Evaluation of the Preservation and Digestion of Seal Meat Processed with Heating and Antioxidant Seal Meat Hydrolysates
Abstract
:1. Introduction
2. Results and Discussion
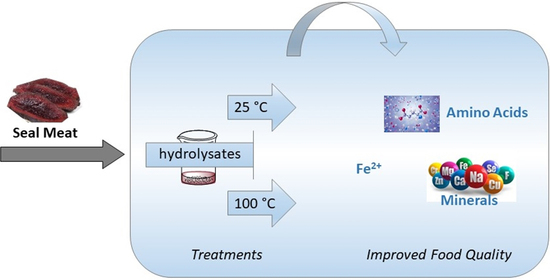
2.1. Seal Meat Hydrolysates as Food Preservatives with Antioxidant Activity
2.2. Amino Acid Profiles of Treated Seal Meats after Digestion
2.3. Mineral Profiles of Treated Seal Meats after Digestion
2.4. Iron Ions in Processed Seal Meat Products after Digestion
3. Methods and Materials
3.1. Chemicals and Biological Materials
3.2. Treatment of Seal Meat with Heat and Seal Meat Hydrolysates
3.3. Determination of Antioxidant Properties
3.4. Digestion of Seal Meat via a Dynamic Gastrointestinal (GI) Model
3.5. Determination of Amino Acids
3.6. Determination of Minerals
3.7. Determination of Fe Ions
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Synowiecki, J. Seal meat: A unique source of muscle food for health and nutrition. Food Rev. Int. 1996, 12, 283–302. [Google Scholar] [CrossRef]
- Hedlund Markussen, N.; Are Øritsland, N. Food energy requirements of the harp seal (Phoca groenlandica) population in the Barents and White seas. Polar Res. 1991, 10, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Fisheries and Oceans Canada. 2019 Status of Northwest Atlantic Harp Seals. Science Advisory Report 2020. 2020. Available online: https://www.dfo-mpo.gc.ca/csas-sccs/Publications/SAR-AS/2020/2020_020-eng.html (accessed on 5 January 2022).
- Blue Economy Strategy. Available online: https://www.dfo-mpo.gc.ca/campaign-campagne/bes-seb/index-eng.html (accessed on 5 January 2022).
- Canadian Seal Products. Available online: https://canadiansealproducts.com/products/seal-oil (accessed on 5 January 2022).
- Shahidi, F.; Synowiecki, J.; Naczk, M. Seal Meat–A potential source of muscle food: Chemical composition, essential amino acids and colour characteristics. Can. Inst. Food Sci. Technol. J. 1990, 23, 137–139. [Google Scholar] [CrossRef]
- Lafrance, D. Canada’s Seal Harvest; Library of Parliament: Ottawa, ON, Canada, 2017. [Google Scholar]
- Robards, M.D.; Reeves, R.R. The global extent and character of marine mammal consumption by humans: 1970–2009. Biol. Conserv. 2011, 144, 2770–2786. [Google Scholar] [CrossRef]
- Anderson, D.M.; Priest, G.; Collins, S.A.; MacIsaac, J.L. Nutritional evaluation of seal by-products as an alternative protein source for use in monogastric animals. Can. J. Anim. Sci. 2019, 100, 77–84. [Google Scholar] [CrossRef]
- Brunborg, L.A.; Julshamn, K.; Nortvedt, R.; Frøyland, L. Nutritional composition of blubber and meat of hooded seal (Cystophora cristata) and harp seal (Phagophilus groenlandicus) from Greenland. Food Chem. 2006, 96, 524–531. [Google Scholar] [CrossRef]
- SeaDNA. Available online: https://www.seadna.ca/nutritional-facts/ (accessed on 5 January 2022).
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rui, X.; Vaugeois, R.; Simpson, B.K. Seal meat enzymatic hydrolysates and its digests: A comparison on protein and minerals profiles. LWT Food Sci. Technol. 2022, 157, 113072. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Caetano da Silva Lannes, S. Lipid oxidation in meat: Mechanisms and protective factors–A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Da Costa De Quadros, C.; Lima, K.O.; Bueno, C.H.L.; dos Santos Fogaça, F.H.; Da Rocha, M.; Prentice, C. Evaluation of the antioxidant and antimicrobial activity of protein hydrolysates and peptide fractions derived from Colossoma macropomum and their effect on ground beef lipid oxidation. J. Aquat. Food Prod. Technol. 2019, 28, 677–688. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Niamah, A.K. Structure, mode of action and application of pediocin natural antimicrobial food preservative: A review. Basrah J. Agric. Sci. 2018, 31, 59–69. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive Peptides: A Promising Alternative to Chemical Preservatives for Food Preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Wal, J.-M.; Bernard, H.; Pentzien, A.-K. Cytotoxic and allergenic potential of bioactive proteins and peptides. Curr. Pharm. Des. 2007, 13, 897–920. [Google Scholar] [CrossRef] [PubMed]
- Ten Have, G.A.; Engelen, M.P.; Luiking, Y.C.; Deutz, N.E. Absorption kinetics of amino acids, peptides, and intact proteins. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S23–S36. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacova-Hanuskova, E.; Buday, T.; Gavliakova, S.; Plevkova, J. Histamine, histamine intoxication and intolerance. Allergol. Iimmunopathol. 2015, 43, 498–506. [Google Scholar] [CrossRef]
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migdał, W.; Kawęcka, A.; Sikora, J.; Migdał, Ł. Meat quality of the native Carpathian goat breed in comparison with the Saanen breed. Animals 2021, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Cobas, N.; Gómez-Limia, L.; Franco, I.; Martínez, S. Amino acid profile and protein quality related to canning and storage of swordfish packed in different filling media. J. Food Compos. Anal. 2022, 107, 104328. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic Sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Pongmalai, P.; Fu, N.; Soponronnarit, S.; Chiewchan, N.; Devahastin, S.; Chen, X.D. Microwave pretreatment enhances the formation of cabbage sulforaphane and its bioaccessibility as shown by a novel dynamic soft rat stomach model. J. Funct. Foods 2018, 43, 186–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Dutilleul, P.; Orsat, V.; Simpson, B.K. Alcalase assisted production of novel high alpha-chain gelatin and the functional stability of its hydrogel as influenced by thermal treatment. Int. J. Biol. Macromol. 2018, 118, 2278–2286. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; Smolders, E.; Delcour, J.A. Mineral bio-accessibility and intrinsic saccharides in breakfast flakes manufactured from sprouted wheat. LWT Food Sci. Technol. 2021, 143, 111079. [Google Scholar] [CrossRef]
- Balavandy, S.K.; Li, F.; Macdonald, N.P.; Maya, F.; Townsend, A.T.; Frederick, K.; Guijt, R.M.; Breadmore, M.C. Scalable 3D printing method for the manufacture of single-material fluidic devices with integrated filter for point of collection colourimetric analysis. Anal. Chim. Acta 2021, 1151, 238101. [Google Scholar] [CrossRef]





| Amino Acids | Numbers (per 1000 Amino Acids) | |||
|---|---|---|---|---|
| Meat (Hydrolysates 25) | Meat (Hydrolysates 100) | Meat (Buffer 25) | Meat (Buffer 100) | |
| Non-essential amino acids (NEAA) | ||||
| Ala | 87 | 87 | 84 | 89 |
| Arg | 51 | 45 | 50 | 50 |
| Asx (Asp+Asn) | 96 | 92 | 100 | 88 |
| Glx (Glu+Gln) | 141 | 131 | 127 | 122 |
| Gly | 85 | 87 | 95 | 141 |
| Pro | 43 | 43 | 48 | 61 |
| Ser | 55 | 54 | 59 | 55 |
| Tyr | 24 | 26 | 27 | 23 |
| Total NEAA | 582 | 565 | 590 | 629 |
| Essential amino acids (EAA) | ||||
| His | 43 | 44 | 40 | 32 |
| Ile | 48 | 50 | 46 | 40 |
| Leu | 91 | 94 | 93 | 79 |
| Lys | 73 | 82 | 77 | 73 |
| Met | 24 | 22 | 19 | 19 |
| Phe | 36 | 41 | 35 | 35 |
| Thr | 47 | 45 | 43 | 41 |
| Val | 56 | 57 | 57 | 52 |
| Total EAA | 418 | 435 | 410 | 371 |
| EAA/NEAA | 0.718 | 0.770 | 0.695 | 0.590 |
| Mineral (mg/kg) | Meat–Hydrolysates 25 °C | Meat–Hydrolysates 100 °C | Meat–Buffer 25 °C | Meat–Buffer 100 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Gastric Digests | Intestinal Digests | Gastric Digests | Intestinal Digests | Gastric Digests | Intestinal Digests | Gastric Digests | Intestinal Digests | |
| Ag | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Al | 11.80 | 21.51 | 26.04 | 7.28 | 25.13 | 22.14 | 26.60 | 17.76 |
| As | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Au | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| B | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Ba | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Be | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Bi | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Ca | 703.82 | 1024.74 | 768.99 | 1107.90 | 2246.46 | 1100.02 | 1206.36 | 750.12 |
| Cr | 36.99 | 60.05 | 48.32 | 40.38 | 63.95 | 65.33 | 44.47 | 64.28 |
| Cd | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Co | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Cu | 2.82 | 3.01 | 1.89 | 2.50 | 5.21 | 5.94 | 1.53 | 2.88 |
| Fe | 884.42 | 781.99 | 736.89 | 486.02 | 880.15 | 769.83 | 124.37 | 400.04 |
| Ga | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Ge | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Hf | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Hg | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| K | 5253.20 | 12,547.02 | 4266.01 | 7236.46 | 15,027.46 | 11,293.84 | 13,885.86 | 13,501.73 |
| Li | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Mg | 1103.74 | 793.99 | 496.35 | 440.31 | 2225.12 | 576.10 | 570.37 | 437.59 |
| Mn | 0.73 | 1.10 | 0.82 | 0.92 | 3.87 | 2.49 | 1.64 | 1.11 |
| Mo | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Na | 21,647.36 | 77,945.86 | 20,824.45 | 50,441.43 | 75,538.30 | 61,061.62 | 93,029.12 | 91,713.28 |
| Ni | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| P | 6132.35 | 4477.88 | 4476.05 | 3592.14 | 15,022.37 | 8100.78 | 3771.29 | 3624.02 |
| Pb | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Pd | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Pt | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| S | 5491.97 | 4058.97 | 5126.93 | 4190.13 | 3856.38 | 6093.86 | 1659.23 | 3450.14 |
| Sb | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Se | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Sn | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Sr | 9.75 | 11.63 | 9.77 | 10.46 | 19.27 | 13.23 | 10.57 | 10.97 |
| Ti | 0.69 | 1.11 | 1.46 | 0.76 | 2.94 | 1.63 | 1.69 | 1.72 |
| TI | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| V | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| W | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| Zn | 72.02 | 84.32 | 66.41 | 90.55 | 102.23 | 88.98 | 67.77 | 82.97 |
| Zr | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Spitzer, L.; Rui, X.; Fernandes, S.C.M.; Vaugeois, R.; Simpson, B.K. Evaluation of the Preservation and Digestion of Seal Meat Processed with Heating and Antioxidant Seal Meat Hydrolysates. Mar. Drugs 2022, 20, 204. https://doi.org/10.3390/md20030204
Zhang Y, Spitzer L, Rui X, Fernandes SCM, Vaugeois R, Simpson BK. Evaluation of the Preservation and Digestion of Seal Meat Processed with Heating and Antioxidant Seal Meat Hydrolysates. Marine Drugs. 2022; 20(3):204. https://doi.org/10.3390/md20030204
Chicago/Turabian StyleZhang, Yi, Lea Spitzer, Xin Rui, Susana C. M. Fernandes, Romy Vaugeois, and Benjamin K. Simpson. 2022. "Evaluation of the Preservation and Digestion of Seal Meat Processed with Heating and Antioxidant Seal Meat Hydrolysates" Marine Drugs 20, no. 3: 204. https://doi.org/10.3390/md20030204
APA StyleZhang, Y., Spitzer, L., Rui, X., Fernandes, S. C. M., Vaugeois, R., & Simpson, B. K. (2022). Evaluation of the Preservation and Digestion of Seal Meat Processed with Heating and Antioxidant Seal Meat Hydrolysates. Marine Drugs, 20(3), 204. https://doi.org/10.3390/md20030204