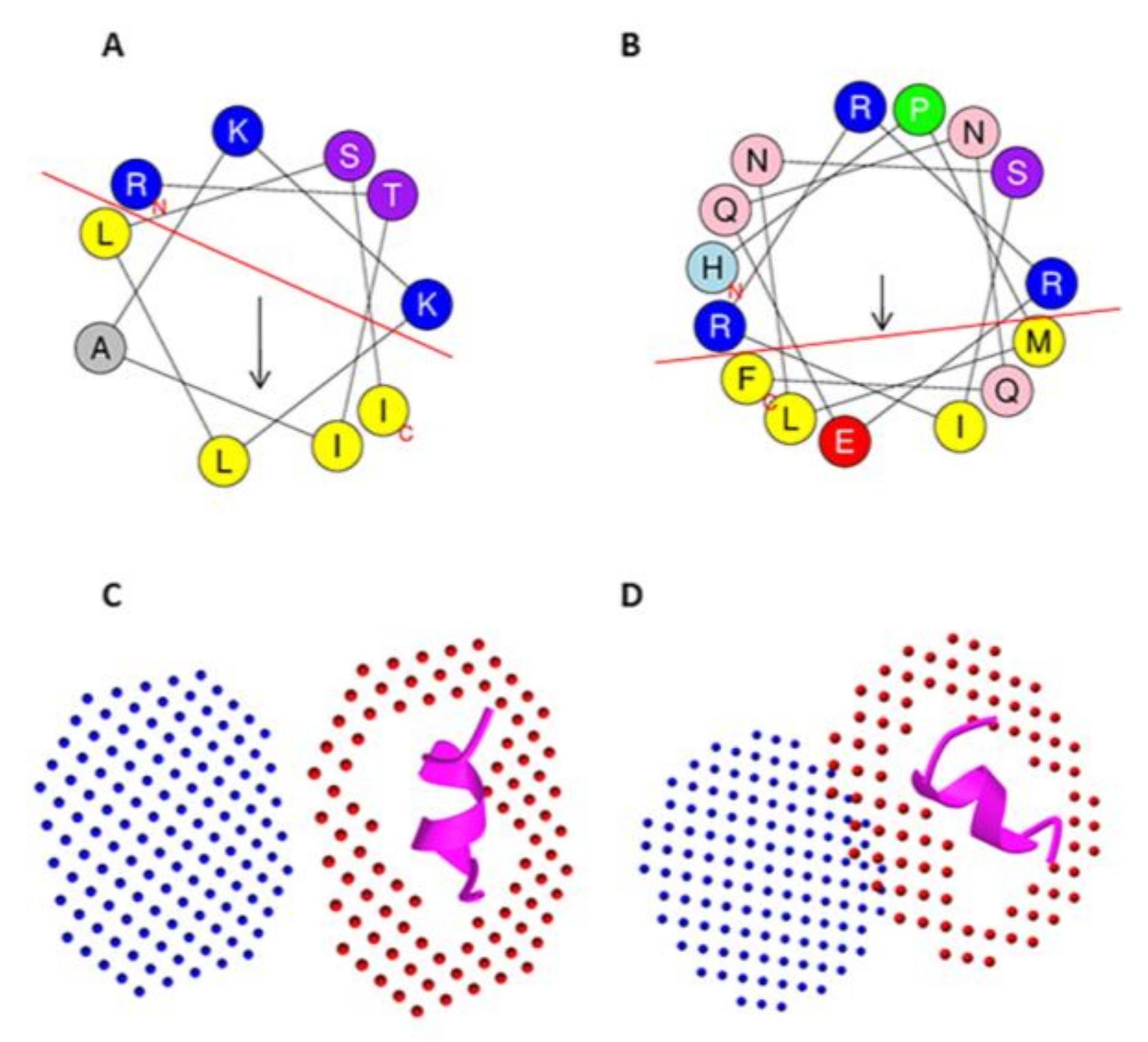
| CPP15-1050 | NRLKENIKFLLKRKT | 5 | 0.399 | 3.47 | √ | Ec, Kn, APCE, IL-4, IL-10, IFN-γ | Q9BPA6 | C. pennaceus |
| CPP20-45 | KRQCRGVRVTRRSLREFSHF | 6.5 | 0.379 | 5.01 | √ | Ec, Kn, BA, IL-4, IL-10, IFN-γ | Q98993 | S. verrucosa |
| CPP20-48 | KKICNDYKLNLQRTIAKKLL | 5 | 0.231 | 2.02 | × | Kn, AVP, APCE, IL-6, IFN-γ | A0A068BD83 | Dendrochirus zebra |
| CPP20-51 | RRFERYQQVLCNKGLSRRHY | 5.5 | 0.189 | 4.70 | √ | AVP, Ec, Kn, TB, BA, IL-10, IFN-γ | A0A068BFX2 | D. zebra |
| CPP20-156 | RAKINLLSKRKPPAERWWRW | 6 | 0.304 | 3.39 | √ | Ec, Kn, TB, AFP, RSV, INFV, HSV, IFN-γ | Q9BHA0 | Conus textile |
| CPP20-184 | VERAGENRSKENIKFLLKRK | 4 | 0.207 | 3.98 | × | AVP, BA, IL-4, IL-10, IFN-γ, HCV | Q9BPB7 | C. textile |
| Peptide Name | Peptide Sequence | DOPC BE (Kcal/mol) | Mit BE (Kcal/mol) | Charge | BBB Score | APCE (ImmunoAdjuvant Score) | Potential ACP Mechanisms | Toxin Uniprot ID | Organism |
|---|---|---|---|---|---|---|---|---|---|
| CPP10-186 | KAYLFRNLAK | −2.5 | −2.5 | 3 | 0.23 | - | MTP (0.57), PDL-1B | Q91453 | Synanceia horrida |
| CPP10-297 | LQRTIAKKLL | −4.0 | - | 3 | - | 0.13 | mIL-2/low IFN | A0A068BD83 | Dendrochirus zebra |
| CPP10-298 | RTIAKKLLSI | −3.7 | - | 3 | 0.10 | - | mIL-2/low IFN, PDL-1B | A0A068BD83 | D. zebra |
| CPP10-299 | TIAKKLLSIR | −3.7 | −3.4 | 3 | 0.11 | 0.27 | MTP (0.62) sIL-2/mIFN, PDL-1B | A0A068BD83 | D. zebra |
| CPP10-421 | ILLPALRKFC | −4.5 | −4.9 | 2 | 0.13 | - | MTP (0.64), sIL-2 | P0C1N6 | Conus textile |
| CPP10-757 | VRWYRNGTCR | −1.8 | - | 3 | 0.34 | 0.02 | THP, Anti-ang | A0A6J2VWA1 | Chanos chanos |
| CPP10-946 | KKGRKNLWRR | −1.9 | - | 6 | 0.43 | 0.5 | Anti-ang sIL-2 | A0A142C1C7 | Conus betulinus |
| CPP10-1389 | RRRRSITRRG | −0.2 | - | 6 | 0.29 | 0.85 | THP, Anti-ang sIL-2/sIFN-γ | Q9BP75 | Conus tessulatus |
| CPP10-1725 | HIFWSKRNCC | −2.7 | - | 2.5 | 0.30 | - | THP, Anti-ang sIL-2, PDL-1B | Q9BPH2 | Conus pennaceus |
| CPP15-4 | KHSMKYRLSFCRKTC | −1.7 | - | 5.5 | 0.24 | 0.29 | THP, Anti-ang mIL-2/low IFN | E2S062 | Synanceia haddoni |
| CPP15-5 | HSMKYRLSFCRKTCG | −1.7 | 4.5 | 0.21 | 0.17 | THP, Anti-ang, mIL-2 | E2S062 | S. haddoni | |
| CPP15-29 | KPKKLRPSTKDYWYI | −2.7 | - | 4 | 0.15 | - | Anti-ang, NLS lowIFN | A0A068BFX2 | D. zebra |
| CPP15-44 | KKQLIRRHYWEIKWS | −2.7 | - | 4 | - | 0.71 | Anti-ang | F2ZAF0 | Pterois volitans |
| CPP15-45 | KQLIRRHYWEIKWSG | −2.7 | - | 3.5 | - | 0.38 | PDL-1B | F2ZAF0 | P. volitans |
| CPP15-58 | LKKKLKTFQKHYERL | −3.0 | - | 5.5 | - | 0.67 | NLS mIL-2/low IFN | A0A2P1BRP3 | Scorpaena plumieri |
| CPP15-144 | KLNLQRTIAKKLLSI | −4.7 | −4.6 | 4 | - | - | MTP (0.69) lowIFN | A0A068BD83 | D. zebra |
| CPP15-423 | VPFFGKRLHHTCPCL | −3.6 | - | 3 | 0.09 | 0.63 | Anti-ang, membrane | A0A6J2VNJ3 | C. chanos |
| CPP20-48 | KKICNDYKLNLQRTIAKKLL | −5.0 | - | 5 | - | 0.32 | mIL-2/sIFN | A0A068BD83 | D. zebra |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmati, S.; Rasekhi Kazerooni, H. Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties. Mar. Drugs 2022, 20, 763. https://doi.org/10.3390/md20120763
Hemmati S, Rasekhi Kazerooni H. Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties. Marine Drugs. 2022; 20(12):763. https://doi.org/10.3390/md20120763
Chicago/Turabian StyleHemmati, Shiva, and Haniyeh Rasekhi Kazerooni. 2022. "Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties" Marine Drugs 20, no. 12: 763. https://doi.org/10.3390/md20120763
APA StyleHemmati, S., & Rasekhi Kazerooni, H. (2022). Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties. Marine Drugs, 20(12), 763. https://doi.org/10.3390/md20120763





