Therapeutic Potential of Marine Bioactive Peptides against Human Immunodeficiency Virus: Recent Evidence, Challenges, and Future Trends
Abstract
:1. Introduction
2. Marine Bioactive Peptides (MBAPs)
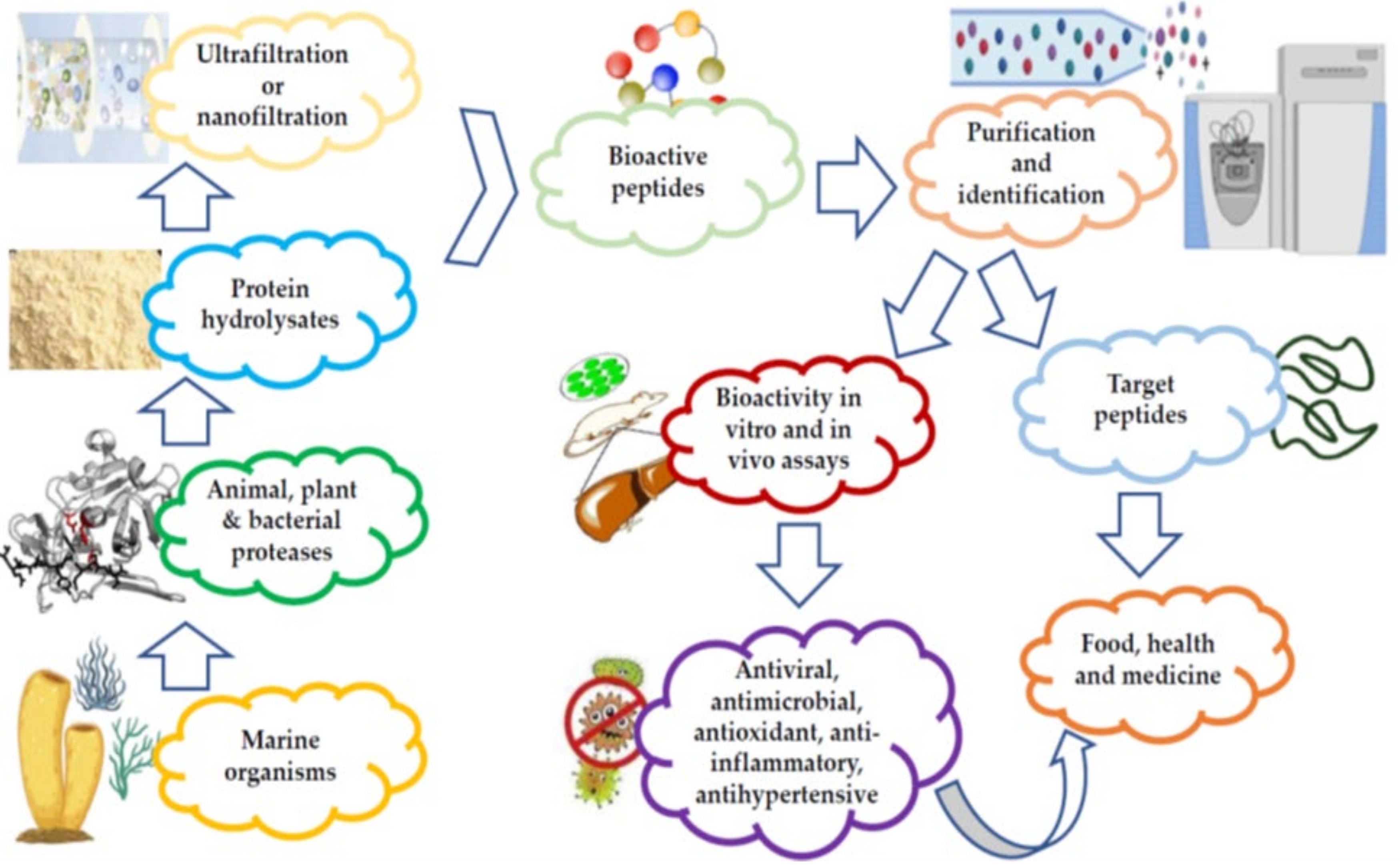
2.1. Techniques Used for the Commercial Preparation and Purification of MBAPs
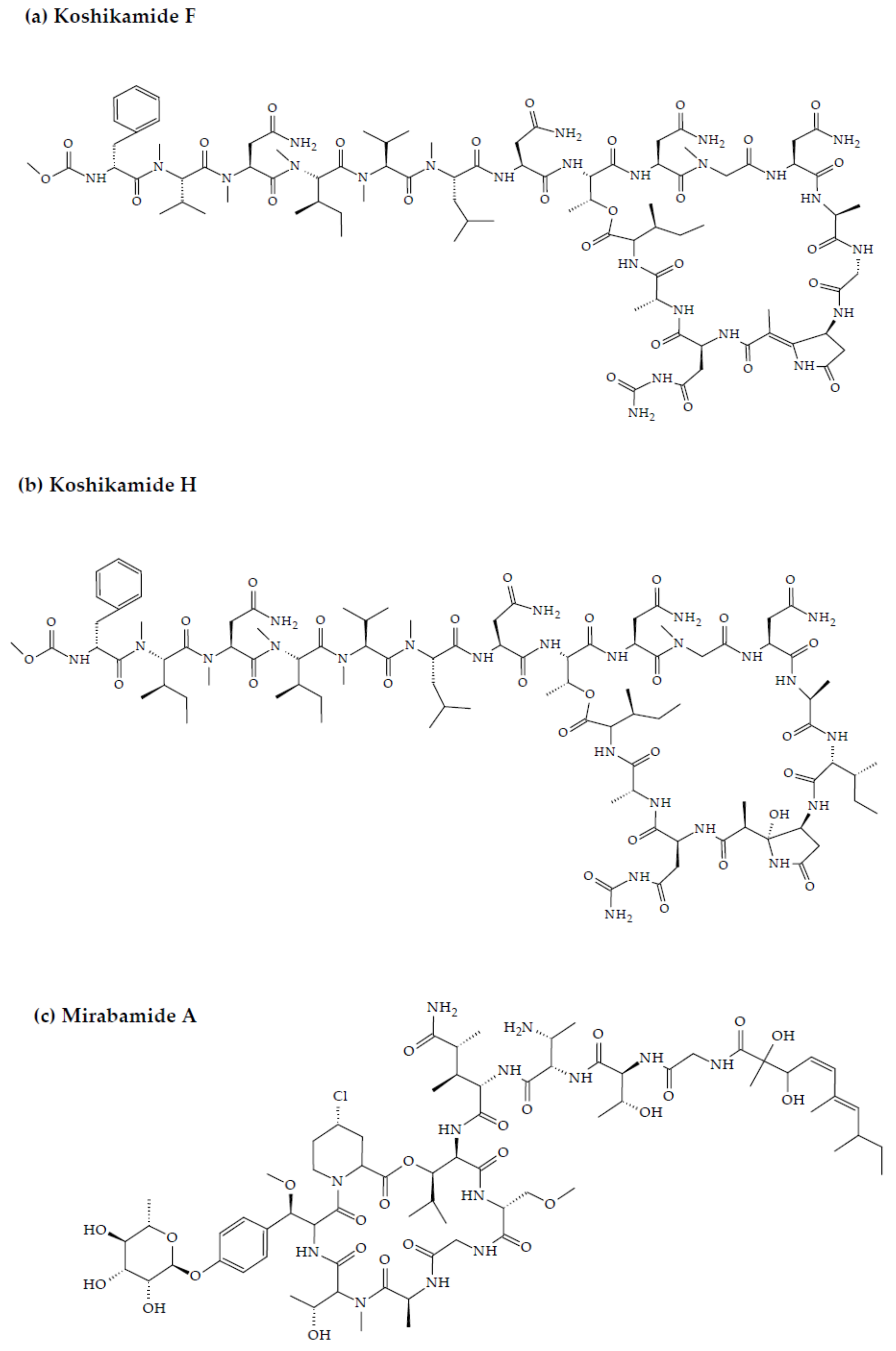
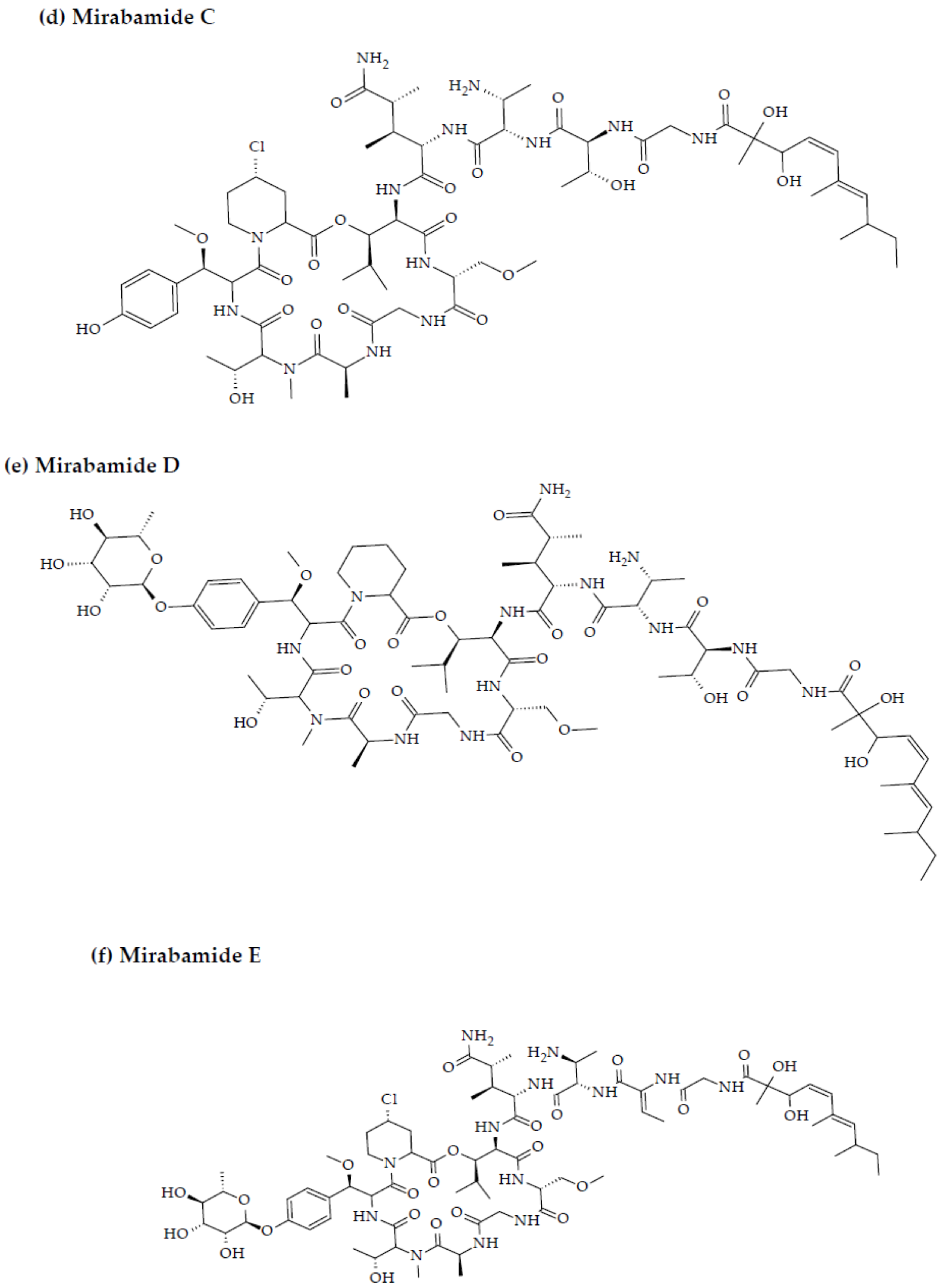
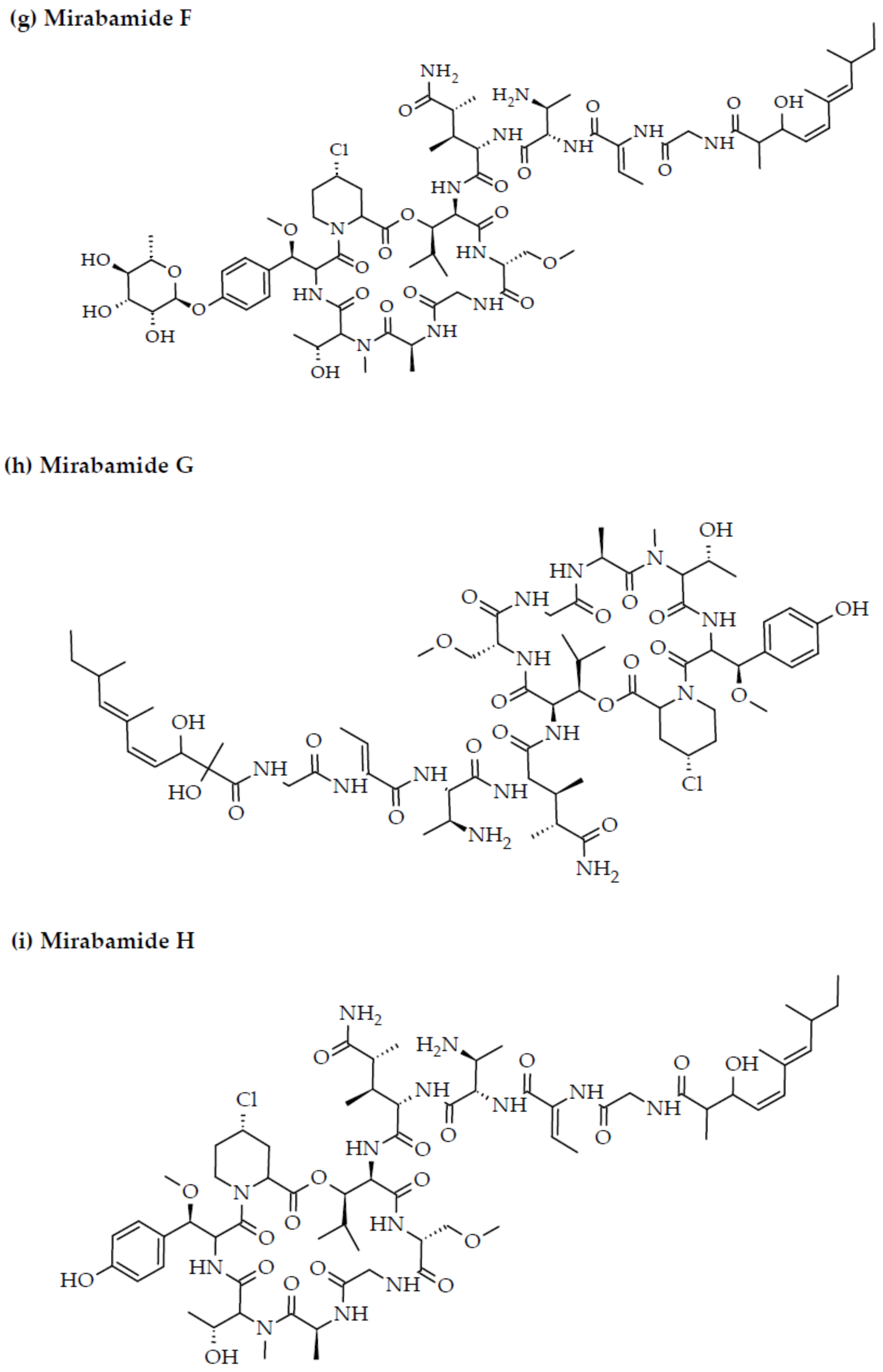
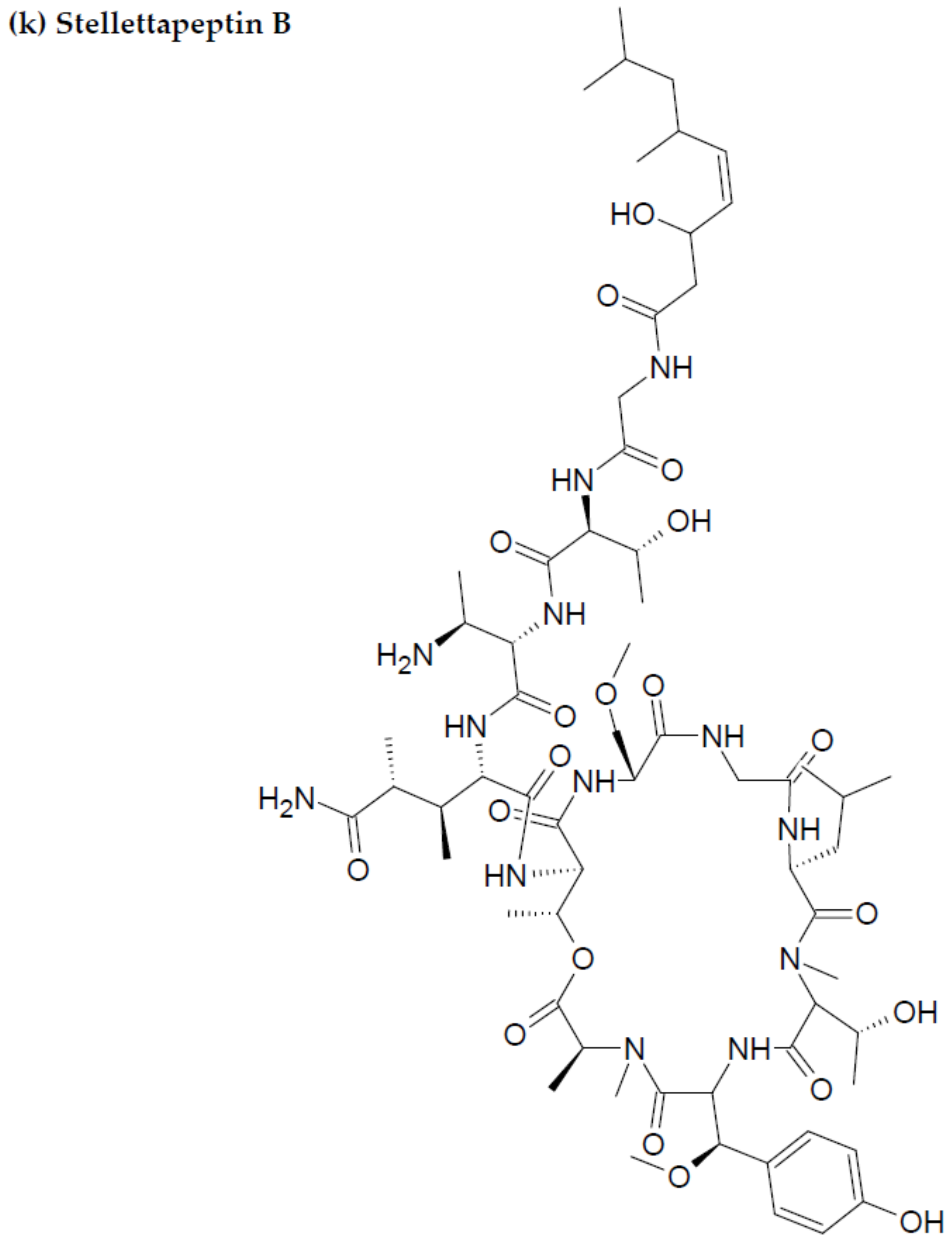
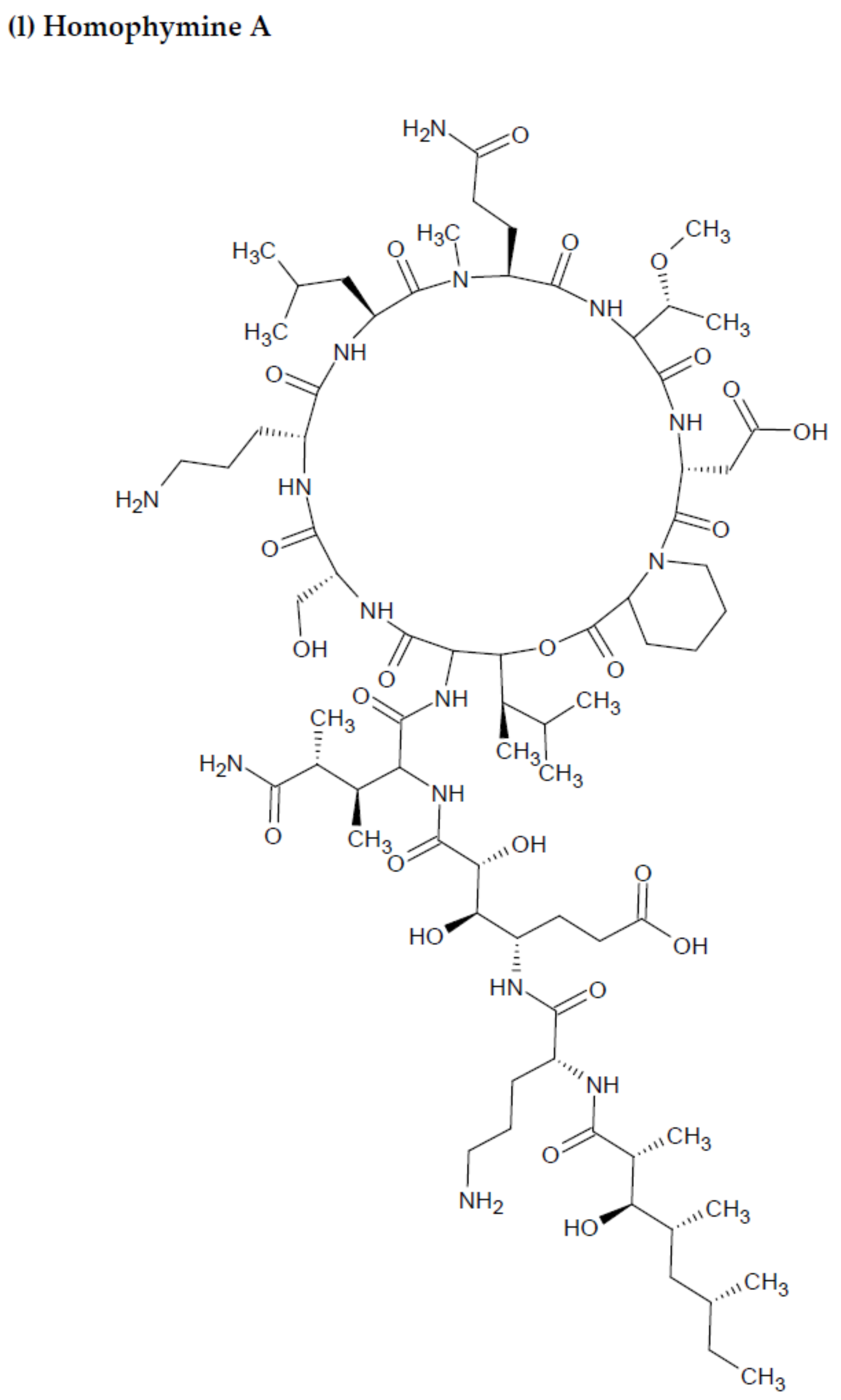
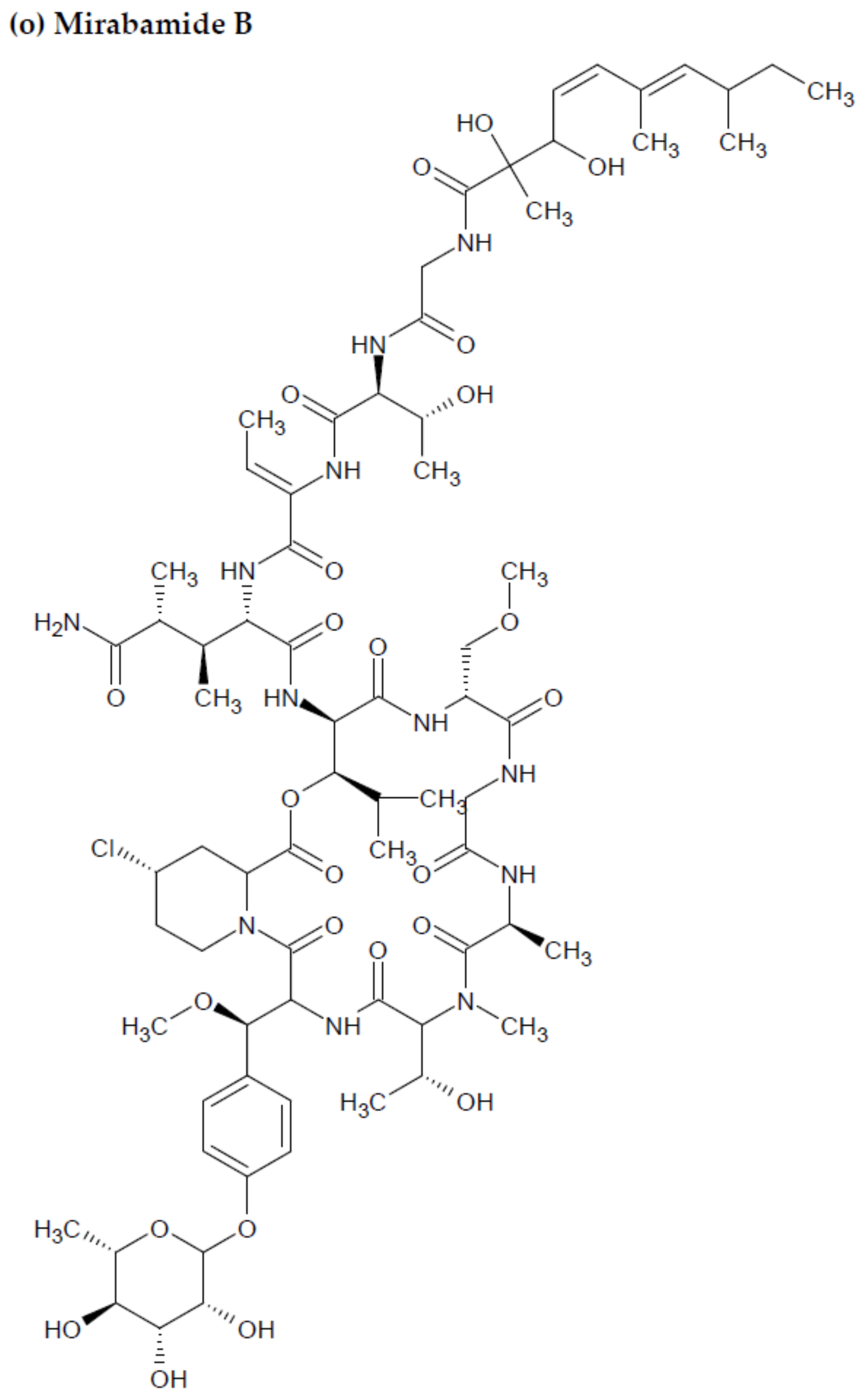
2.2. Marine Sponge-Derived Bioactive Peptides against HIV
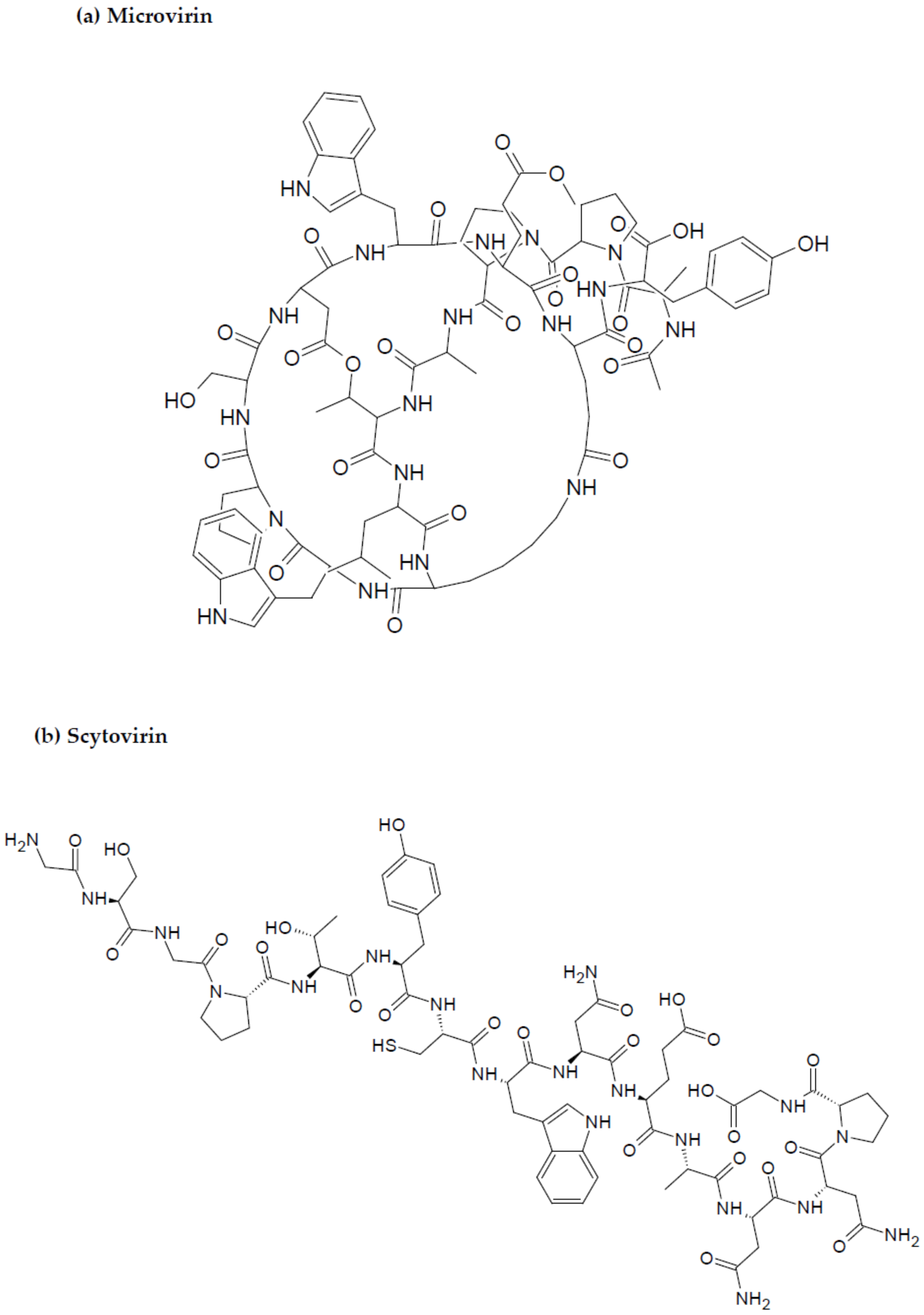
2.3. Marine Cyanobacteria-Associated Compounds with Anti-HIV Properties
2.4. Marine Seaweed Originated Bioactive Peptides with Anti-HIV Properties
2.5. Marine Mussels, Ascidians, and Oyster-Derived Bioactive Peptides against HIV
2.6. Anti-HIV Bioactive Peptides Isolated from Marine Bacteria and Fungi
3. Processing Techniques for Marine Bioactive Peptides
4. Conclusions, Limitations, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni, Z.A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural bioactive compounds useful in clinical management of metabolic syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- HIV/AIDS, Global HIV & Camp; AIDS Statistics—2018 Fact Sheet, UNAIDS. Available online: http://www.unaids.org/en/resources/fact-sheet (accessed on 8 August 2018).
- Ariën, K.K.; Jespers, V.; Vanham, G. HIV sexual transmission and microbicides. Rev. Med. Virol. 2011, 21, 110–133. [Google Scholar] [CrossRef]
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Prévost, J.; Tolbert, W.D.; Medjahed, H.; Sherburn, R.T.; Madani, N.; Zoubchenok, D.; Gendron-Lepage, G.; Gaffney, A.E.; Grenier, M.C.; Kirk, S.; et al. The HIV-1 Env gp120 inner domain shapes the Phe43 cavity and the CD4 binding site. MBio 2020, 11, e00280-20. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Arai, H.; Yoshimura, K.; Harada, S.; Nomura, W.; Matsushita, S.; Tamamura, H. Small molecular CD4 mimics as HIV entry inhibitors. Bioorg. Med. Chem. 2011, 19, 6735–6742. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.B.; Kim, J.; Shin, C.G. Distribution and fate of HIV-1 unintegrated DNA species: A comprehensive update. AIDS Res. Ther. 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.L.; Kutluay, S.B. Going beyond integration: The emerging role of HIV-1 Integrase in virion morphogenesis. Viruses 2020, 12, 1005. [Google Scholar] [CrossRef]
- Laskey, S.B.; Siliciano, R.F. A mechanistic theory to explain the efficacy of antiretroviral therapy. Nat. Rev. Microbiol. 2014, 12, 772–780. [Google Scholar] [CrossRef]
- Mirani, A.G.; Shah, T.K.; Patravale, V.B. Marine source-derived anti-HIV therapeutics. In Encyclopedia of Marine Biotechnology, 1st ed.; Se-Kwon, K., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 2725–2753. [Google Scholar]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, preparation and purification of marine bioactive peptides. Biomed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sato, Y.; Ito, K.; Fujiwara, Y.; Iwamoto, Y.; Makino, H.; Kawakubo, A. Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology 2007, 17, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High mannose binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corzo, L.; Fernández Novoa, L.; Carrera, I.; Martínez, O.; Rodríguez, S.; Alejo, R.; Cacabelos, R. Nutrition, health and disease: Role of selected marine and vegetal nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef] [Green Version]
- Ande, M.P.; Syamala, K.; Rao, P.; Mohan, K.; Lingam, S.S. Marine nutraceuticals. Aquac. Times 2017, 3, 6–9. [Google Scholar]
- Anas, K.; Mathew, S. Marine Nutraceuticals; ICAR-Central Institute of Fisheries Technology: Kerala, India, 2018. [Google Scholar]
- Ovchinnikova, T.V. Structure, function and therapeutic potential of marine bioactive peptides. Mar. Drugs 2019, 17, 505. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo Benavent, M.; Harnedy Rothwell, P.; Fitz Gerald, R.J. Current knowledge on the extraction, purification, identification and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Mayer, A.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar]
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 81–196. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, J. Recent advances in antibacterial and antiendotoxic peptides or proteins from marine resources. Mar. Drugs 2018, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, F.; Mancera-Andrade, E.I.; Iqbal, M.N.H. Marine derived bioactive peptides for biomedical sectors: A review. Protein Pept. Lett. 2017, 24, 109–117. [Google Scholar] [CrossRef]
- Mayer, A.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2020, 18, 5. [Google Scholar]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; Hamed, A.N.E.S.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Wang, H. Cytotoxic natural products from marine sponge derived microorganisms. Mar. Drugs 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells. Oxid. Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajapakse, N.; Jung, W.K.; Mendis, E.; Moon, S.H.; Kim, S.K. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005, 76, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.Y.; Jung, W.K.; Kim, S.K. Purification and characterization of a novel anticoagulant peptide from marine echiuroid worm, Urechis unicinctus. Process Biochem. 2008, 43, 179–184. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Barkia, I.; Al-Haj, L.; Abdul Hamid, A.; Zakaria, M.; Saari, N.; Zadjali, F. Indigenous marine diatoms as novel sources of bioactive peptides with antihypertensive and antioxidant properties. Int. J. Food Sci. Technol. 2019, 54, 1514–1522. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.C.; Kim, H.S.; Yang, H.W.; Ahn, G.; Lee, S.C.; Lee, T.G.; Lee, J.S.; Jeon, Y.J. Structural evidence for antihypertensive effect of an antioxidant peptide purified from the edible marine animal Styela clava. J. Med. Food 2020, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S. Antioxidants from crustaceans: A panacea for lipid oxidation in marine-based foods. Food Rev. Int. 2022, 38, 1–31. [Google Scholar] [CrossRef]
- Florean, C.; Dicato, M.; Diederich, M. Immune modulating and anti-inflammatory marine compounds against cancer. Semin. Cancer Biol. 2020, 80, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Byun, H.G.; Jung, W.K.; Kim, S.K. Angiotensin I converting enzyme (ACE) inhibitory peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Bioresour. Technol. 2005, 96, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Se-Kwon, K.; Niranjan, R.; Fereidoon, S. Production of bioactive chitosan oligosaccharides and their potential use as nutraceuticals. In Marine Nutraceuticals and Functional Foods; Colin, B., Fereidoon, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 183–195. [Google Scholar]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Clare, D.; Swaisgood, H. Bioactive milk peptides: A prospectus. J. Dairy Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.; Carneiro, J.A.; Costa, R.A.; Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci 2021, 8, 889. [Google Scholar] [CrossRef]
- Chai, Q.Y.; Yang, Z.; Lin, H.W.; Han, B.N. Alkynyl containing peptides of marine origin: A review. Mar. Drugs 2016, 14, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.C.; Duttagupta, I.; Bose, C.; Banerjee, P.; Gayen, A.K.; Sinha, S. Synthesis and anticancer activities of proline containing cyclic peptides and their linear analogs and congeners. Synth. Commun. 2019, 49, 221–236. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Brown, K.L.; Mookherjee, N. Host defence peptides from invertebrates emerging antimicrobial strategies. Immunobiology 2006, 211, 315–322. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmal, N.; Santivarangkna, C.; Rajput, M.; Benjakul, S.; Maqsood, S. Valorization of fish byproducts: Sources to end product applications of bioactive protein hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammad, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood discards: A potent source of enzymes and biomacromolecules with nutritional and nutraceutical significance. Front Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive peptides from marine ascidians and future drug development–A review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Núñez, M.A.; López, V.E.L. Nonribosomal peptides synthetases and their applications in industry. Sustain. Chem. Process. 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Le Gouic, A.; Harnedy, P.; Fitz Gerald, R. Bioactive peptides from fish protein byproducts. In Bioactive Molecules in Food, 1st ed.; Jean-Michel, M., Ramawat, K.G., Eds.; Springer: New York, NY, USA, 2018; pp. 1–35. [Google Scholar]
- Atef, M.; Chait, Y.A.; Ojagh, S.M.; Latifi, A.M.; Esmaeili, M.; Hammami, R.; Udenigwe, C.C. Anti-Salmonella activity and peptidomics profiling of peptide fractions produced from sturgeon fish skin collagen (Huso huso) using commercial enzymes. Nutrients 2021, 13, 2657. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersson, B.; Rydén, L.; Janson, J.C. Protein Purification: Principles, High Resolution Methods, and Applications, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 1–22. [Google Scholar]
- Lee, J.W.; Jeffries, T.W. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour. Technol. 2011, 102, 5884–5890. [Google Scholar] [CrossRef]
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T. Membrane Filtration in Advanced Physicochemical Treatment Processes; Humana Press: Totowa, NJ, USA, 2006; pp. 203–259. [Google Scholar]
- Bai, C.; Wei, Q.; Ren, X. Selective extraction of collagen peptides with high purity from cod skins by deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 7220–7227. [Google Scholar] [CrossRef]
- Anbuchezian, R.; Ravichandran, S.; Karthick Rajan, D.; Tilivi, S.; Prabha-Devi, S. Identification and functional characterization of antimicrobial peptide from the marine crab Dromia dehaani. Microb. Pathog. 2018, 125, 60–65. [Google Scholar] [CrossRef]
- Anand, M.; Alagar, M.; Ranjitha, J.; Selvaraj, V. Total synthesis and anticancer activity of a cyclic heptapeptide from marine sponge using water soluble peptide coupling agent EDC. Arab. J. Chem. 2019, 12, 2782–2787. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zainal Abidin, N.; Zarei, M.; Tan, C.P.; Saari, N. Identification, structure activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE 2019, 14, e0197644. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Cui, Y.; Zhang, R.; Zhang, X. Purification and identification of anti-obesity peptides derived from Spirulina platensis. J. Funct. Foods. 2018, 47, 350–360. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Int. Food Res. 2017, 100, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and identification of antioxidant peptide from underutilized Dunaliella salina protein: Extraction, in vitro gastrointestinal digestion, and fractionation. Biomed Res. Int. 2019, 2019, 6424651. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Ye, L.; Tang, Y.; Zheng, J.; Tian, X.; Yang, Y.; Yang, Z. Preparation and purification of an immunoregulatory peptide from Stolephorus chinensis of the East Sea of China. Process Biochem. 2020, 98, 151–159. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Int. Food Res. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S. Marine sponges as a drug treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Vitali, A. Antimicrobial peptides derived from marine sponges. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1006. [Google Scholar]
- Wu, Q.; Nay, B.; Yang, M.; Ni, Y.; Wang, H.; Yao, L.; Li, X. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharm. Sin. B. 2019, 9, 237–257. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
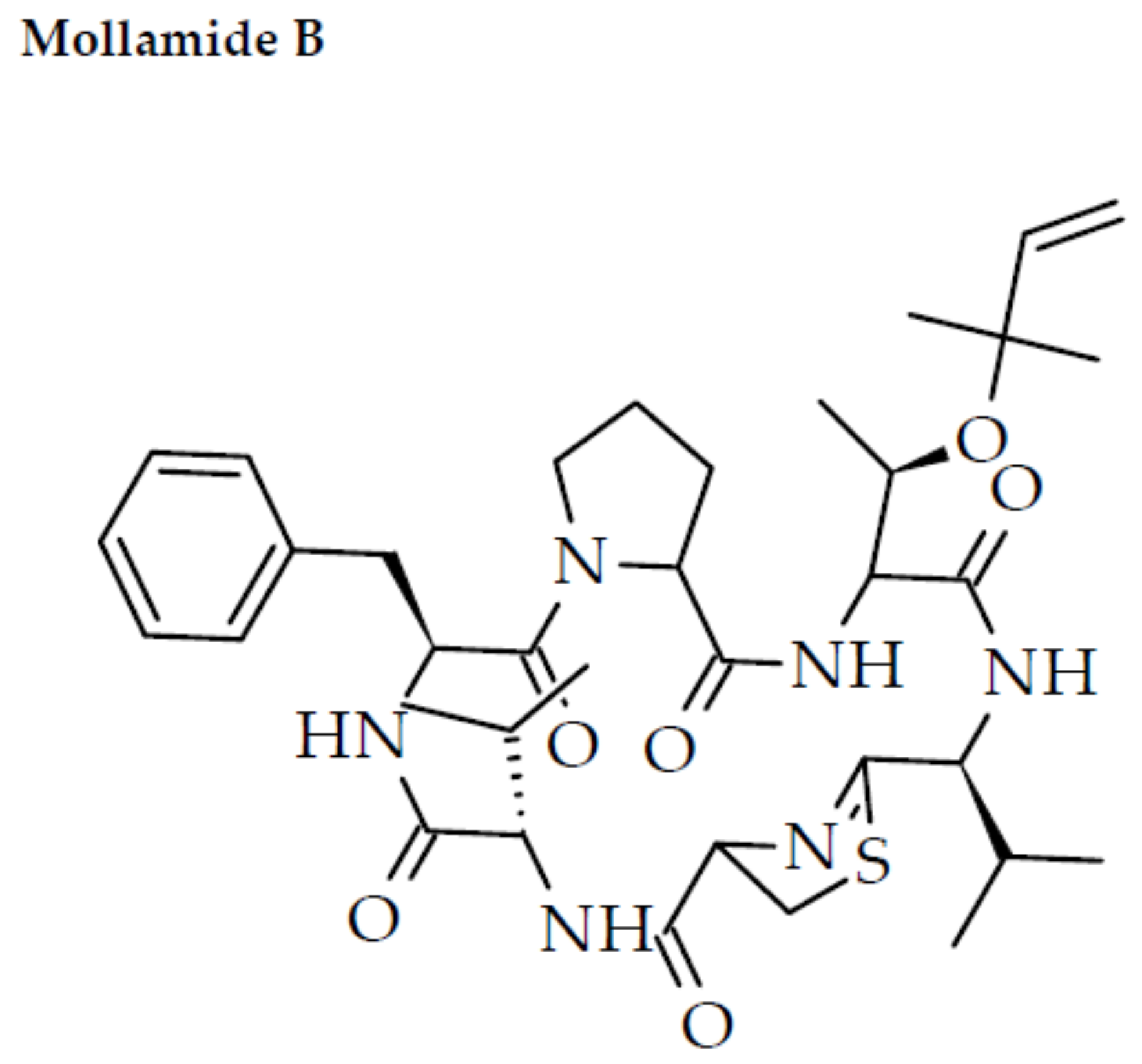
- Plaza, A.; Bifulco, G.; Masullo, M.; Lloyd, J.R.; Keffer, J.L.; Colin, P.L.; Hooper, J.N.; Bell, L.J.; Bewley, C.A. Mutremdamide A and koshikamides C-H, peptide inhibitors of HIV-1 entry from different Theonella species. J. Org. Chem. 2010, 75, 4344–4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Choi, M.; Seo, C.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, N.; Gustafson, K.R.; Cartner, L.K.; Wilson, J.A.; Shigematsu, N.; Hess, S.; Pannell, L.K.; Boyd, M.R.; McMahon, J.B. Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod. 2004, 67, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Andavan, G.S.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.; Kim, S. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [Green Version]
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P.; D’Auria, M.; Cresteil, T. Homophymines B–E and A1–E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Computational pharmacokinetics Report, ADMET study and conceptual DFT-based estimation of the chemical reactivity properties of marine cyclopeptides. Chem. Open 2021, 10, 1142–1149. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N. Biotechnological applications of bioactive peptides from marine sources. Adv. Microb. Physiol. 2018, 73, 171–220. [Google Scholar] [PubMed]
- Sukmarini, L. Antiviral peptides (AVPs) of marine origin as propitious therapeutic drug candidates for the treatment of human viruses. Molecules 2022, 27, 2619. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef]
- Chun, T.W.; Moir, S.; Fauci, A.S. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat. Immunol. 2015, 16, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tietjen, I.; Chen, M.; Williams, D.E.; Daoust, J.; Brockman, M.A.; Andersen, R.J. Sesterterpenoids isolated from the sponge Phorbas sp. activate latent HIV-1 provirus expression. J. Org. Chem. 2016, 81, 11324–11334. [Google Scholar] [CrossRef]
- Maina, E.K.; Adan, A.A.; Mureithi, H.; Muriuki, J.; Lwembe, R.M. A review of current strategies towards the elimination of latent HIV-1 and subsequent HIV-1 cure. Curr. HIV Res. 2021, 19, 14–26. [Google Scholar]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-and-lock strategies to cure HIV infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Richard, K.; Williams, D.E.; De Silva, E.D.; Brockman, M.A.; Brumme, Z.L.; Andersen, R.J.; Tietjen, I. Identification of novel HIV-1 latency-reversing agents from a library of marine natural products. Viruses 2018, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Silipo, A.; Molinaro, A.; Molteni, M.; Rossetti, C.; Parrilli, M.; Lanzetta, R. Full structural characterization of an extracellular polysaccharide produced by the freshwater cyanobacterium Oscillatoria planktothrix. Eur. J. Org. Chem. 2010, 2010, 5594–5600. [Google Scholar] [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: A review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazalovska, M.; Kouokam, J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. Biomed Res. Int. 2018, 2018, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Trivedi, J.; Mitra, D. High yield production of recombinant cyanovirin-N (antiviral lectin) exhibiting significant anti-HIV activity, from a rationally selected Escherichia coli strain. Process Biochem. 2020, 93, 1–11. [Google Scholar] [CrossRef]
- Lotfi, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. Bioimpacts 2018, 8, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da-Silva, D.M.; Cannon, P.M.; Kast, W.M. Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: Implications for prevention and treatment. AIDS Patient Care STDS 2016, 30, 291–306. [Google Scholar] [CrossRef] [Green Version]
- Vamvaka, E.; Evans, A.; Ramessar, K.; Krumpe, L.R.; Shattock, R.J.; Keefe, B.R.; Christou, P.; Capell, T. Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Rep. 2016, 35, 1309–1319. [Google Scholar] [CrossRef]
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel α (1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Qadir, A.; Yang, J.; Ahmad, I.; Zahid, H.; Mirza, S.; Windisch, M.P.; Shahzad-ul-Hussan, S. An engineered microvirin variant with identical structural domains potently inhibits human immunodeficiency virus and hepatitis C virus cellular entry. Viruses 2020, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Armario-Najera, V.; Blanco-Perera, A.; Shenoy, S.R.; Sun, Y.; Marfil, S.; Muñoz-Basagoiti, J.; Perez-Zsolt, D.; Blanco, J.; Izquierdo-Useros, N.; Capell, T.; et al. Physicochemical characterization of the recombinant lectin scytovirin and microbicidal activity of the SD1 domain produced in rice against HIV-1. Plant Cell Rep. 2022, 41, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivi. Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.H.; McClelland, E.E.; McFeeters, H.; McFeeters, R.L. Novel antifungal activity for the lectin scytovirin: Inhibition of Cryptococcus neoformans and Cryptococcus gattii. Front. Microbiol. 2017, 8, 755. [Google Scholar] [CrossRef]
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; O’Keefe, B.R.; Botos, I.; Wlodawer, A.; McMahon, J.B. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 2006, 46, 233–239. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae-A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Ziółkowska, N.E.; Wlodawer, A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006, 53, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Harnedy, P.A.; Fitz Gerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Serna-Arbeláez, M.S.; Florez-Sampedro, L.; Orozco, L.P.; Ramírez, K.; Galeano, E.; Zapata, W. Natural products with inhibitory activity against human immunodeficiency virus type 1. Adv. Virol. 2021, 2021, 5552088. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Falkenhagen, A.; Joshi, S. HIV entry and its inhibition by bifunctional antiviral proteins. Mol. Ther. Nucleic Acids 2018, 13, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Lee, C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: From discovery to clinical application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [Green Version]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, K.; Nguyen, K.; LiWang, P. Griffithsin retains anti-HIV-1 potency with changes in gp120 glycosylation and complements broadly neutralizing antibodies PGT121 and PGT126. Antimicrob. Agents Chemother. 2019, 64, e01084-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [Green Version]
- Férir, G.; Palmer, K.E.; Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology 2011, 417, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Hamorsky, K.T.; Grooms-Williams, T.W.; Husk, A.S.; Bennett, L.J.; Palmer, K.E.; Matoba, N. Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides. Antimicrob. Agents Chemother. 2013, 57, 2076–2086. [Google Scholar] [CrossRef] [Green Version]
- Férir, G.; Huskens, D.; Noppen, S.; Koharudin, L.M.; Gronenborn, A.M.; Schols, D. Broad anti-HIV activity of the Oscillatoria agardhii agglutinin homologue lectin family. J. Antimicrob. Chem. 2014, 69, 2746–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammari, N.; Krier, Y.; Albert, Q.; Devocelle, M.; Varbanov, M.; on behalf of the OEMONOM. Plant-derived antimicrobial peptides as potential antiviral agents in systemic viral infections. J. Pharma. 2021, 14, 774. [Google Scholar] [CrossRef]
- Hirayama, M.; Shibata, H.; Imamura, K.; Sakaguchi, T.; Hori, K. High-mannose specific lectin and its recombinants from a carrageenophyta Kappaphycus alvarezii represent a potent anti-HIV activity through high-affinity binding to the viral envelope glycoprotein gp120. Mar. Biotechnol. 2016, 18, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.M.; Liu, Y. Marine mollusks: Food with benefits. Compr. Rev. Food Sci. Food Saf. 2019, 18, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Lee, M.J.; Kim, Y.O.; Nam, B.H.; Kong, H.J.; Kim, J.W.; An, C.M.; Kim, D.G. Isolation and purification of antimicrobial peptide from hard-shelled mussel, Mytilus coruscus. J. Life Sci. 2016, 26, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Cunha Neves, A. Extraction, Purification and Characterisation of Biofunctional Peptides from Marine Processing Co-Products. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2015. [Google Scholar]
- Lee, T.G.; Maruyama, S. Isolation of HIV-1 protease-inhibiting peptides from thermolysin hydrolysate of oyster proteins. Biochem. Biophys. Res. Commun. 1998, 253, 604–608. [Google Scholar] [CrossRef]
- Ulagesan, S.; Krishnan, S.; Nam, T.J.; Choi, Y.H. A review of bioactive compounds in oyster shell and tissues. Front. Bioeng. Biotechnol. 2020, 10, 913839. [Google Scholar] [CrossRef]
- Wang, G.; Watson, K.M.; Peterkofsky, A.; Buckheit, R.W. Identification of novel human immunodeficiency virus type 1-Inhibitory peptides based on the antimicrobial peptide database. Antimicrob. Agents Chemotherap. 2010, 54, 1343–1346. [Google Scholar] [CrossRef] [Green Version]
- Vilas-Boas, L.; Campos, M.L.; Berlanda, R.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef]
- Reveillaud, J.; Maignien, L.; Eren, A.M.; Huber, J.A.; Apprill, A.; Sogin, M.L.; Vanreusel, A. Host-specificity among abundant and rare taxa in the sponge microbiome. ISME J. 2014, 8, 1198–1209. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef]
- Gustafson, K.; Roman, M.; Fenical, W. The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 1989, 111, 7519–7524. [Google Scholar] [CrossRef]
- Singh, S.B.; Jayasuriya, H.; Salituro, G.M.; Zink, D.L.; Shafiee, A.; Heimbuch, B.; Silverman, K.C.; Lingham, R.B.; Genilloud, O.; Teran, A.; et al. The complestatins as HIV-1 integrase inhibitors. Efficient isolation, structure elucidation and inhibitory activities of isocomplestatin, chloropeptin I, new complestatins, A and B, and acid-hydrolysis products of chloropeptin I. J. Nat. Prod. 2001, 64, 874–882. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuzaki, K.; Nakashima, H.; Ogino, T.; Matsumoto, A.; Ikeda, H.; Woodruff, H.B.; Omura, S. Chloropeptins, new anti-HIV antibiotics inhibiting gp120-CD4 binding from Streptomyces sp. I. Taxonomy, fermentation, isolation, and physico-chemical properties and biological activities. J. Antibiot. 1997, 50, 58–65. [Google Scholar] [CrossRef] [Green Version]
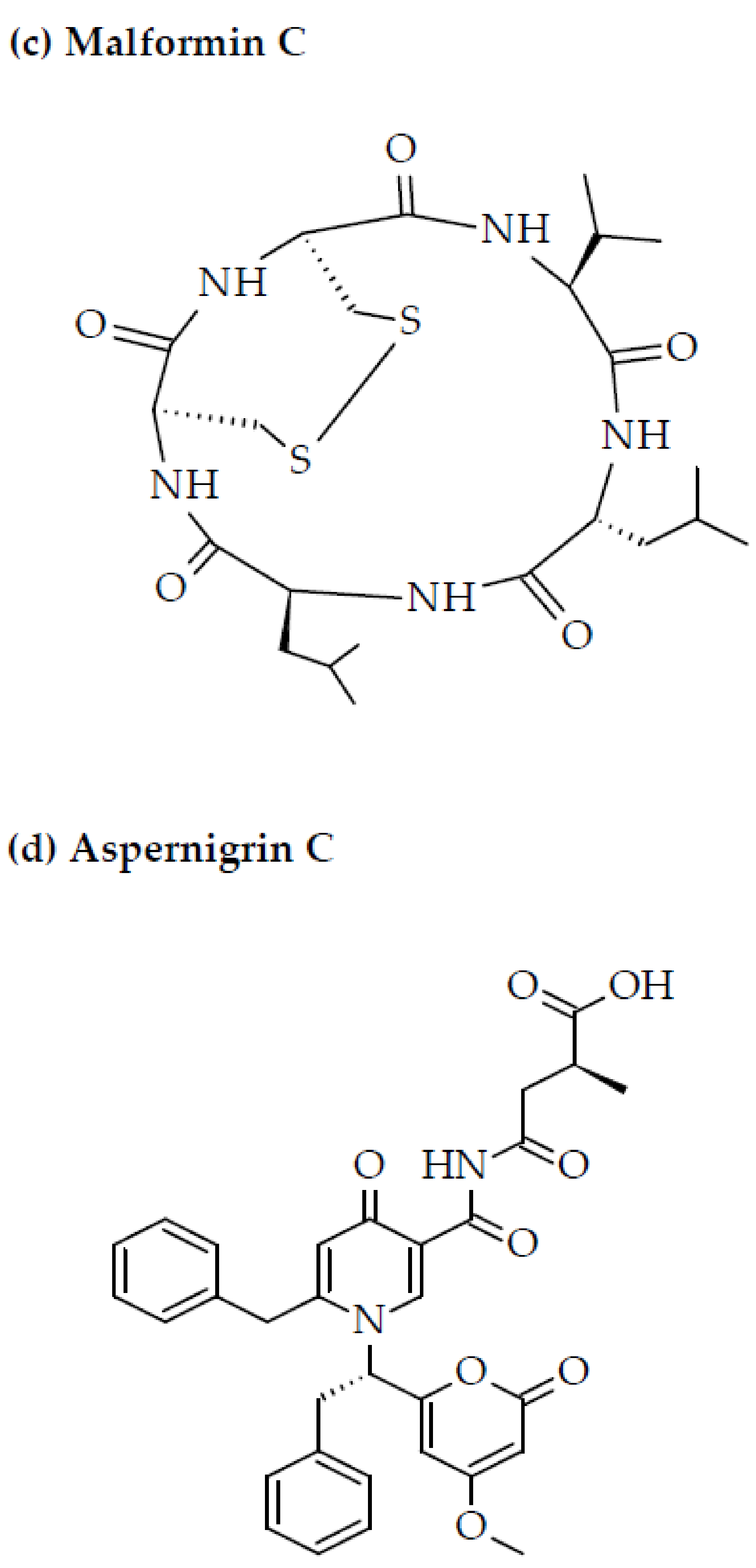
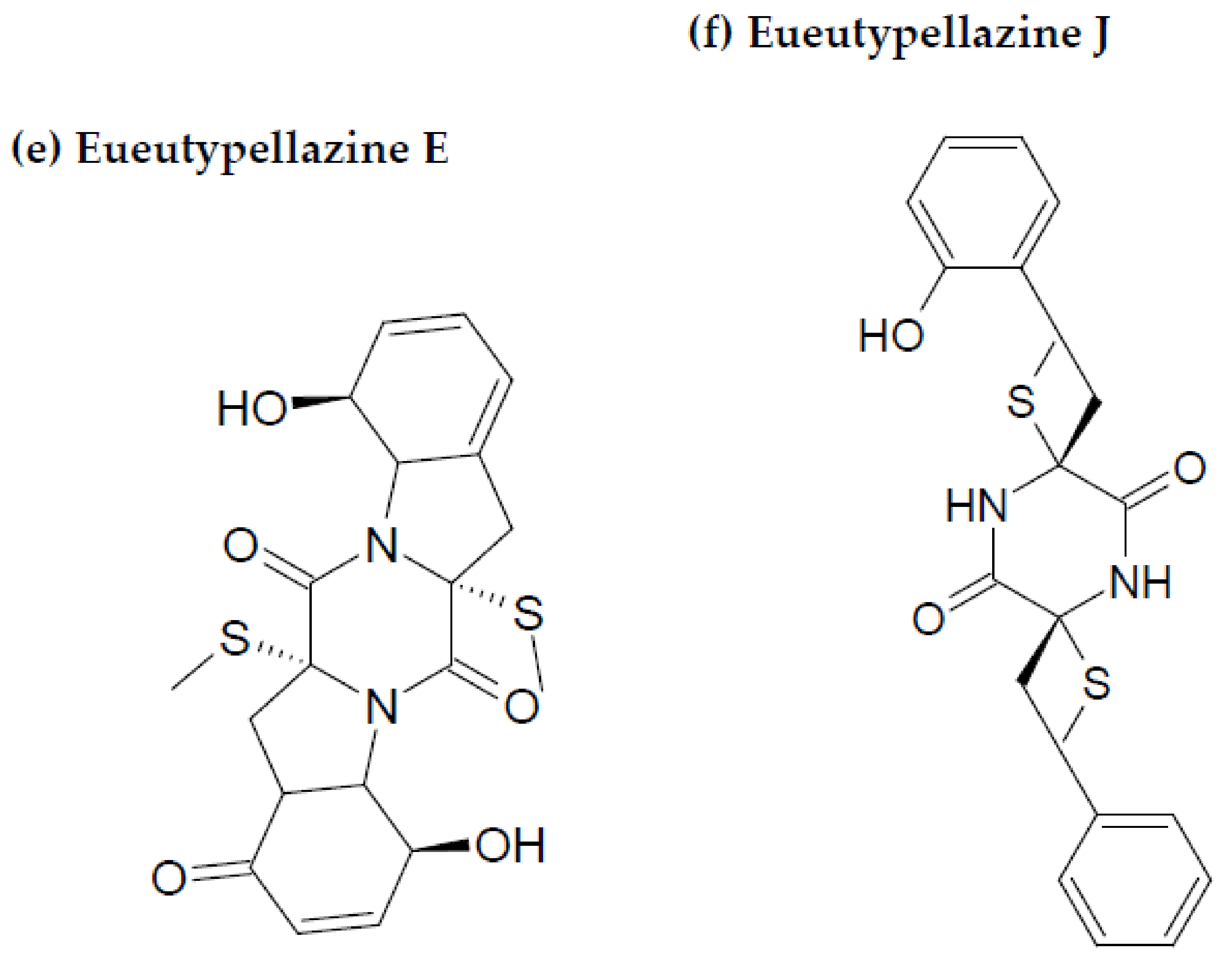
- Wittine, K.; Saftić, L.; Peršurić, Ž.; Kraljević Pavelić, S. Novel antiretroviral structures from marine organisms. Molecule 2019, 24, 3486. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A–M, thiodiketopiperazine-type alkaloids from deep sea derived fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdéz., J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L.J. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Eds.; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Tsiaka, T.; Zoumpoulakis, P.; Sinanoglou, V.J.; Makris, C.; Heropoulos, G.A.; Calokerinos, A.C. Response surface methodology toward the optimization of high-energy carotenoid extraction from Aristeus antennatus shrimp. Anal. Chim. Acta 2015, 877, 100–110. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.P.; Mishra, H.N. Thin layer modeling of convective and microwave-convective drying of oyster mushroom (Pleurotus ostreatus). J. Food Sci. Technol. 2015, 52, 2013–2022. [Google Scholar] [CrossRef] [Green Version]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Z.; Hao, Y.; Zhang, W. Marine products as a promising resource of bioactive peptides: Update of extraction strategies and their physiological regulatory effects. J. Agric. Food Chem. 2022, 70, 3081–3095. [Google Scholar] [CrossRef]
- Kent, S.B. Novel protein science enabled by total chemical synthesis. Protein Sci. 2019, 28, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Vijaykrishnaraj, M.; Prabhasankar, P. Marine protein hydrolysates: Their present and future perspectives in food chemistry-a review. RSC Adv. 2015, 5, 34864–34877. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Aisha; Chang, C.I.; Aulanni Chen, H.H.; Huang, T.C.; Hsu, J.L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteom. 2013, 94, 359–369. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Deng, C.; Pan, B.; Connor, O.A. Brentuximab vedotin. Clin. Cancer Res. 2013, 19, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Techniques | General Properties | Type of Peptides | Sources | Solvents/ Chemicals Applied |
|---|---|---|---|---|
| Preparation Techniques | ||||
| Solvent extraction | Less effective and time-consuming | Collagen peptides | Cod skin, marine crab, hemolymph | Ethanol, methanol, acetone, ethyl acetate, hexane butanol, and methanol |
| Microwave-assisted extraction (MAE) | Uses electromagnetic radiations of 300 MHz to 300 GHz, enhanced yield, rapid and selective extraction | Applied to fish, shrimp, brown seaweed, and oyster | Utilizes a series of solvents from heptane to water | |
| Chemical Hydrolysis | Simple and inexpensive | Fish protein hydrolysates | Applied to fish, bycatch fish | Sulfuric acid, hydrochloric acid, nitric acid, malic acid, oxalic acid, and phosphoric acids are majorly used |
| Enzyme Hydrolysis | Alcalase, flavourzyme, neutrase, trypsin, pepsin, papain, and bromelain are primarily used, more controllable and reproducible method, temperature, time, pH, enzyme concentration, and water/matter ratio are the major variables | Two purified dipeptides Tyr-Arg (337.2 Da) and Ile-Arg (287.2 Da), crude protein hydrolysates, papain hydrolysates, YVMRF peptide | Marine algae, marine sponge (Stylotella aurantium), Bellerochea and Nitzschia species, Stolephorus chinensis | |
| Increased cost and fouling are the main problems, MF (pore size is 0.1–10 µm), UF (pore size is 0.001–0.1 µm) | UF is used for nonhydrolyzed proteins and macro peptides | Generally applied to mackerel, shrimp, Spirulina platensis | ||
| Gel Filtration Chromatography | Simple and mild method, separate based on size, varying elution conditions, higher selectivity, and resolution, sample time consumption is the major limitation | Applied to fish and marine plants | Aqueous buffer (pH 6–8) | |
| Ion-Exchange Chromatography | It captures target protein and bulk impurities, Capto, MacroCap, and Monobeads are some of the mediums used | Applied to the mussel | Aqueous solutions or buffers containing organic solvents such as methanol and acetonitrile | |
| * HPLC including RP-HPLC, MS, LC-MS/MS, ESI, MALDI-TOF, HPLC-ELSD, UHPLC-MS/MS and RRLC–MS. | Higher resolution an sensitivity, ease of operation, rapid, expensive and environment unfriendly | SAITAPGGAM peptide, collagen peptides, cyclic heptapeptide Euryjanicin A, ALGPGPT, LVPPLA, LAPPTM, GVLIG and GHPVL, ILTLAALGGL, IITGGL, AAPSTVL, and TVAPPGA | Applied to cyanobacteria, fish, sponge, snail and Palmaria palmata, cod skin, Prosuberites laughlini, Stonefish, Dunaliella salina | Choline-oxalic acid based on eutectic solvent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khayri, J.M.; Asghar, W.; Khan, S.; Akhtar, A.; Ayub, H.; Khalid, N.; Alessa, F.M.; Al-Mssallem, M.Q.; Rezk, A.A.-S.; Shehata, W.F. Therapeutic Potential of Marine Bioactive Peptides against Human Immunodeficiency Virus: Recent Evidence, Challenges, and Future Trends. Mar. Drugs 2022, 20, 477. https://doi.org/10.3390/md20080477
Al-Khayri JM, Asghar W, Khan S, Akhtar A, Ayub H, Khalid N, Alessa FM, Al-Mssallem MQ, Rezk AA-S, Shehata WF. Therapeutic Potential of Marine Bioactive Peptides against Human Immunodeficiency Virus: Recent Evidence, Challenges, and Future Trends. Marine Drugs. 2022; 20(8):477. https://doi.org/10.3390/md20080477
Chicago/Turabian StyleAl-Khayri, Jameel Mohammed, Waqas Asghar, Sipper Khan, Aqsa Akhtar, Haris Ayub, Nauman Khalid, Fatima Mohammed Alessa, Muneera Qassim Al-Mssallem, Adel Abdel-Sabour Rezk, and Wael Fathi Shehata. 2022. "Therapeutic Potential of Marine Bioactive Peptides against Human Immunodeficiency Virus: Recent Evidence, Challenges, and Future Trends" Marine Drugs 20, no. 8: 477. https://doi.org/10.3390/md20080477









