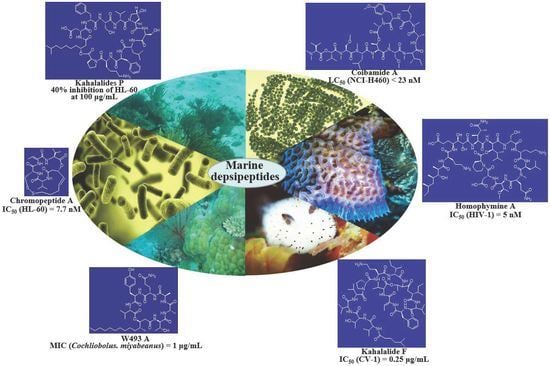
Marine Organisms as a Prolific Source of Bioactive Depsipeptides
Abstract
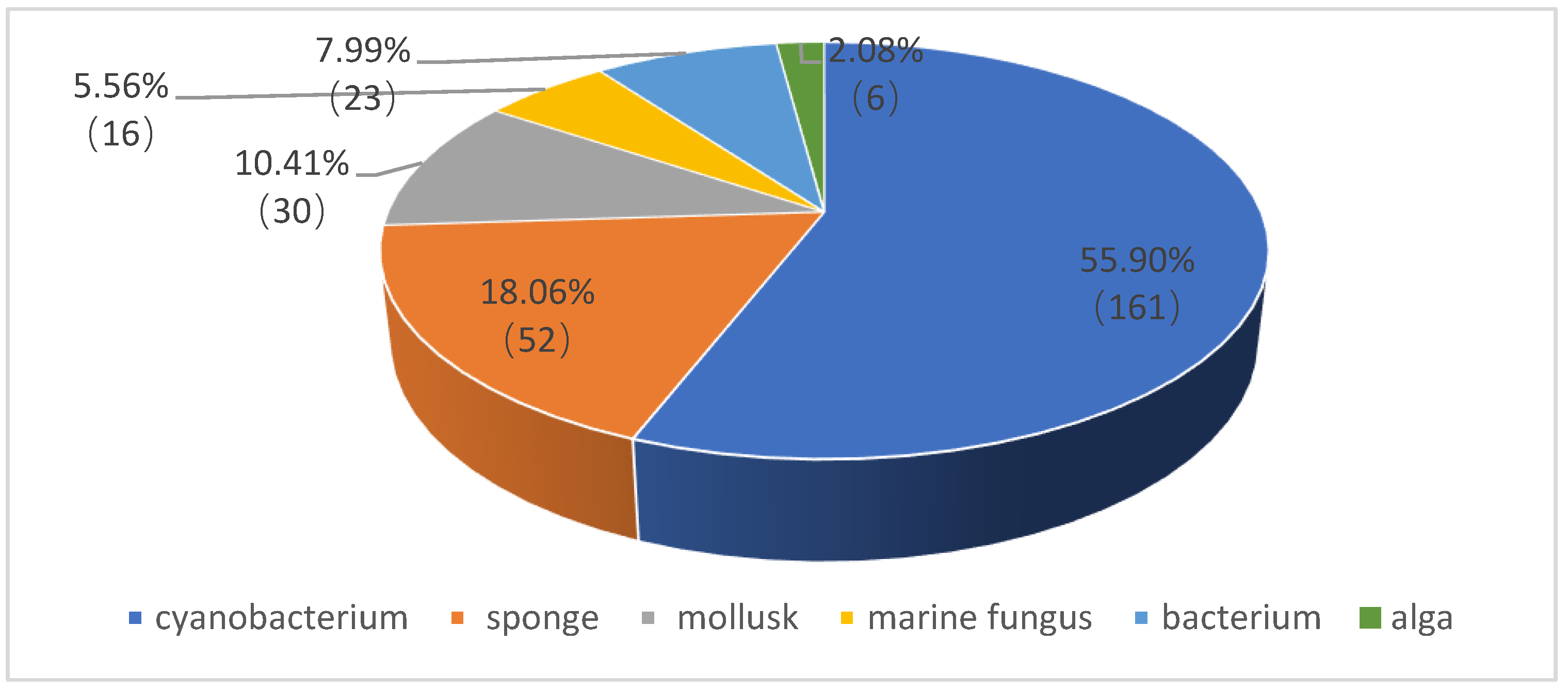
:1. Introduction
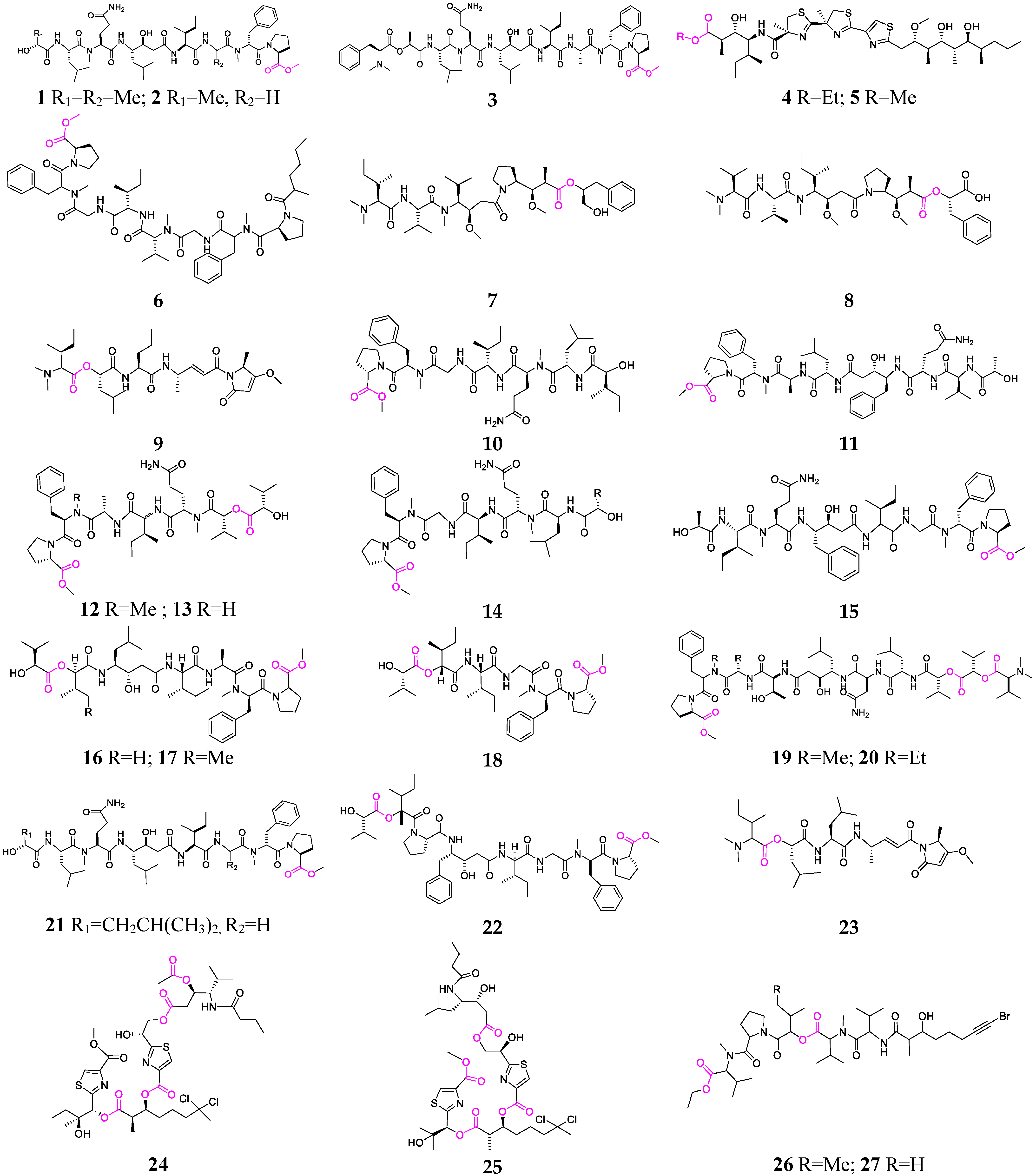
2. Marine Cyanobacteria
2.1. Linear Depsipeptides
2.2. Cyclic Depsipeptides
2.2.1. Cyclopentadepsipeptides
2.2.2. Cyclohexadepsipeptide
2.2.3. Cycloheptadepsipeptide
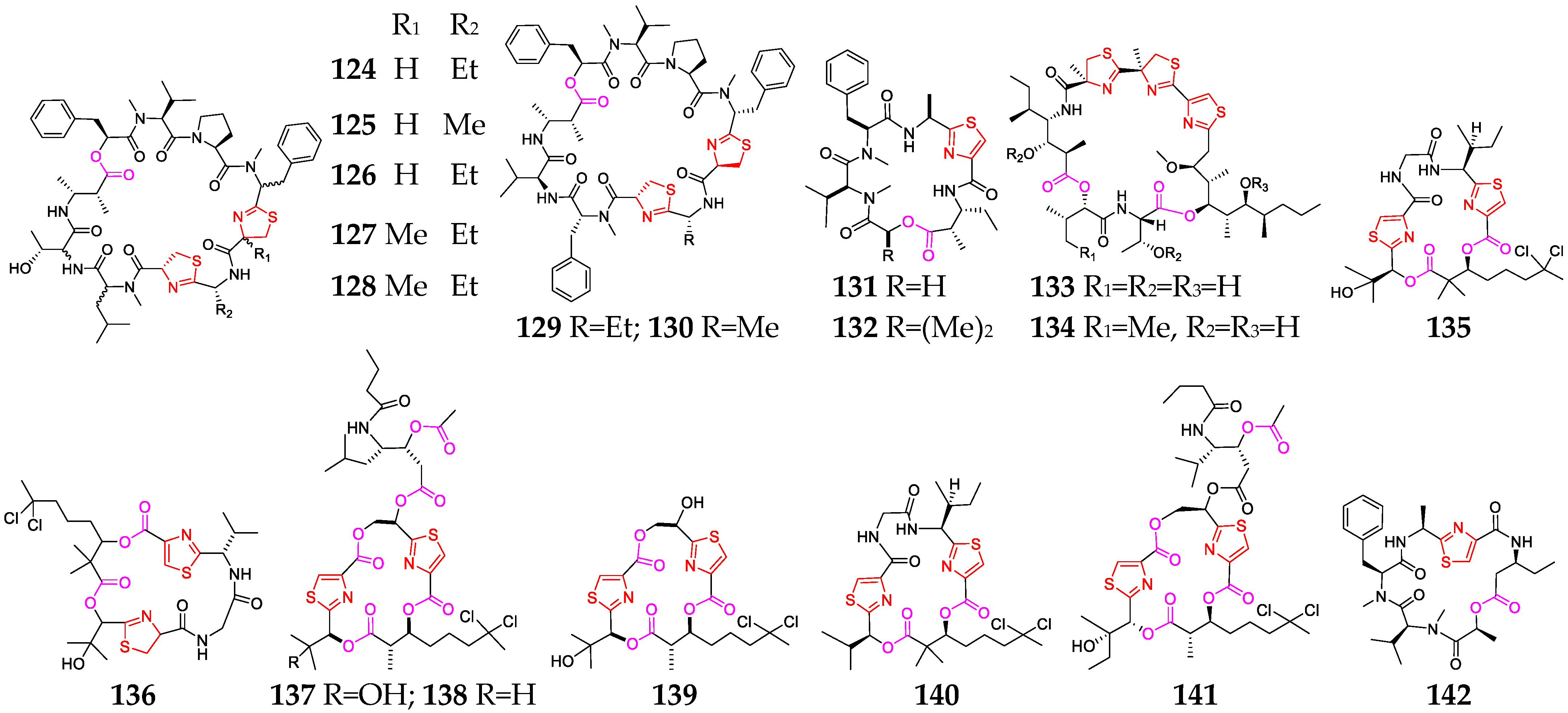
2.2.4. Thiazole-Containing Cyclodepsipeptides
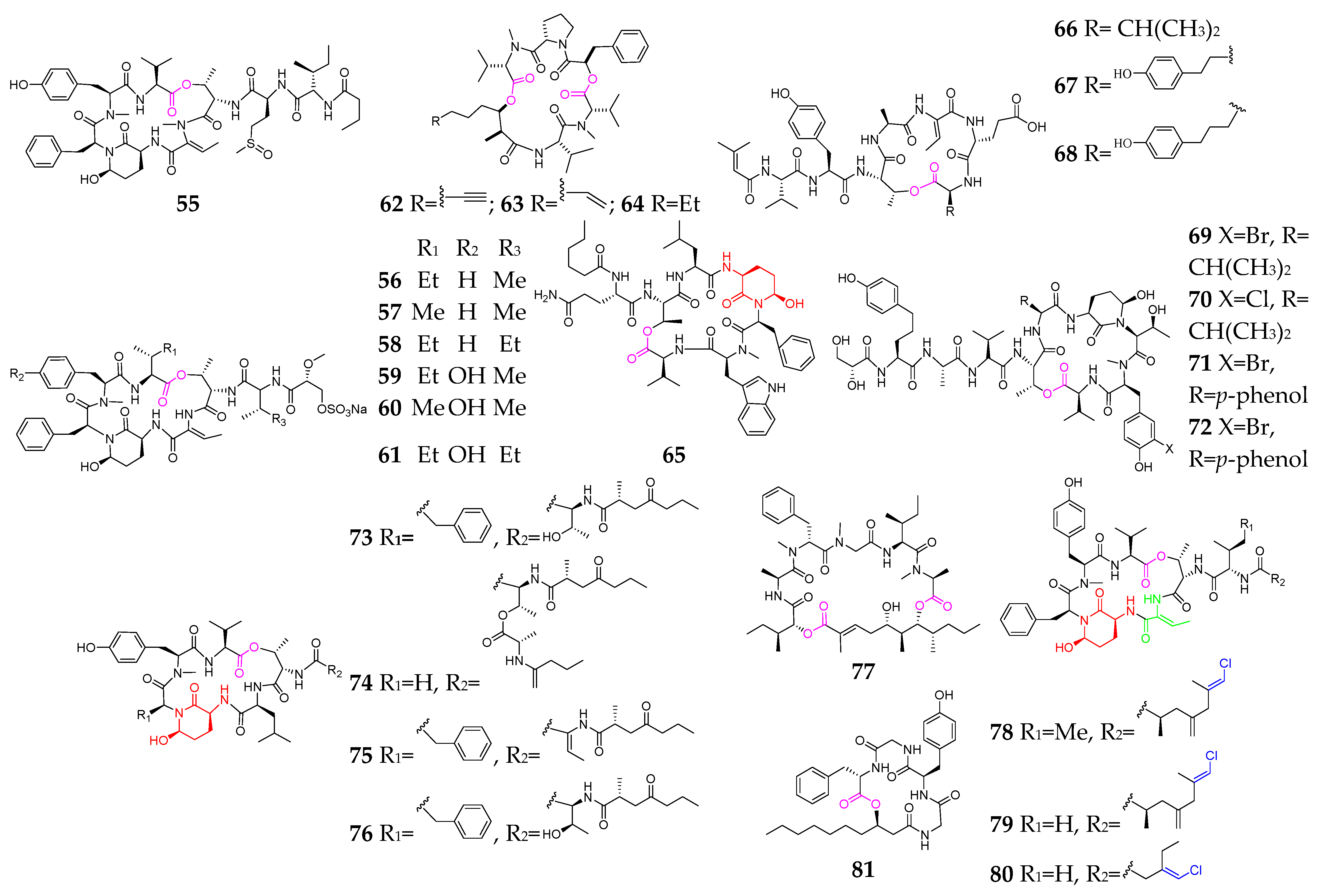
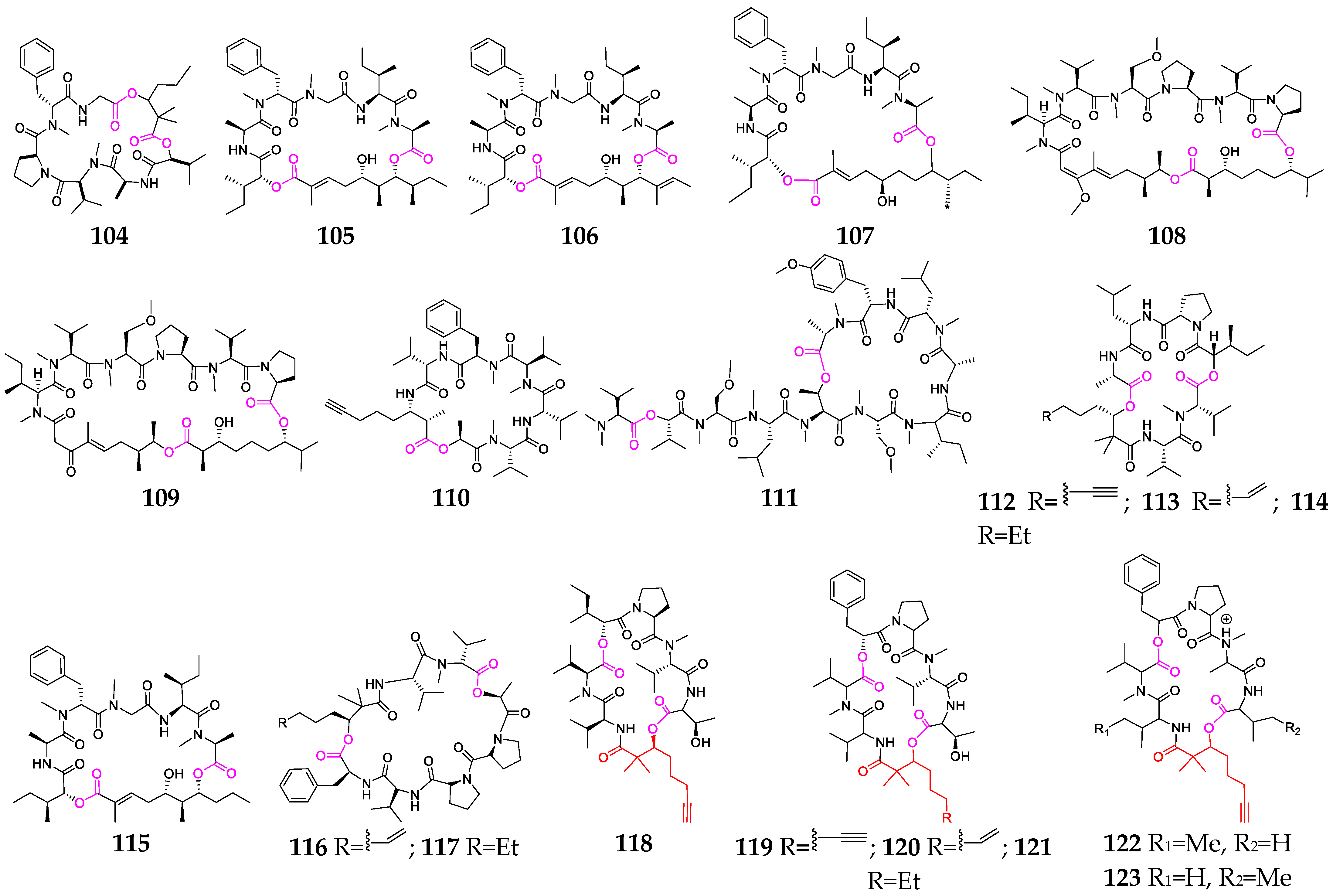
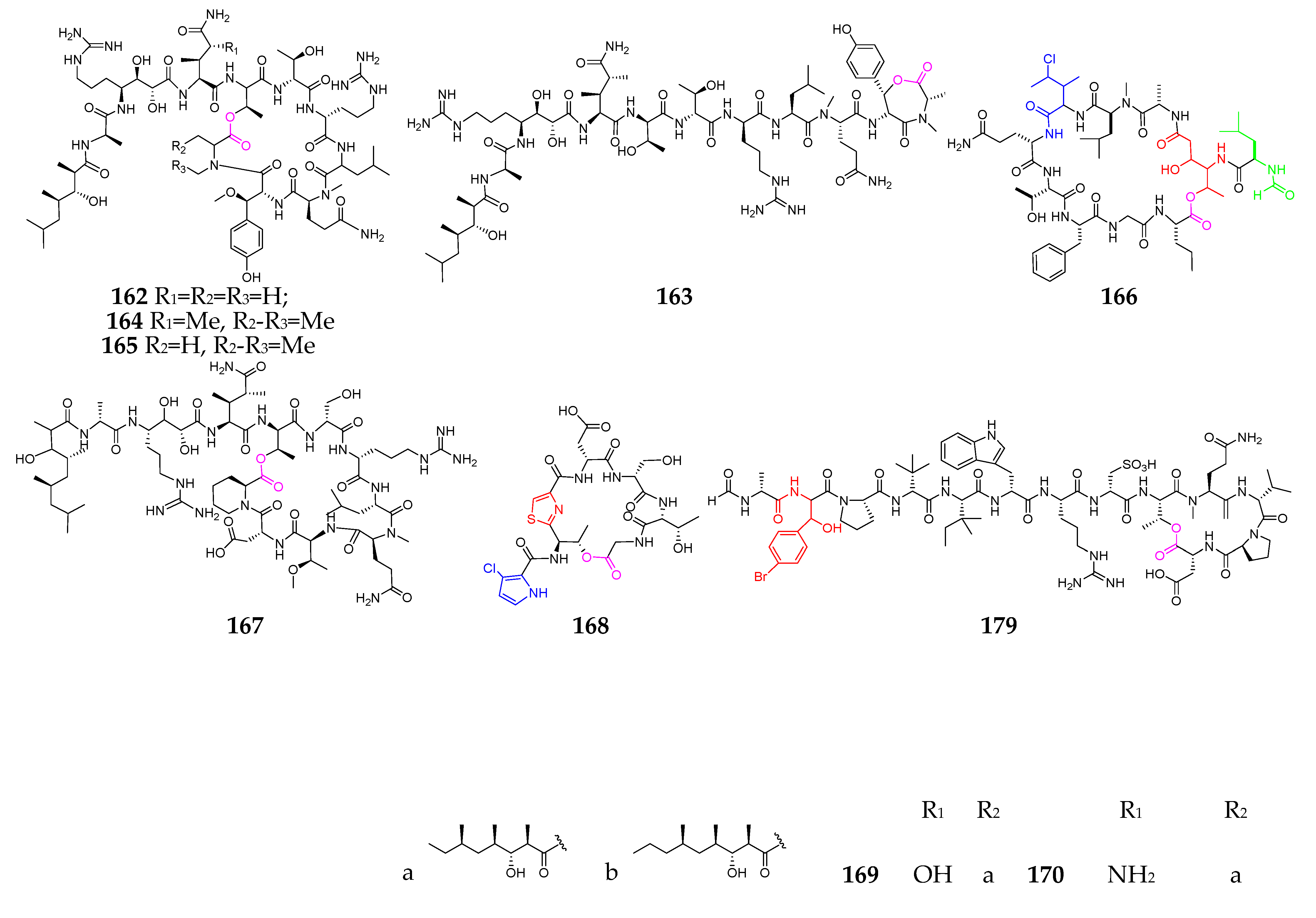
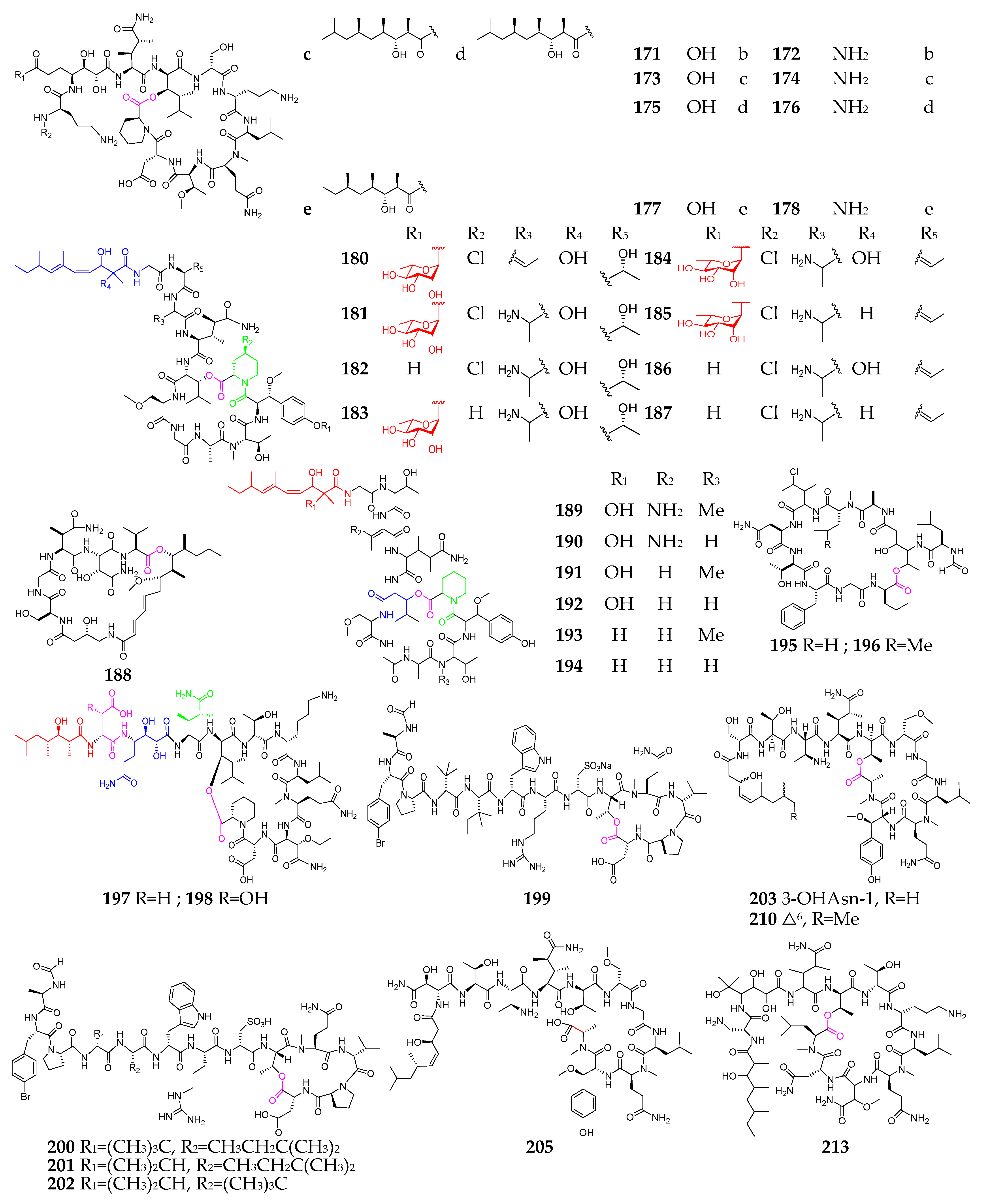
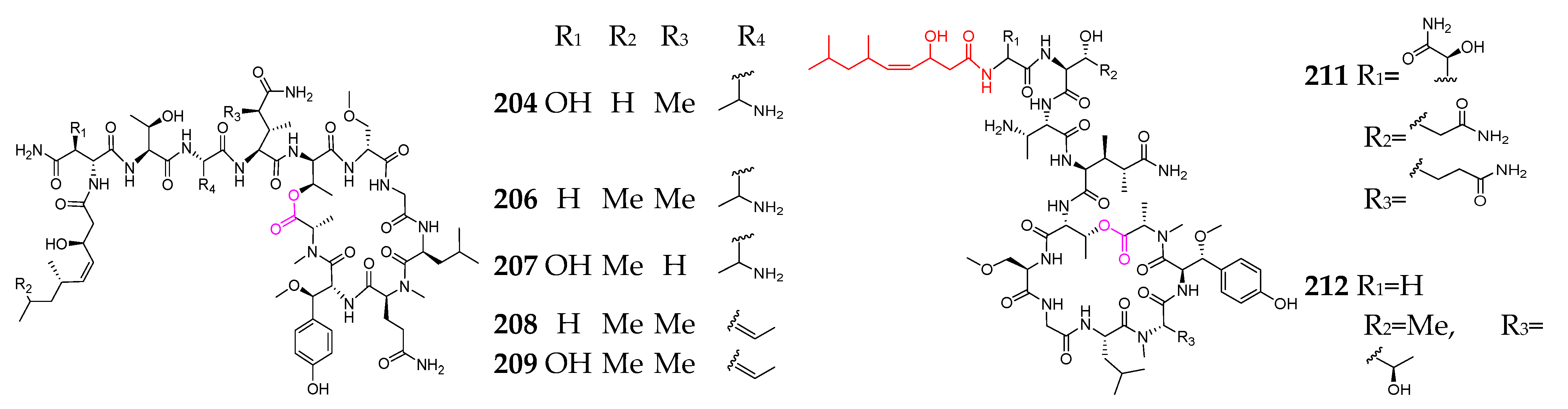
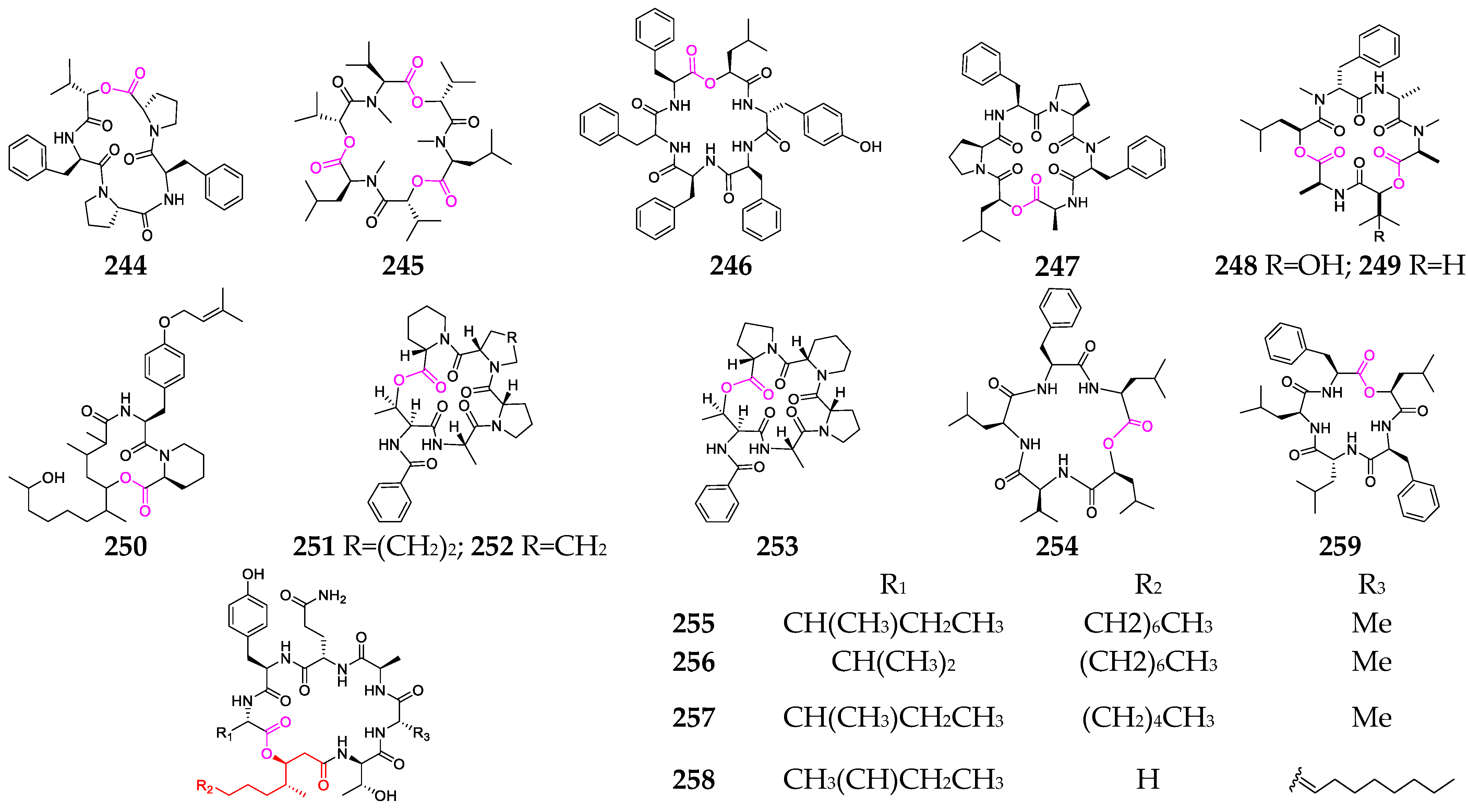
2.2.5. Other Cyclodepsipeptides
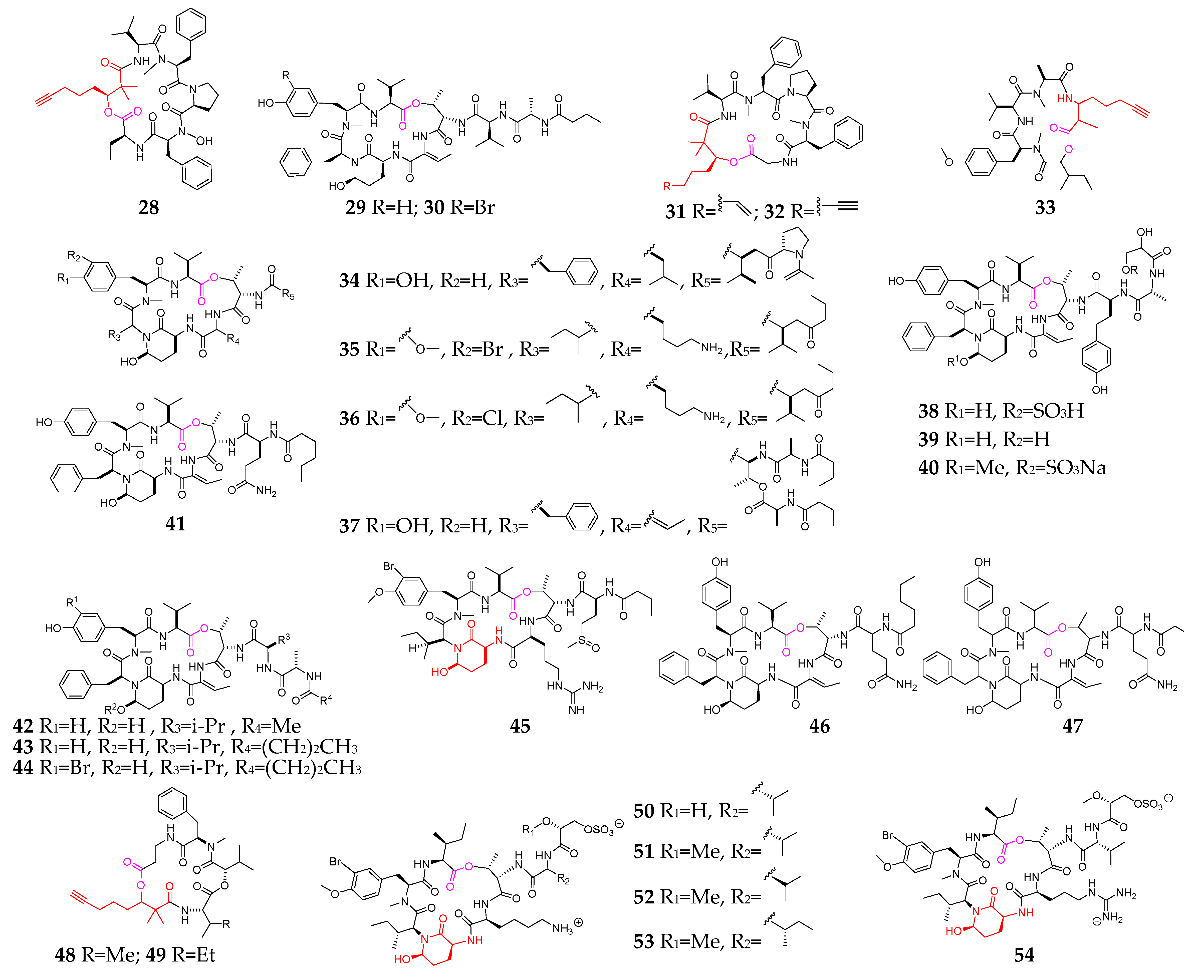
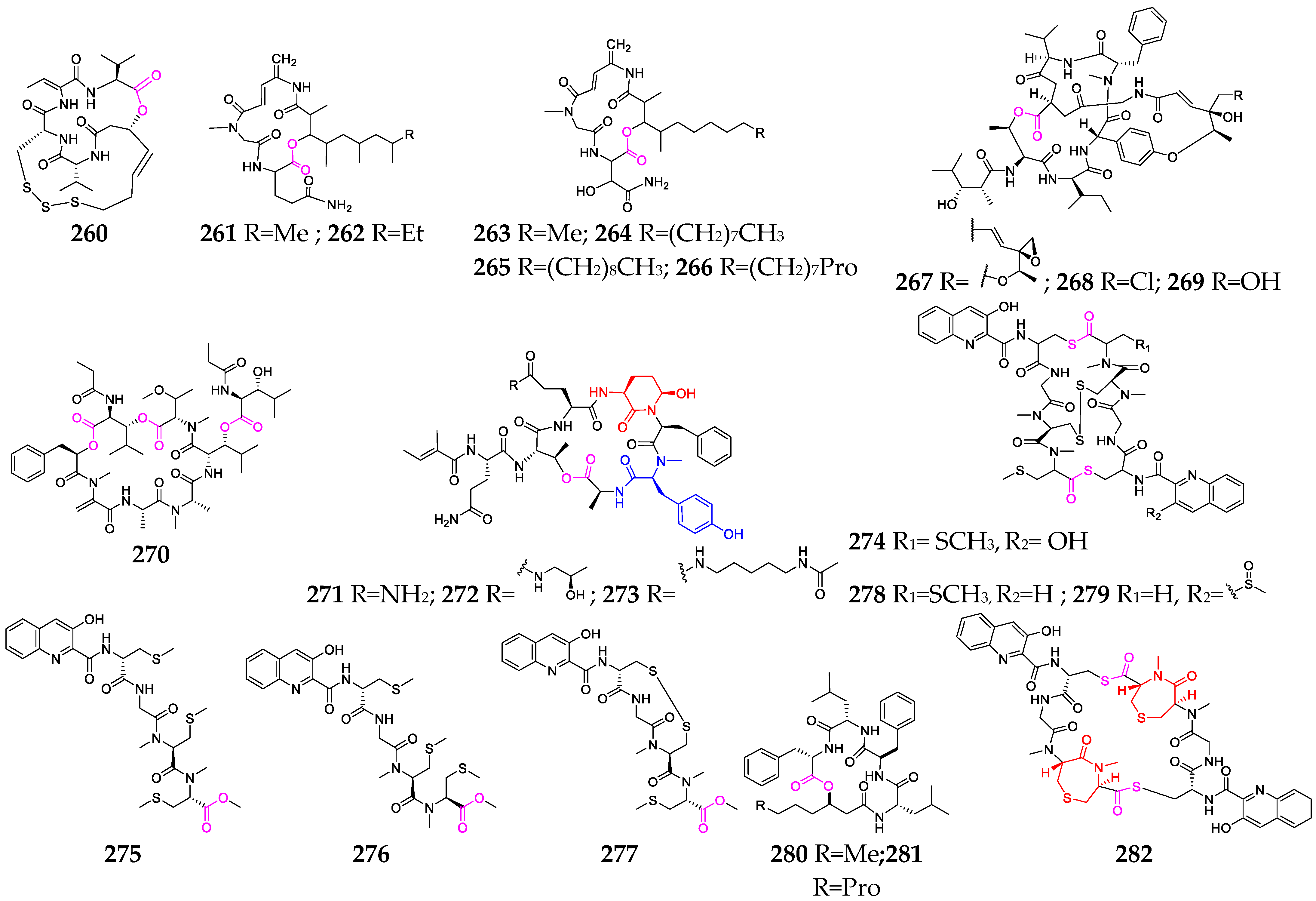
3. Marine Sponges
4. Marine Mollusks
5. Marine Fungi
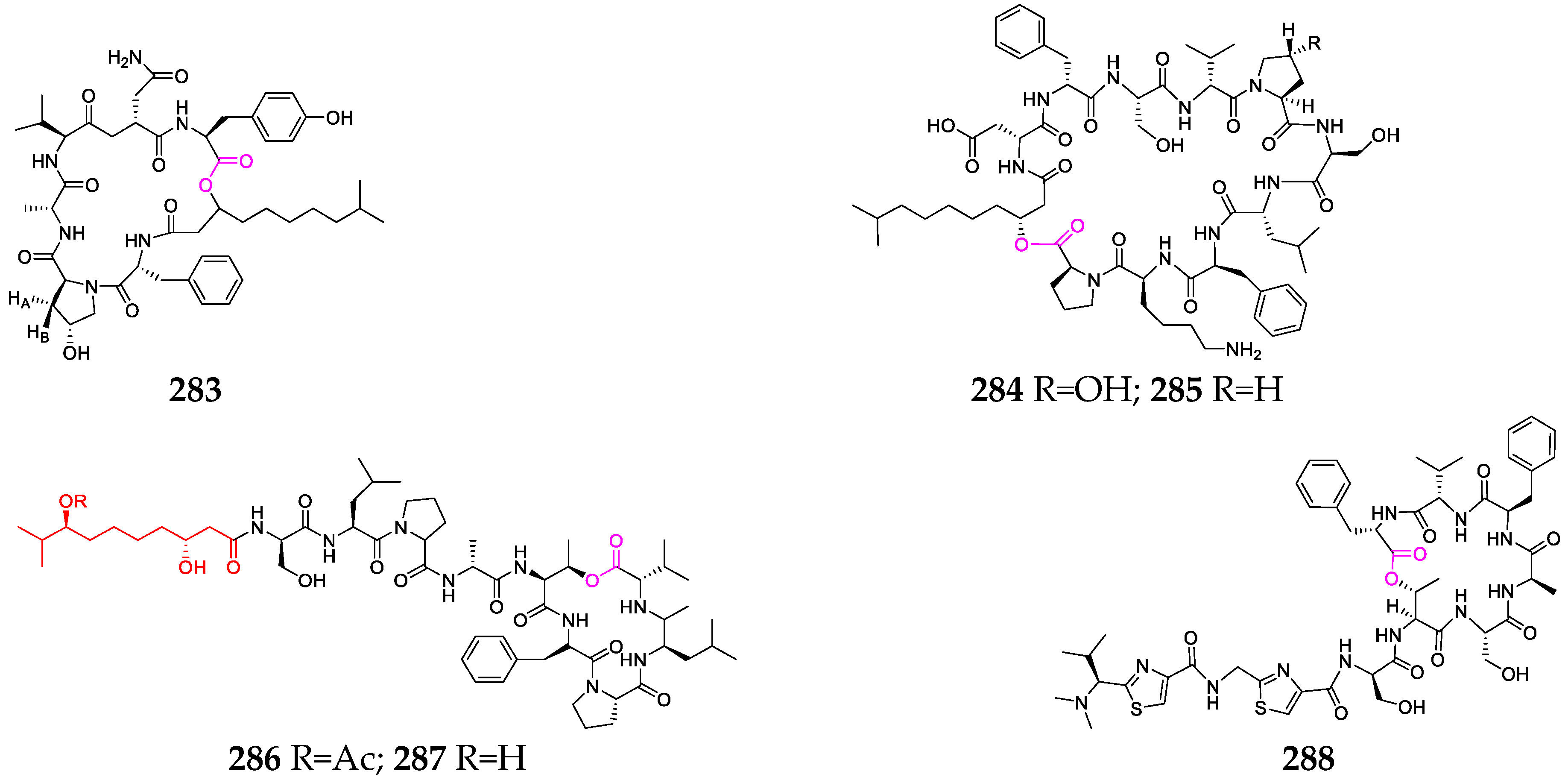
6. Marine Bacteria
7. Marine Algae
8. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Papon, N.; Copp, B.R.; Courdavault, V. Marine drugs: Biology, pipelines, current and future prospects for production. Biotechnol. Adv. 2022, 54, 0734–9750. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring marine as a rich source of bioactive peptides: Challenges and opportunities from marine pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef]
- Alonzo, D.A.; Schmeing, T.M. Biosynthesis of depsipeptides, or Depsi: The peptides with varied generations. Protein Sci. 2020, 29, 2316–2347. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Camus, V.; Thieblemont, C.; Sibon, D.; Casasnovas, R.O.; Ysebaert, L.; Damaj, G.; Guidez, S.; Pica, G.M.; Kim, W.S.; et al. Romidepsin Plus CHOP Versus CHOP in Patients with Previously Untreated Peripheral T-Cell Lymphoma: Results of the Ro-CHOP Phase III Study (Conducted by LYSA). J. Clin. Oncol. 2022, 40, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Broggini, M.; Marchini, S.V.; Galliera, E.; Borsotti, P.; Taraboletti, G.; Erba, E.; Sironi, M.; Jimeno, J.; Faircloth, G.T.; Giavazzi, R.; et al. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia 2003, 17, 52–59. [Google Scholar] [CrossRef]
- Miguel-Lillo, B.; Valenzuela, B.; Peris-Ribera, J.E.; Soto-Matos, A.; Pérez-Ruixo, J.J. Population pharmacokinetics of kahalalide F in advanced cancer patients. Cancer Chemother. Pharm. 2015, 76, 365–374. [Google Scholar] [CrossRef]
- Wyer, S.; Townsend, D.M.; Ye, Z.; Kourtidis, A.; Choo, Y.M.; de Barros, A.L.B.; Donia, M.S.; Hamann, M.T. Recent advances and limitations in the application of kahalalides for the control of cancer. Biomed. Pharmacother. 2022, 148, 112676. [Google Scholar] [CrossRef]
- Heath, E.I.; Weise, A.; Vaishampayan, U.; Danforth, D.; Ungerleider, R.S.; Urata, Y. Phase Ia dose escalation study of OBP-801, a cyclic depsipeptide class I histone deacetylase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 300–307. [Google Scholar] [CrossRef]
- Gao, F.-x.; Tian, M. Research progress on the depsipeptides. Chin. J. Antibiot. 2018, 43, 777–785. [Google Scholar]
- Rangel, M.; de Santana, C.J.; Pinheiro, A.; Dos Anjos, L.; Barth, T.; Pires, O.R.; Fontes, W.; Castro, M.S. Marine depsipeptides as promising pharmacotherapeutic agents. Curr. Protein Pept. Sci. 2017, 18, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhang, J.R.; He, S.; Yan, X.J. New peptides isolated from marine cyanobacteria, an overview over the past decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, F.H.; Law, B.K.; Paul, V.J.; Luesch, H. Grassystatins D–F, potent aspartic protease inhibitors from marine cyanobacteria as potential antimetastatic agents targeting invasive breast cancer. J. Nat. Prod. 2017, 80, 2969–2986. [Google Scholar] [CrossRef]
- Choi, H.; Pereira, A.R.; Cao, Z.; Shuman, C.F.; Engene, N.; Byrum, T.; Matainaho, T.; Murray, T.F.; Mangoni, A.; Gerwick, W.H. The hoiamides, structurally intriguing neurotoxic lipopeptides from Papua New Guinea marine cyanobacteria. J. Nat. Prod. 2010, 73, 1411–1421. [Google Scholar] [CrossRef]
- Malloy, K.L.; Choi, H.; Fiorilla, C.; Valeriote, F.A.; Matainaho, T.; Gerwick, W.H. Hoiamide D, a marine cyanobacteria-derived inhibitor of p53/MDM2 interaction. Bioorg. Med. Chem. Lett. 2012, 22, 683–688. [Google Scholar] [CrossRef]
- Horgen, F.D.; Kazmierski, E.B.; Westenburg, H.E.; Yoshida, W.Y.; Scheuer, P.J. Malevamide D: Isolation and structure determination of an isodolastatin H analogue from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 2002, 65, 487–491. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Symplostatin 3, a new dolastatin 10 analogue from the marine cyanobacterium Symploca sp. VP452. J. Nat. Prod. 2002, 65, 16–20. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasiamide, a cytotoxic peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2002, 65, 1336–1339. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. The isolation and structure elucidation of Tasiamide B, a 4-amino-3-hydroxy-5-phenylpentanoic acid containing peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2003, 66, 1006–1009. [Google Scholar] [CrossRef]
- Mevers, E.; Haeckl, F.P.; Boudreau, P.D.; Byrum, T.; Dorrestein, P.C.; Valeriote, F.A.; Gerwick, W.H. Lipopeptides from the tropical marine cyanobacterium Symploca sp. J. Nat. Prod. 2014, 77, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, F.H.; Ratnayake, R.; Paul, V.J.; Luesch, H. Tasiamide F, a potent inhibitor of cathepsins D and E from a marine cyanobacterium. Bioorg. Med. Chem. 2016, 24, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Iwasaki, A.; Sumimoto, S.; Matsubara, T.; Sato, T.; Suenaga, K. Izenamides A and B, statine-containing depsipeptides, and an analogue from a marine cyanobacterium. J. Nat. Prod. 2018, 81, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A–C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Suda, S.; Suenaga, K. Maedamide, a novel chymotrypsin inhibitor from a marine cyanobacterial assemblage of Lyngbya sp. Tetrahedron Lett. 2014, 55, 4126–4128. [Google Scholar] [CrossRef]
- Williams, P.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Continuing studies on the cyanobacterium Lyngbya sp.: Isolation and structure determination of 15-norlyngbyapeptin A and lyngbyabellin D. J. Nat. Prod. 2003, 66, 595–598. [Google Scholar] [CrossRef]
- Petitbois, J.G.; Casalme, L.O.; Lopez, J.A.V.; Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Yoshimura, E.; Nogata, Y.; Umezawa, T.; Matsuda, F.; et al. Serinolamides and Lyngbyabellins from an Okeania sp. Cyanobacterium Collected from the Red Sea. J. Nat. Prod. 2017, 80, 2708–2715. [Google Scholar] [CrossRef]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.D.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.-T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef]
- Rubio, B.K.; Parrish, S.M.; Yoshida, W.; Schupp, P.J.; Schils, T.; Williams, P.G. Depsipeptides from a guamanian marine cyanobacterium, Lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron Lett. 2010, 51, 6718–6721. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Owle, C.S.; Montaser, R.; Luesch, H.; Paul, V.J. Malyngamide 3 and cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from cocos lagoon, guam. J. Nat. Prod. 2011, 74, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Sitachitta, N.; Gerwick, W.H. The guineamides, novel cyclic depsipeptides from a papua new guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2003, 66, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008, 71, 1625–1629. [Google Scholar] [CrossRef]
- Al-Awadhi, F.H.; Salvador, L.A.; Law, B.K.; Paul, V.J.; Luesch, H. Kempopeptin C, a novel marine-derived serine protease inhibitor targeting invasive breast cancer. Mar. Drugs 2017, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Sumimoto, S.; Ohno, O.; Suda, S.; Suenaga, K. Kurahamide, a cyclic depsipeptide analog of dolastatin 13 from a marine cyanobacterial assemblage of Lyngbya sp. Bull. Chem. Soc. Jpn. 2014, 87, 609–613. [Google Scholar] [CrossRef]
- Matthew, S.; Ross, C.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007, 70, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5-7, potent elastase inhibitors from floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Taori, K.; Paul, V.J.; Luesch, H. Lyngbyastatins 8-10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef]
- Matthew, S.; Ross, C.; Paul, V.J.; Luesch, H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar] [CrossRef]
- Ozaki, K.; Iwasaki, A.; Suenaga, K.; Teruya, T. Kyanamide, a new Ahp-containing depsipeptide from marine cyanobacterium Caldora penicillata. Tetrahedron 2019, 75, 3382–3386. [Google Scholar] [CrossRef]
- Gallegos, D.A.; Sauri, J.; Cohen, R.D.; Wan, X.; Videau, P.; Vallota-Eastman, A.O.; Shaala, L.A.; Youssef, D.T.A.; Williamson, R.T.; Martin, G.E.; et al. Jizanpeptins, cyanobacterial protease inhibitors from a Symploca sp. cyanobacterium collected in the red sea. J. Nat. Prod. 2018, 81, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, G.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Paul, V.J. Symplostatin 2: A dolastatin 13 analogue from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 1999, 62, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.A.; Taori, K.; Biggs, J.S.; Jakoncic, J.; Ostrov, D.A.; Paul, V.J.; Luesch, H. Potent elastase inhibitors from cyanobacteria: Structural basis and mechanisms mediating cytoprotective and anti-inflammatory effects in bronchial epithelial cells. J. Med. Chem. 2013, 56, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Phyo, M.Y.; Katermeran, N.P.; Goh, J.X.; Tan, L.T. Trikoveramides A-C, cyclic depsipeptides from the marine cyanobacterium Symploca hydnoides. Phytochemistry 2021, 190, 112879. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Bewley, C.A. Largamides A-H, unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J. Org. Chem. 2006, 71, 6898–6907. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, F.H.; Paul, V.J.; Luesch, H. Structural diversity and anticancer activity of marine-derived elastase inhibitors: Key features and mechanisms mediating the antimetastatic effects in invasive breast cancer. ChemBioChem 2018, 19, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Miller, M.W.; Kwan, J.C.; Luesch, H.; Paul, V.J. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010, 73, 459–462. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Okeania sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J.; Sikandar, A.; Naman, C.B.; Glukhov, E.; Luo, D.; Duggan, B.M.; et al. Tutuilamides A-C: Vinyl-chloride-containing cyclodepsipeptides from marine cyanobacteria with potent elastase inhibitory properties. ACS Chem. Biol. 2020, 15, 751–757. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, cancer cell toxins from a papua new guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Bunyajetpong, S.; Yoshida, W.Y.; Sitachitta, N.; Kaya, K. Trungapeptins A-C, cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.A.; Biggs, J.S.; Paul, V.J.; Luesch, H. Veraguamides A-G, cyclic hexadepsipeptides from a dolastatin 16-producing cyanobacterium Symploca cf. hydnoides from Guam. J. Nat. Prod. 2011, 74, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Shiota, I.; Sumimoto, S.; Matsubara, T.; Sato, T.; Suenaga, K. Kohamamides A, B, and C, cyclic depsipeptides from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2017, 80, 1948–1952. [Google Scholar] [CrossRef]
- Luo, D.; Putra, M.Y.; Ye, T.; Paul, V.J.; Luesch, H. Isolation, structure elucidation and biological evaluation of Lagunamide D: A new cytotoxic macrocyclic depsipeptide from marine cyanobacteria. Mar. Drugs 2019, 17, 83. [Google Scholar] [CrossRef]
- Phyo, M.Y.; Goh, J.X.; Tan, L.T. Triproamide and Pemukainalides, cyclic depsipeptides from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 2022, 85, 485–492. [Google Scholar] [CrossRef]
- Boudreau, P.D.; Byrum, T.; Liu, W.T.; Dorrestein, P.C.; Gerwick, W.H. Viequeamide A, a cytotoxic member of the kulolide superfamily of cyclic depsipeptides from a marine button cyanobacterium. J. Nat. Prod. 2012, 75, 1560–1570. [Google Scholar] [CrossRef]
- Kwan, J.C.; Ratnayake, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Grassypeptolides A-C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides. J. Org. Chem. 2010, 75, 8012–8023. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.; McPhail, K.L. Cyclic depsipeptides, grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Ratnayake, R.; Wilson, J.A.; Beutler, J.A.; Colburn, N.H.; Henrich, C.J.; McMahon, J.B.; McKee, T.C. Grassypeptolides F and G, cyanobacterial peptides from Lyngbya majuscula. J. Nat. Prod. 2011, 74, 1686–1691. [Google Scholar] [CrossRef]
- Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Hoiamide a, a sodium channel activator of unusual architecture from a consortium of two papua new Guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; McPhail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and structure of five lyngbyabellin derivatives from a papua new guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron 2005, 61, 11723–11729. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Isolation and structure determination of obyanamide, a novel cytotoxic cyclic depsipeptide from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2002, 65, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Gross, H.; McPhail, K.L.; Goeger, D.; Maier, C.S.; Gerwick, W.H. Wewakamide A and guineamide G, cyclic depsipeptides from the marine cyanobacteria Lyngbya semiplena and Lyngbya majuscula. J. Microbiol. Biotechnol. 2011, 21, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Davies-Coleman, M.T.; Dzeha, T.M.; Gray, C.A.; Hess, S.; Pannell, L.K.; Hendricks, D.T.; Arendse, C.E. Isolation of homodolastatin 16, a new cyclic depsipeptide from a kenyan collection of Lyngbya majuscula. J. Nat. Prod. 2003, 66, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, G.G.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Park, P.U.; Biggs, J.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H.; Valeriote, F.A. Isolation, structure determination, and biological activity of dolastatin 12 and lyngbyastatin 1 from Lyngbya majuscula/Schizothrix calcicola cyanobacterial assemblages. J. Nat. Prod. 1998, 61, 1221–1225. [Google Scholar] [CrossRef]
- Williams, P.G.; Moore, R.E.; Paul, V.J. Isolation and structure determination of lyngbyastatin 3, a lyngbyastatin 1 homologue from the marine cyanobacterium Lyngbya majuscula. Determination of the configuration of the 4-amino-2,2-dimethyl-3-oxopentanoic acid unit in majusculamide C, dolastatin 12, lyngbyastatin 1, and lyngbyastatin 3 from cyanobacteria. J. Nat. Prod. 2003, 66, 1356–1363. [Google Scholar]
- Han, B.; Goeger, D.; Maier, C.S.; Gerwick, W.H. The wewakpeptins, cyclic depsipeptides from a papua new guinea collection of the marine cyanobacterium Lyngbya semiplena. J. Org. Chem. 2005, 70, 3133–3139. [Google Scholar] [CrossRef]
- Adams, B.; Porzgen, P.; Pittman, E.; Yoshida, W.Y.; Westenburg, H.E.; Horgen, F.D. Isolation and structure determination of malevamide E, a dolastatin 14 analogue, from the marine cyanobacterium symploca laete-viridis. J. Nat. Prod. 2008, 71, 750–754. [Google Scholar] [CrossRef]
- Vining, O.B.; Medina, R.A.; Mitchell, E.A.; Videau, P.; Li, D.; Serrill, J.D.; Kelly, J.X.; Gerwick, W.H.; Proteau, P.J.; Ishmael, J.E.; et al. Depsipeptide companeramides from a Panamanian marine cyanobacterium associated with the coibamide producer. J. Nat. Prod. 2015, 78, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, K.; Burgoyne, D.L.; Moore, R.E.; Patterson, G.M.L.; Smith, C.D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem. 2002, 59, 7219–7226. [Google Scholar] [CrossRef]
- Kanamori, Y.; Iwasaki, A.; Sumimoto, S.; Suenaga, K. Urumamide, a novel chymotrypsin inhibitor with a β-amino acid from a marine cyanobacterium Okeania sp. Tetrahedron Lett. 2016, 57, 4213–4216. [Google Scholar] [CrossRef]
- Zampella, A.; D’Auria, M.V.; Paloma, L.G.; Casapullo, A.; Minale, L.; Debitus, C.; Henin, Y. Callipeltin A, an anti-HIV cyclic depsipeptide from the new caledonian lithistida sponge Callipelta sp. J. Am. Chem. Soc. 1996, 118, 6202–6209. [Google Scholar] [CrossRef]
- Stierhof, M.; Hansen, K.Ø.; Sharma, M.; Feussner, K.; Subko, K.; Díaz-Rullo, F.F.; Isaksson, J.; Pérez-Victoria, I.; Clarke, D.; Hansen, E.; et al. New cytotoxic callipeltins from the Solomon Island marine sponge Asteropus sp. Tetrahedron 2016, 72, 6929–6934. [Google Scholar] [CrossRef]
- Clark, D.P.; Carroll, J.; Naylor, S.; Crews, P. An antifungal cyclodepsipeptide, Cyclolithistide A, from the sponge Theonella swinhoei. J. Org. Chem. 1998, 63, 8757–8764. [Google Scholar] [CrossRef]
- Urda, C.; Fernandez, R.; Rodriguez, J.; Perez, M.; Jimenez, C.; Cuevas, C. Daedophamide, a cytotoxic cyclodepsipeptide from a Daedalopelta sp. sponge collected in indonesia. J. Nat. Prod. 2017, 80, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, G.; Fernández, R.; Cruz, P.G.; Pérez, M.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Combining JBCA and Marfey’s methodology to determine the absolute configuration of threonines: The case of gunungamide A, a new cyclic depsipeptide containing chloropyrrole from the sponge Discodermia sp. Org. Chem. Front. 2019, 6, 15–21. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.C.; D’Auria, M.V.; Jepsen, T.; Petek, S.; Adeline, M.T.; Laprevote, O.; et al. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Petek, S.; Poupat, C.; Ahond, A. Homophymines B-E and A1-E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Cartner, L.K.; Shigematsu, N.; Pannell, L.K.; Boyd, M.R. Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J. Nat. Prod. 2001, 64, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Gustchina, E.; Baker, H.L.; Kelly, M.; Bewley, C.A. Mirabamides A-D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J. Nat. Prod. 2007, 70, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; Dilip de Silva, E.; Lassota, P.; Allen, T.M.; et al. Papuamides A−D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in papua new guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Coello, L.; Reyes, F.; Martin, M.J.; Cuevas, C.; Fernandez, R. Isolation and structures of pipecolidepsins A and B, cytotoxic cyclic depsipeptides from the madagascan sponge Homophymia lamellosa. J. Nat. Prod. 2014, 77, 298–303. [Google Scholar] [CrossRef]
- Feng, Y.; Carroll, A.R.; Pass, D.M.; Archbold, J.K.; Avery, V.M.; Quinn, R.J. Polydiscamides B-D from a marine sponge Ircinia sp. as potent human sensory neuron-specific G protein coupled receptor agonists. J. Nat. Prod. 2008, 71, 8–11. [Google Scholar] [CrossRef]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef]
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586. [Google Scholar] [CrossRef]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Fujita, T.; Takada, N.; Hayamizu, K.; Takagi, M.; Irifune, T.; Kigoshi, H.; et al. Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: Isolation, structure determination, synthesis, and biological activity. Tetrahedron 2004, 60, 8509–8527. [Google Scholar] [CrossRef]
- Sone, H.; Nemoto, T.; Ishiwata, H.; Ojika, M.; Yamada, K. Isolation, structure, and synthesis of dolastatin D, a cytotoxic cyclic depsipeptide from the sea hare Dolabella abricularia. Tetrahedron Lett. 1993, 34, 8449–8452. [Google Scholar] [CrossRef]
- Mutou, T.; Kondo, T.; Ojika, M.; Yamada, K. Isolation and stereostructures of dolastatin G and nordolastatin G, cytotoxic 35-membered cyclodepsipeptides from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1996, 61, 6340–6345. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Dufresne, C.; Bates, R.B.; Schmidt, J.M.; Cerny, R.L.; Kizu, H. Antineoplastic agents. 190. isolation and structure of the cyclodepsipeptide dolastatin 14. J. Org. Chem 2002, 55, 2989–2990. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Devi, P.; Carbone, M.; Mathieu, V.; Kiss, R.; Casapullo, A.; Gavagnin, M. Kahalalide F analogues from the mucous secretion of Indian sacoglossan mollusc Elysia ornata. Tetrahedron 2016, 72, 625–631. [Google Scholar] [CrossRef]
- Kimura, J.; Takada, Y.; Inayoshi, T.; Nakao, Y.; Goetz, G.; Yoshida, W.Y.; Scheuer, P.J. Kulokekahilide-1, a cytotoxic depsipeptide from the cephalaspidean mollusk Philinopsis speciosa. J. Org. Chem. 2002, 67, 1760–1767. [Google Scholar] [CrossRef]
- Nakao, Y.; Yoshida, W.Y.; Takada, Y.; Kimura, J.; Yang, L.; Mooberry, S.L.; Scheuer, P.J. Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciosa. J. Nat. Prod. 2004, 67, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshida, W.Y.; Szabo, C.M.; Baker, B.J.; Scheuer, P.J. More peptides and other diverse constituents of the marine mollusk Philinopsis speciosa. J. Org. Chem. 1998, 63, 3272–3280. [Google Scholar] [CrossRef]
- Rodríguez, J.; Fernández, R.; Quiñoá, E.; Riguera, R.; Debitus, C.; Bouchet, P. Onchidin: A cytotoxic depsipeptide with C2 symmetry from a marine mollusc. Tetrahedron Lett. 1994, 35, 9239–9242. [Google Scholar] [CrossRef]
- Fernández, R.; Rodríguez, J.; Quiñoá, E.; Riguera, R.; Muñoz, L.; Fernández-Suárez, M.; Debitus, C. Onchidin B: A new cyclodepsipeptide from the mollusc Onchidium sp. J. Am. Chem. Soc. 1996, 118, 11635–11643. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Wu, X.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G. A novel compound Enniatin G from the mangrove fungus Halosarpheia sp. (strain #732) from the south China sea. Aust. J. Chem. 2002, 55, 225–227. [Google Scholar]
- Zhu, X.; Zhong, Y.; Xie, Z.; Wu, M.; Hu, Z.; Ding, W.; Li, C. Fusarihexins A and B: Novel cyclic hexadepsipeptides from the mangrove endophytic fungus Fusarium sp. R5 with antifungal activities. Planta Med. 2018, 84, 1355–1362. [Google Scholar] [CrossRef]
- Feng, Y.; Blunt, J.W.; Cole, A.L.; Munro, M.H. A novel cyclodepsipeptide, HA23, from a Fusarium sp. Org. Lett. 2002, 4, 2095–2096. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Jensen, P.R.; Fenical, W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999, 40, 2913–2916. [Google Scholar] [CrossRef]
- Nihei, K.; Itoh, H.; Hashimoto, K.; Miyairi, K.; Okuno, T. Antifungal cyclodepsipeptides, W493 A and B, from Fusarium sp.: Isolation and structural determination. Biosci. Biotechnol. Biochem. 1998, 62, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Daletos, G.; Lin, W.; Proksch, P. Two new cyclic depsipeptides from the endophytic fungus Fusarium sp. Nat. Prod. Commun. 2015, 10, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Haygood, M.G. Light organ symbioses in fishes. Crit. Rev. Microbiol. 1993, 19, 191–216. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhang, H.; Sun, J.; Zheng, L.; Liu, H.; Wang, J.; Shen, A.; Geng, M.; Guo, Y. Chromopeptide A, a highly cytotoxic depsipeptide from the marine sediment-derived bacterium Chromobacterium sp. HS-13-94. Acta. Pharm. Sin. B 2015, 5, 62–66. [Google Scholar] [CrossRef]
- Kodani, S.; Komaki, H.; Hemmi, H.; Miyake, Y.; Kaweewan, I.; Dohra, H. Streptopeptolin, a cyanopeptolin-type peptide from Streptomyces olivochromogenes. ACS. Omega 2018, 3, 8104–8110. [Google Scholar] [CrossRef]
- Oku, N.; Kawabata, K.; Adachi, K.; Katsuta, A.; Shizuri, Y. Unnarmicins A and C, new antibacterial depsipeptides produced by marine bacterium Photobacterium sp. MBIC06485. J. Antibiot. 2008, 61, 11–17. [Google Scholar] [CrossRef]
- Nair, V.; Kim, M.C.; Golen, J.A.; Rheingold, A.L.; Castro, G.A.; Jensen, P.R.; Fenical, W. Verrucosamide, a cytotoxic 1,4-thiazepane-containing thiodepsipeptide from a marine-derived Actinomycete. Mar. Drugs 2020, 18, 549. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Matsubara, T.; Shimada, S.; Sato, T.; Suenaga, K. Mebamamides A and B, cyclic lipopeptides isolated from the Green Alga Derbesia marina. J. Nat. Prod. 2015, 78, 901–908. [Google Scholar] [CrossRef]
- Zhang, J.N.; Xia, Y.X.; Zhang, H.J. Natural cyclopeptides as anticancer agents in the last 20 Years. Int. J. Mol. Sci. 2021, 22, 3973. [Google Scholar] [CrossRef]
- Love, T.M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Battershill, P.E.; Clissold, S.P. Octreotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs 1989, 38, 658–702. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.C.; Williams, S.; Yoong, F.F. Vasopressin and terlipressin: Pharmacology and its clinical relevance. Anaesthesia 2004, 59, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.P. Vancomycin. Mayo Clin. Proc. 1991, 66, 1165–1170. [Google Scholar] [CrossRef]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Tran, T.B.; Velkov, T.; Nation, R.L.; Forrest, A.; Tsuji, B.T.; Bergen, P.J.; Li, J. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: Are we there yet? Int. J. Antimicrob. Agents 2016, 48, 592–597. [Google Scholar] [CrossRef]
- Barshes, N.R.; Goodpastor, S.E.; Goss, J.A. Pharmacologic immunosuppression. Front. Biosci. 2004, 9, 411–420. [Google Scholar] [CrossRef]
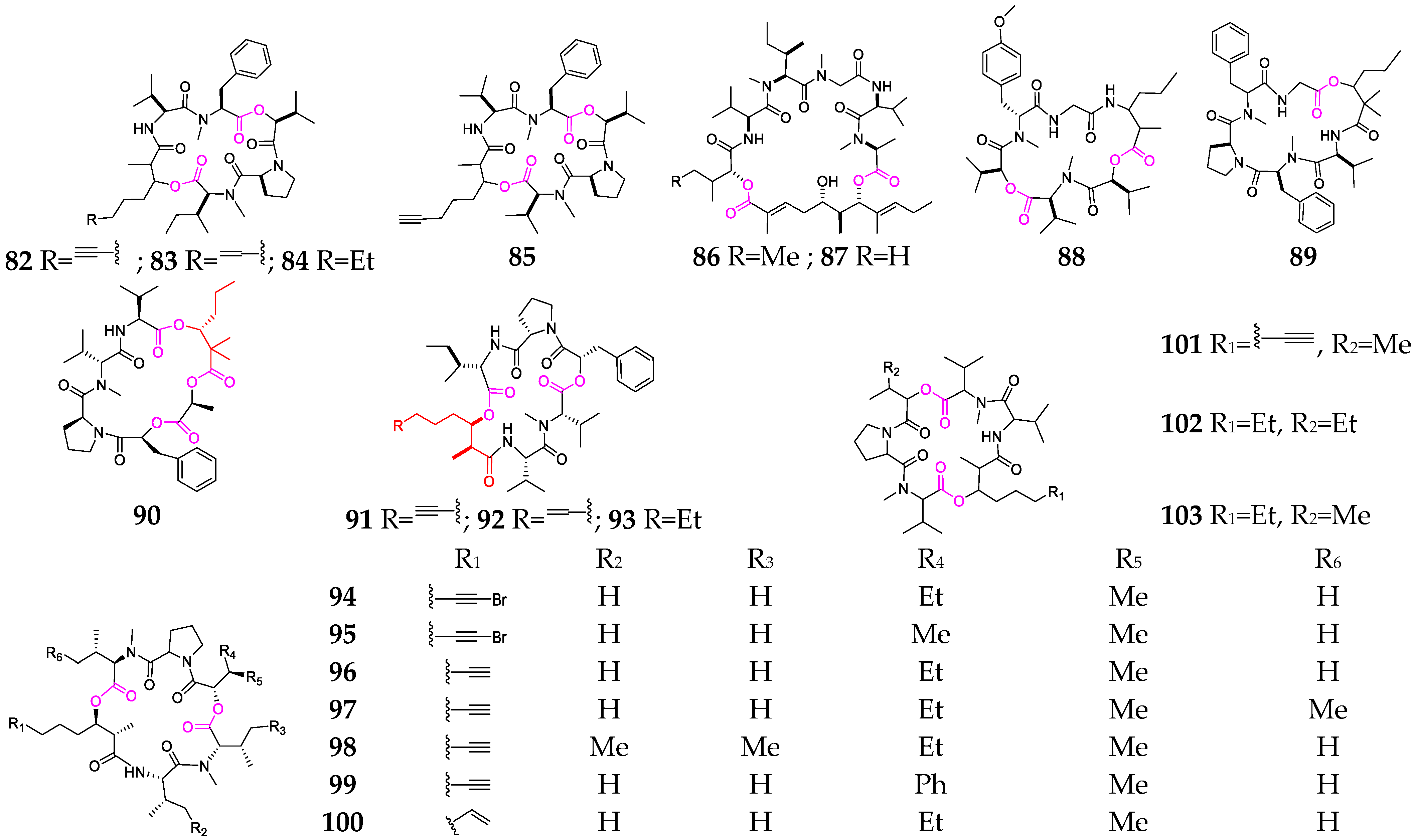
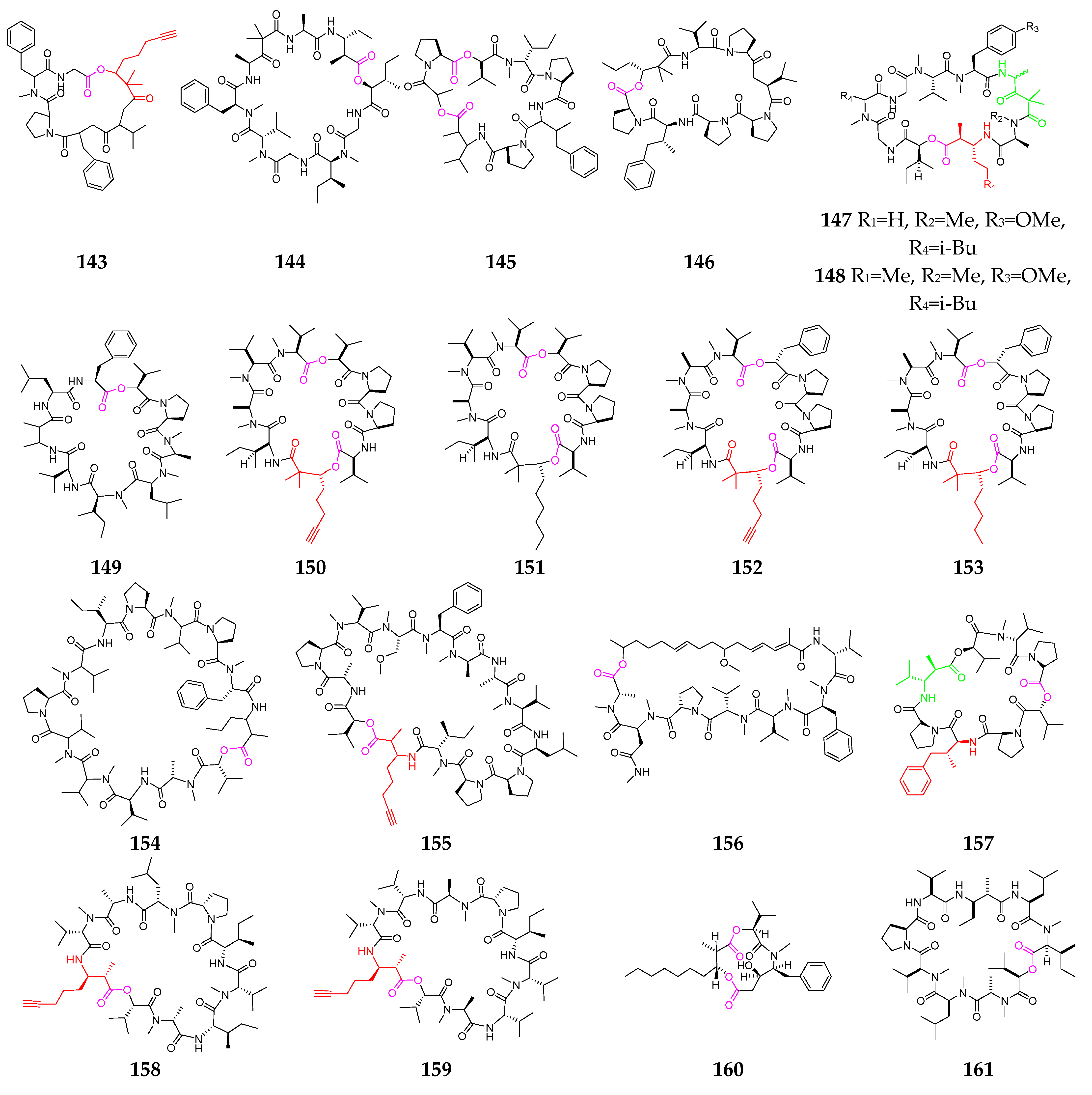
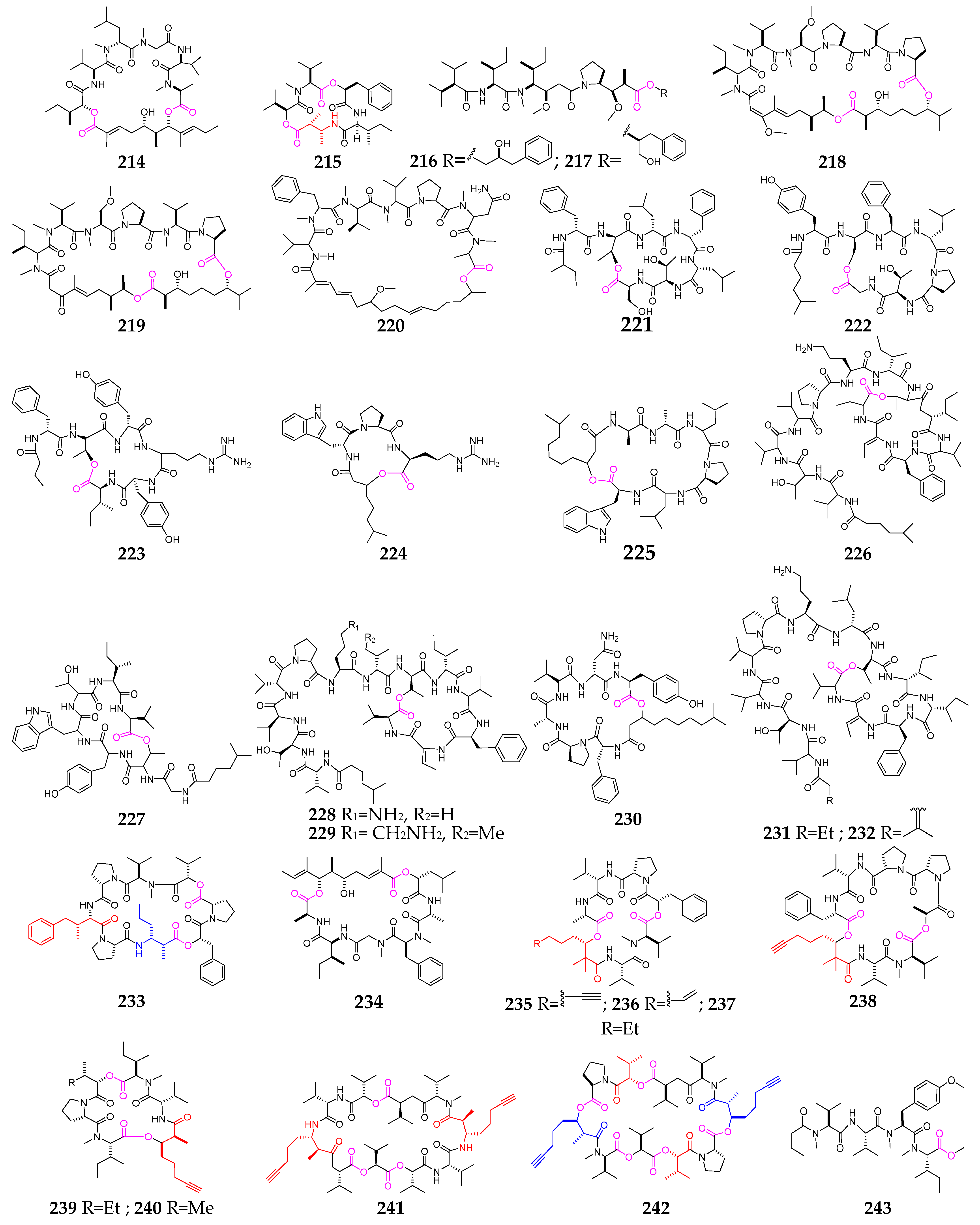
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Tao, J.; Xu, S.; Bai, X.; Zhang, H. Marine Organisms as a Prolific Source of Bioactive Depsipeptides. Mar. Drugs 2023, 21, 120. https://doi.org/10.3390/md21020120
Zeng M, Tao J, Xu S, Bai X, Zhang H. Marine Organisms as a Prolific Source of Bioactive Depsipeptides. Marine Drugs. 2023; 21(2):120. https://doi.org/10.3390/md21020120
Chicago/Turabian StyleZeng, Mingyuan, Jianyun Tao, Shuang Xu, Xuelian Bai, and Huawei Zhang. 2023. "Marine Organisms as a Prolific Source of Bioactive Depsipeptides" Marine Drugs 21, no. 2: 120. https://doi.org/10.3390/md21020120
APA StyleZeng, M., Tao, J., Xu, S., Bai, X., & Zhang, H. (2023). Marine Organisms as a Prolific Source of Bioactive Depsipeptides. Marine Drugs, 21(2), 120. https://doi.org/10.3390/md21020120