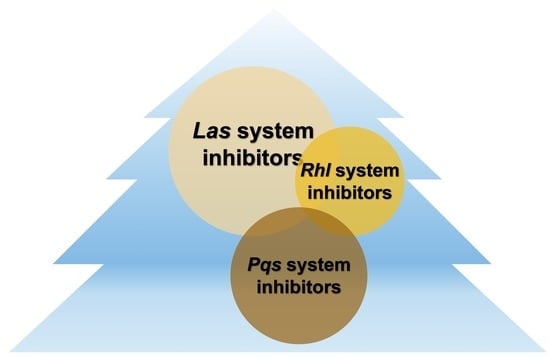
The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors
Abstract
:1. Introduction
2. Inhibitors of the las System
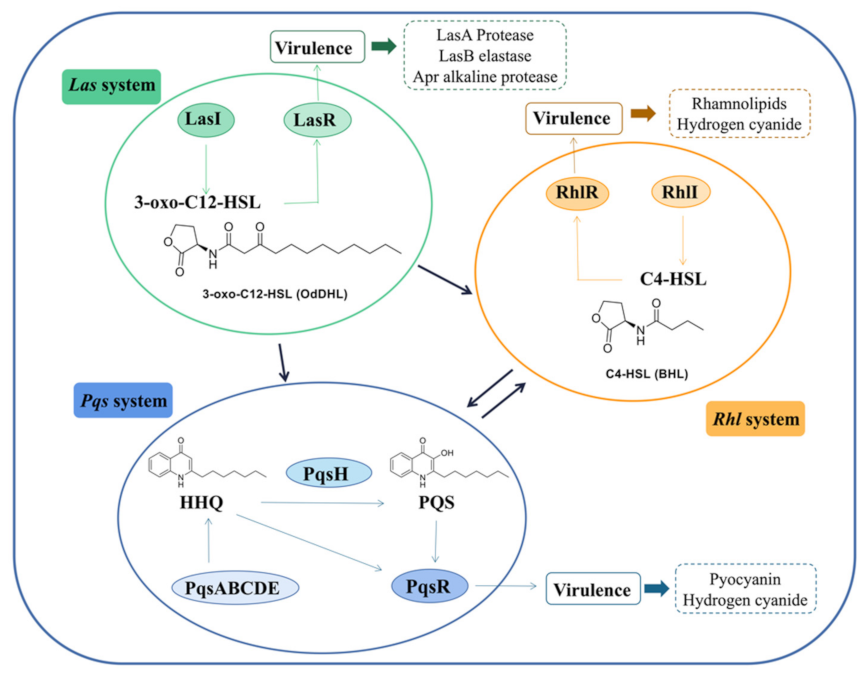
2.1. The Introduction of las
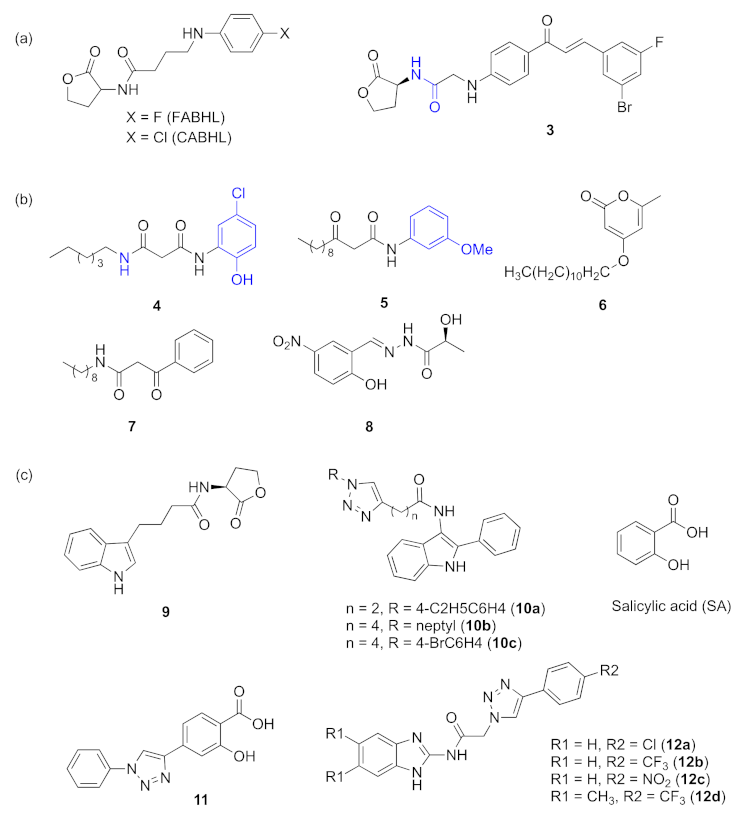
2.2. Inhibitors Acting on las
3. Inhibitors of the rhl System
3.1. The Introduction of rhl
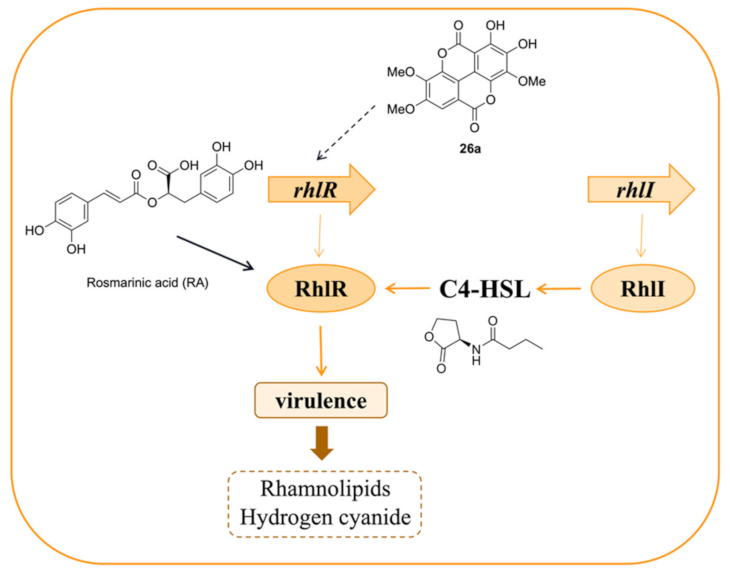
3.2. Inhibitors Acting on rhl
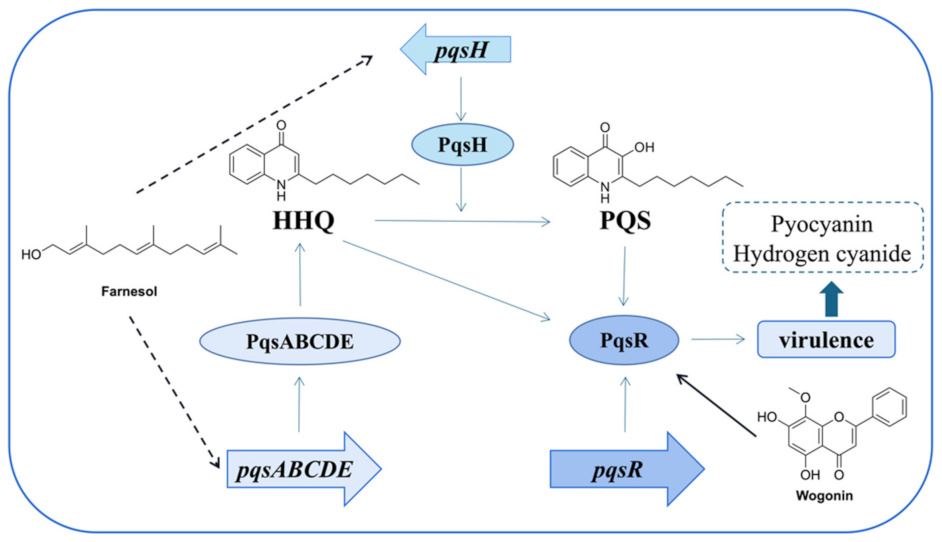
4. Inhibitors of the pqs System
4.1. The Introduction of pqs
4.2. Inhibitors Acting on pqs
5. Inhibitors Acting on Multiple QS Systems
6. Inhibition Mechanism Undetermined
7. QS Inhibitors from Marine Resources
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ouedraogo, V.; Kiendrebeogo, M. Methanol extract from Anogeissus leiocarpus (DC) Guill. et Perr. (Combretaceae) stem bark quenches the quorum sensing of Pseudomonas aeruginosa PAO1. Medicines 2016, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, I.; Sěegan, S.; Andrić, F.; Zlatović, M.; Moric, I.; Opsenica, D.M.; Senerovic, L. Long-chain 4-aminoquinolines as quorum sensing inhibitors in Serratia marcescens and Pseudomonas aeruginosa. ACS Chem. Biol. 2017, 12, 1425–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, B.N.; Iskander, G.M.; Mielczarek, M.; Yu, T.T.; Black, D.S.; Kumar, N. Alkyne-substituted fimbrolide analogues as novel bacterial quorum-sensing inhibitors. Aust. J. Chem. 2018, 71, 708–715. [Google Scholar]
- Swift, S.; Allan Downie, J.; Whitehead, N.A. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 2001, 45, 199–270. [Google Scholar] [PubMed]
- Givskov, M.; Nys, R.D.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yin, B.; Qian, L.; Zeng, Z.R.; Yang, Z.L.; Li, H.X.; Lu, Y.J.; Zhou, S.N. Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J. Med. Microbiol. 2011, 60, 1827–1834. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Bjarnsholt, T.; Jensen, P.; Givskov, M.; Høiby, N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013, 8, 901–921. [Google Scholar] [CrossRef]
- Bijtenhoorn, P.; Mayerhofer, H.; Muller-Dieckmann, J.; Utpatel, C.; Schipper, C.; Hornung, C.; Szesny, M.; Grond, S.; Thurmer, A.; Brzuszkiewicz, E.; et al. A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS ONE 2011, 6, e26278. [Google Scholar] [CrossRef]
- Chourasiya, S.S.; Kathuria, D.; Singh, S.; Sonawane, V.C.; Chakraborti, A.K.; Bharatam, P.V. Design, synthesis and biological evaluation of novel unsymmetrical azines as quorum sensing inhibitors. RSC Adv. 2015, 5, 80027–80038. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.; Ham, S.-Y.; Nam, S.; Kim, M.; Lee, K.Y.; Park, H.D. Recent advance in small molecules targeting RhlR of Pseudomonas aeruginosa. Antibiotics 2022, 11, 274. [Google Scholar] [CrossRef]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef] [Green Version]
- Vijendra Kumar, N.V.; Murthy, P.S.; Manjunatha, J.R.; Bettadaiah, B.K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef]
- Grabski, H.; Hunanyan, L.; Tiratsuyan, S.; Vardapetyan, H. Interaction of quercetin with transcriptional regulator LasR of Pseudomonas aeruginosa: Mechanistic insights of the inhibition of virulence through quorum sensing. bioRxiv 2017, 239996. [Google Scholar] [CrossRef] [Green Version]
- Hernando-Amado, S.; Alcalde-Rico, M.; Gil-Gil, T.; Valverde, J.R.; Martínez, J.L. Naringenin inhibition of the Pseudomonas aeruginosa quorum sensing response is based on its time-dependent competition with N-(3-Oxo-dodecanoyl)-L-homoserine lactone for LasR binding. Front. Mol. Biosci. 2020, 7, 25–35. [Google Scholar] [CrossRef]
- Baldelli, V.; D’Angelo, F.; Pavoncello, V.; Fiscarelli, E.V.; Visca, P.; Rampioni, G.; Leoni, L. Identification of FDA-approved antivirulence drugs targeting the Pseudomonas aeruginosa quorum sensing effector protein PqsE. Virulence 2020, 11, 652–668. [Google Scholar] [CrossRef]
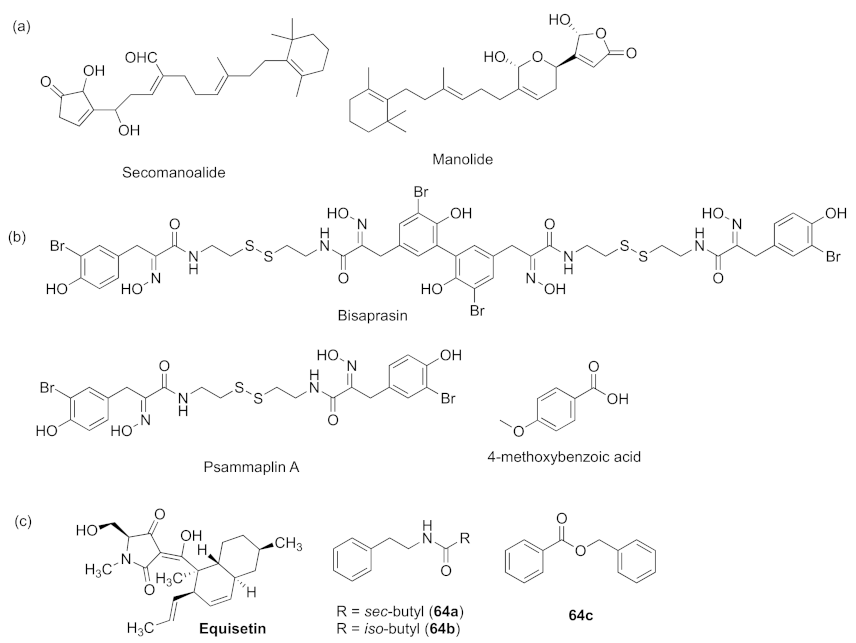
- Zhang, M.M.; Wang, M.J.; Zhu, X.C.; Yu, W.G.; Gong, Q.H. Equisetin as potential quorum sensing inhibitor of Pseudomonas aeruginosa. Biotechnol. Lett. 2018, 40, 865–870. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Santos, Y.L.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169–30180. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.G.; Ansari, M.A.; Khan, H.M.; Jalal, M.; Mahdi, A.A.; Cameotra, S.S. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. J Basic Microbiol. 2017, 57, 193–203. [Google Scholar] [CrossRef]
- Pangastuti, A.; Sari, S.A.; Nugraheni, E.R.; Astuti, R.T. Inhibition of Pseudomonas aeruginosa virulence factors expression regulated by quorum sensing system using ethyl acetate rxtract of Temu Ireng (Curcuma aeruginosa). IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012031–012038. [Google Scholar] [CrossRef]
- Ganin, H.; Rayo, J.; Amara, N.; Levy, N.; Krief, P.; Meijler, M.M. Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. Med. Chem. Comm. 2013, 4, 175–179. [Google Scholar] [CrossRef]
- Bai, A.J.; Vittal, R.R. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014, 28, 269–292. [Google Scholar] [CrossRef]
- Zhong, L.; Ravichandran, V.; Zhang, N.; Wang, H.L.; Bian, X.Y.; Zhang, Y.M.; Li, A.Y. Attenuation of Pseudomonas aeruginosa quorum sensing by natural products: Virtual screening, evaluation and biomolecular interactions. Int. J. Mol. Sci. 2020, 21, 2190. [Google Scholar] [CrossRef] [Green Version]
- Baburam, S.; Ramasamy, S.; Shanmugam, G.; Mathanmohun, M. Quorum sensing inhibitory potential and molecular docking studies of Phyllanthus emblica phytochemicals against Pseudomonas aeruginosa. Appl. Biochem. Biotechnol. 2022, 194, 434–444. [Google Scholar] [CrossRef]
- Borges, A.; Sousa, P.; Gaspar, A.; Vilar, S.; Borges, F.; Simões, M. Furvina inhibits the 3-oxo-C12-HSL-based quorum sensing system of Pseudomonas aeruginosa and QS-dependent phenotypes. Biofouling 2017, 33, 156–168. [Google Scholar] [CrossRef]
- Hançer Aydemir, D.; Çifci, G.; Aviyente, V.; Boşgelmez-Tinaz, G. Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J Appl Microbial. 2018, 125, 731–739. [Google Scholar] [CrossRef]
- Onem, E.; Soyocak, A.; Muhammed, M.T.; Ak, A. In vitro and in silico assessment of the potential of Niaouli essential oil as a quorum densing inhibitor of biofilm formation and its effects on fibroblast cell viability. Braz. Arch. Biol. Technol. 2021, 64, 782–792. [Google Scholar] [CrossRef]
- Fazzini, R.A.B.; Skindersoe, M.E.; Bielecki, P.; Puchałka, J.; Givskov, M.; Martins dos Santos, V.A.P. Protoanemonin: A natural quorum sensing inhibitor that selectively activates iron starvation response. Environ. Microbiol. 2012, 15, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.S.; Naik, D.; Bhat, C.; Tilve, S.; Tilvi, S.; D’Souza, L. Synthesis of (R)-norbgugaine and its potential as quorum sensing inhibitor against Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2013, 23, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Shastry, R.P.; Ghate, S.D.; Kumar, B.S.; Srinath, B.S.; Kumar, V. Vanillin derivative inhibits quorum sensing and biofilm formation in Pseudomonas aeruginosa: A study in a Caenorhabditis elegans infection model. Nat. Prod. Res. 2021, 36, 1610–1615. [Google Scholar] [CrossRef]
- Kalaiarasan, E.; Kottha, T.; Harish, B.N.; Gnanasambandam, V.; Sali, V.K.; John, J. Inhibition of quorum sensing-controlled biofilm formation in Pseudomonas aeruginosa by quorum-sensing inhibitors. Microb. Pathog. 2017, 111, 99–107. [Google Scholar] [CrossRef]
- Luo, C.Y.; Li, P.; Liu, H.Y.; Feng, P.X.; Li, J.M.; Zhao, L.T.; Wu, C.-L. Synthesis of novel chalcone-based L-homoserine lactones and theirquorum sensing inhibitory activity evaluation. Russ. J. Bioorg. Chem. 2020, 46, 139–148. [Google Scholar]
- Jadhav, G.P.; Chhabra, S.R.; Telford, G.; Hooi, D.S.W.; Righetti, K.; Williams, P.; Kellam, B.; Pritchard, D.I.; Fischer, P.M. Immunosuppressive but non-LasR-inducing analogues of the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. J. Med. Chem. 2011, 54, 3348–3359. [Google Scholar] [CrossRef]
- Baker, Y.R.; Galloway, W.R.J.D.; Hodgkinson, J.T.; Spring, D.R. Design and synthesis of a biotinylated chemical probe for detecting the molecular targets of an inhibitor of the production of the Pseudomonas aeruginosa virulence factor pyocyanin. Molecules 2013, 18, 11783–11796. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, H.-S.; Ok, K.; Kim, Y.H.; Park, H.-D.; Byun, Y. Design, synthesis and biological evaluation of 4-(alkyloxy)-6-methyl-2H-pyran-2-one derivatives as quorum sensing inhibitors. Bioorg. Med. Chem. Lett. 2015, 5, 54–58. [Google Scholar] [CrossRef]
- Moore, J.D.; Rossi, F.M.; Welsh, M.A.; Nyffffeler, K.E.; Blackwell, H.E. A comparative analysis of synthetic quorum sensing modulators in Pseudomonas aeruginosa: New insights into mechanism, active efflux susceptibility, phenotypic response, and next-generation ligand design. J. Am. Chem. Soc. 2015, 137, 14626–14639. [Google Scholar] [CrossRef]
- Heidari, A.; Haghi, F.; Noshiranzadeh, N.; Bikas, R. (S,E)-2-hydroxy-N-(2-hydroxy-5-nitrobenzylidene)propane hydrazide as a quorum sensing inhibitor of Pseudomonas aeruginosa. Med. Chem. Res. 2017, 17, 1908–1915. [Google Scholar] [CrossRef]
- Srinivasarao, S.; Nizalapur, S.; Yu, T.T.; Wenholz, D.S.; Trivedi, P.; Ghosh, B.; Rangan, K.; Kumar, N.; Sekhar, K.V.G.C. Design, synthesis and biological evaluation of triazole-containing 2-phenylindole and salicylic acid as quorum sensing inhibitors against Pseudomonas aeruginosa. ChemistrySelect 2018, 3, 9170–9180. [Google Scholar] [CrossRef]
- Srinivasarao, S.; Nandikolla, A.; Nizalapur, S.; Yu, T.T.; Pulya, S.; Ghosh, B.; Murugesan, S.; Kumar, N.; Sekhar, K.V.G.C. Design, synthesis and biological evaluation of 1,2,3-triazole based 2-aminobenzimidazoles as novel inhibitors of LasR dependent quorum sensing in Pseudomonas aeruginosa. RSC Adv. 2019, 9, 29273–29292. [Google Scholar] [CrossRef] [Green Version]
- Almohaywi, B.; Taunk, A.; Wenholz, D.S.; Nizalapur, S.; Biswas, N.N.; Ho, K.K.K.; Rice, S.A.; Iskander, G.; Black, D.S.; Griffith, R.; et al. Design and Synthesis of Lactams Derived from Mucochloric and Mucobromic Acids as Pseudomonas aeruginosa Quorum Sensing Inhibitors. Molecules 2018, 23, 1106. [Google Scholar] [CrossRef] [Green Version]
- Capilato, J.N.; Philippi, S.V.; Reardon, T.; McConnell, A.; Oliver, D.C.; Warren, A.; Adams, J.S.; Wu, C.; Perez, L.J. Development of a novel series of non-natural triaryl agonists and antagonists of the Pseudomonas aeruginosa LasR quorum sensing receptor. Bioorg. Med. Chem. 2016, 25, 153–165. [Google Scholar] [CrossRef]
- O’Brien, K.T.; Noto, J.G.; Nichols-O’Neill, L.; Perez, L.J. Potent Irreversible Inhibitors of LasR Quorum Sensing in Pseudomonas aeruginosa. ACS Med. Chem. Lett. 2015, 6, 162–167. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.C.; Blackwell, H.E. Structure-based design and biological evaluation of triphenyl scaffold-based hybrid compounds as hydrolytically stable modulators of a LuxR-type quorum sensing receptor. ACS Infect. Dis. 2016, 2, 32–38. [Google Scholar] [CrossRef]
- Tan, S.Y.-Y.; Chua, S.-L.; Chen, Y.C.; Rice, S.A.; Kjelleberg, S.; Nielsen, T.E.; Yang, L.; Givskov, M. Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother. 2013, 57, 5629–5641. [Google Scholar] [CrossRef] [Green Version]
- Thanh, T.T.; Xuan, H.L.; Quoc, T.N. Benzo[d]thiazole-2-thiol bearing 2-oxo-2- substituted-phenylethan-1-yl as potent selective lasB quorum sensing inhibitors of Gram-negative bacteria. RSC Adv. 2021, 11, 28797–28808. [Google Scholar] [CrossRef]
- Ulusoy, S.; Şenkardeş, S.; Coşkun, İ.; Boşgelmez-Tınaz, G.; Soulère, L.; Queneau, Y.; Küçükgüzel, Ş.G. Quorum aensing inhibitor activities of Celecoxib derivatives in Pseudomonas aeruginosa. Lett. Drug Des. Discov. 2017, 14, 613–618. [Google Scholar]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Chan, D.S.H.; Ho, K.K.K.; Rice, S.; Black, D.S.; Griffith, R.; Kumar, N. Dihydropyrrolones as bacterial quorum sensing inhibitors. Bioorg Med Chem Lett. 2019, 29, 1054–1059. [Google Scholar] [CrossRef]
- Mohanvel, S.K.; Ravichandran, V.; Kamalanathan, C.; Satish, A.S.; Ramesh, S.; Lee, J.; Rajasekharan, S.K. Molecular docking and biological evaluation of novel urea-tailed mannich base against Pseudomonas aeruginosa. Microb. Pathog. 2019, 130, 104–111. [Google Scholar] [CrossRef]
- Xie, Y.X.; Chen, J.X.; Wang, B.; Peng, A.-Y.; Mao, Z.-W.; Xia, W. Inhibition of quorum-sensing regulator from Pseudomonas aeruginosa using a flavone derivative. Molecules. 2022, 27, 2439. [Google Scholar] [CrossRef]
- Magalhães, R.P.; Vieira, T.F.; Melo, A.; Sousa, S.F. Identification of novel candidates for inhibition of LasR, a quorum-sensing receptor of multidrug resistant Pseudomonas aeruginosa, through a specialized multi-level in silico approach. Mol. Syst. Des. Eng. 2022, 7, 434–446. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.-F.; Chong, Y.-M.; Tan, L.Y.; Chong, T.M.; Chan, K.-G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014, 4, 7245–7252. [Google Scholar] [CrossRef] [PubMed]
- Boursier, M.E.; Moore, J.D.; Heitman, K.M.; Shepardson-Fungairino, S.P.; Combs, J.B.; Koenig, L.C.; Shin, D.; Brown, E.C.; Nagarajan, R.; Blackwell, H.E. Structure-function analyses of the N-butanoyl L-homoserine lactone quorum-sensing signal define features critical to activity in RhlR. ACS Chem. Biol. 2018, 13, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yao, J.N.; Li, H.M. Plantain herb extracts significantly attenuate the quorum sensing-controlled virulence factors and inhibit biofilm formation in Pseudomonas aeruginosa PAO1. E3S Web Conf. 2019, 78, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-W.; Muhammad, J.; Sun, B.; Yang, R.; Wadood, A.; Wang, J.-S.; Jia, A.-Q. Metabolomic analysis of quorum sensing inhibitor hordenine on Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 6271–6285. [Google Scholar]
- Ahmed, A.A.; Salih, F.A. Quercus infectoria gall extracts reduce quorum sensing-controlled virulence factors production and biofilm formation in Pseudomonas aeruginosa recovered from burn wounds. BMC Complement. Altern. Med. 2019, 19, 177. [Google Scholar]
- Naik, V.; Mahajan, G. Quorum sensing: A non-conventional target for antibiotic discovery. Nat. Prod. Commun. 2013, 8, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Corral-Lugo, A.; Krell, T. The plant compound rosmarinic acid induces a broad quorum sensing response in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 2018, 20, 4230–4244. [Google Scholar]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar]
- Boursier, M.E.; Combs, J.B.; Blackwell, H.E. N-acyl L-homocysteine thiolactones are potent and stable synthetic modulators of the RhlR quorum sensing receptor in Pseudomonas aeruginosa. ACS Chem. Biol. 2019, 14, 186–191. [Google Scholar]
- Qiu, M.-N.; Wang, F.; Chen, S.-Y.; Wang, P.-C.; Fu, Y.-H.; Liu, Y.-Y.; Wang, X.; Wang, F.-B.; Wang, C.; Yang, H.-W.; et al. Novel 2, 8-bit derivatives of quinolines attenuate Pseudomonas aeruginosa virulence and biofilm formation. Bioorg. Med. Chem. Lett. 2019, 29, 749–754. [Google Scholar] [CrossRef]
- Nam, S.J.; Ham, S.-Y.; Kwon, H.; Kim, H.-S.; Moon, S.; Lee, J.-H.; Lim, T.; Son, S.-H.; Park, H.-D.; Byun, Y. Discovery and characterization of pure RhlR antagonists against Pseudomonas aeruginosa infections. J. Med. Chem. 2020, 63, 8388–8407. [Google Scholar] [CrossRef]
- Lin, J.S.; Cheng, J.L. Quorum sensing in Pseudomonas aeruginosa and its relationship to biofilm development. In Introduction to Biofilm Engineering; American Chemical Society: Washington, DC, USA, 2019; pp. 1–16. [Google Scholar]
- Sahner, J.H.; Empting, M.; Kamal, A.; Weidel, E.; Groh, M.; Borger, C.; Hartmann, R.W. Exploring the chemical space of ureidothiophene-2-carboxylic acids as inhibitors of the quorum sensing enzyme PqsD from Pseudomonas aeruginosa. Eur. J. Med. Chem. 2015, 96, 14–21. [Google Scholar] [CrossRef]
- Aleksic, I.; Jeremic, J.; Milivojevic, D.; Ilic-Tomic, T.; Šegan, S.; Zlatovic, M.; Opsenica, D.M.; Senerovic, L. N-benzyl derivatives of long-chained 4-amino-7-chloro-quionolines as inhibitors of pyocyanin production in Pseudomonas aeruginosa. ACS Chem. Biol. 2019, 14, 2800–2809. [Google Scholar] [CrossRef]
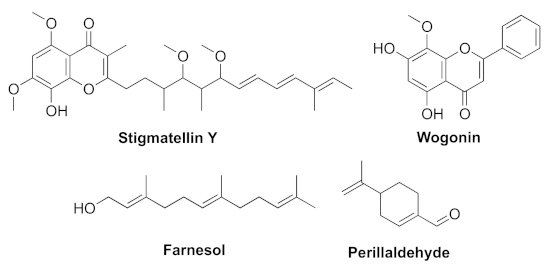
- Boopathi, S.; Vashisth, R.; Manoharan, P.; Kandasamy, R.; Sivakumar, N. Stigmatellin Y- an anti-biofilm compound from Bacillus subtilis BR4 possibly interferes in PQS-PqsR mediated quorum sensing system in Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2017, 27, 2113–2118. [Google Scholar] [CrossRef]
- Wang, S.W.; Feng, Y.Q.; Han, X.F.; Cai, X.Y.; Yang, L.; Liu, C.L.; Shen, L.X. Inhibition of virulence factors and biofilm formation by wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting pqs quorum-sensing system. Int. J. Mol. Sci. 2021, 22, 12699. [Google Scholar] [CrossRef]
- Li, W.-R.; Zeng, T.-H.; Xie, X.-B.; Shi, Q.-S.; Li, C.-L. Inhibition of the pqsABCDE and pqsH in the pqs quorum sensing system and related virulence factors of the Pseudomonas aeruginosa PAO1 strain by farnesol. Int. Biodeter. Biodegr. 2020, 151, 104956–104967. [Google Scholar] [CrossRef]
- Benny, A.T.; Rathinam, P.; Dev, S.; Mathew, B.; Radhakrishnan, E.K. Perillaldehyde mitigates virulence factors and biofilm formation of Pseudomonas aeruginosa clinical isolates, by acting on the quorum sensing mechanism in vitro. J. Appl. Microbiol. 2021, 1–15. [Google Scholar] [CrossRef]
- Lu, C.; Kirsch, B.; Zimmer, C.; de Jong, J.C.; Henn, C.; Maurer, C.K.; Müsken, M.; Häussler, S.; Steinbach, A.; Hartmann, R.W. Discovery of antagonists of PqsR, a key player in 2-alkyl-4-quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem. Biol. 2012, 19, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Grossman, S.; Soukarieh, F.; Richardson, W.; Liu, R.L.; Mashabi, A.; Emsley, J.; Williams, P.; Camara, M.; Stocks, M.J. Novel quinazolinone inhibitors of the Pseudomonas aeruginosa quorum sensing transcriptional regulator PqsR. Eur. J. Med. Chem. 2020, 208, 112778–112792. [Google Scholar] [CrossRef]
- Murray, E.J.; Dubern, J.-F.; Chan, W.C.; Chhabra, S.R.; Williams, P. A Pseudomonas aeruginosa PQS quorum-sensing system inhibitor with anti-staphylococcal activity sensitizes polymicrobial biofilms to tobramycin. Cell Chem. Biol. 2022, 29, 1187–1199.e6. [Google Scholar] [CrossRef]
- Trognon, J.; Vera, G.; Rima, M.; Stigliani, J.-L.; Amielet, L.; Hage, S.E.; Lajoie, B.; Roques, C.; Garah, F.E. Investigation of direct and retro chromone-2-carboxamides based analogs of Pseudomonas aeruginosa quorum sensing signal as new anti-biofilm agents. Pharmaceuticals 2022, 15, 417. [Google Scholar] [CrossRef]
- Sahner, J.H.; Brengel, C.; Storz, M.P.; Groh, M.; Plaza, A.; Müller, R.; Hartmann, R.W. Combining in silico and biophysical methods for the development of Pseudomonas aeruginosa quorum sensing inhibitors: An alternative approach for structure-based drug design. J. Med. Chem. 2013, 56, 8656–8664. [Google Scholar] [CrossRef]
- Hinsberger, S.; de Jong, J.C.; Groh, M.; Haupenthal, J.; Hartmann, R.W. Benzamidobenzoic acids as potent PqsD inhibitors for the treatment of Pseudomonas aeruginosa infections. Eur. J. Med. Chem. 2014, 76, 343–351. [Google Scholar] [CrossRef]
- Storz, M.P.; Allegretta, G.; Kirsch, B.; Empting, M.; Hartmann, R.W. From in vitro to in cellulo: Structure-activity relationship of (2-nitrophenyl) methanol derivatives as inhibitors of PqsD in Pseudomonas aeruginosa. Org. Biomol. Chem. 2014, 12, 6094–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegretta, G.; Weidel, E.; Empting, M.; Hartmann, R.W. Catechol-based substrates of chalcone synthase as a scaffold for novel inhibitors of PqsD. Eur. J. Med. Chem. 2015, 90, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, J.-S.; Chang, Y.-Q.; Peng, L.-J.; Zhang, X.-Y.; Miao, Z.-Y.; Sun, P.-H.; Lin, J.; Chen, W.-M. New pqsquorum sensing system inhibitor as an antibacterial synergist against multidrug-resistant Pseudomonas aeruginosa. J. Med. Chem. 2022, 65, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Soukarieh, F.; Oton, E.V.; Dubern, J.-F.; Gomes, J.; Halliday, N.; Crespo, M.D.P.; Ramírez-Prada, J.; Insuasty, B.; Abonia, R.; Quiroga, J.; et al. In silico and in vitro-guided identification of inhibitors of alkylquinolone-dependent quorum sensing in Pseudomonas aeruginosa. Molecules 2018, 23, 257. [Google Scholar] [CrossRef] [Green Version]
- Maura, D.; Rahme, L.G. Pharmacological inhibition of the Pseudomonas aeruginosa MvfR quorum sensing system interferes with biofilm formation and potentiates antibiotic-mediated biofilm disruption. Antimicrob. Agents Chemother. 2017, 61, 1362–1378. [Google Scholar] [CrossRef] [Green Version]
- Soukarieh, F.; Liu, R.L.; Romero, M.; Roberston, S.N.; Richardson, W.; Lucanto, S.; Oton, E.V.; Qudus, N.R.; Mashabi, A.; Grossman, S.; et al. Hit identification of new potent PqsR antagonists as inhibitors of quorum sensing in planktonic and biofilm grown Pseudomonas aeruginosa. Front. Chem. 2020, 8, 204–217. [Google Scholar] [CrossRef]
- Schütz, C.; Ho, D.-K.; Hamed, M.M.; Abdelsamie, A.S.; Röhrig, T.; Herr, C.; Kany, A.M.; Rox, K.; Schmelz, S.; Siebenbürger, L.; et al. A New PqsR inverse agonist potentiates tobramycin efficacy to eradicate Pseudomonas aeruginosa biofilms. Adv. Sci. 2021, 8, 2004369–2004380. [Google Scholar] [CrossRef]
- Soltani, S.; Bazzaz, B.S.F.; Hadizadeh, F.; Roodbari, F.; Soheil, V. New insight into Vitamin E and K1 as anti-quorum sensing agents against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2021, 65, 1342–1361. [Google Scholar] [CrossRef]
- Vieira, T.F.; Magalhães, R.P.; Simões, M.; Sousa, S.F. Drug repurposing targeting Pseudomonas aeruginosa MvfR using docking, virtual screening, molecular dynamics, and free-energy calculations. Antibiotics 2022, 11, 185. [Google Scholar] [CrossRef]
- Beury-Cirou, A.; Tannières, M.; Minard, C.; Soulère, L.; Rasamiravaka, T.; Dodd, R.H.; Queneau, Y.; Dessaux, Y.; Guillou, C.; Vandeputte, O.M.; et al. At a supra-physiological concentration, human sexual hormones act as quorum-sensing inhibitors. PLoS ONE 2013, 8, e83564. [Google Scholar]
- Abinaya, M.; Gayathri, M. Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P. aeruginosa. Bioorg. Chem. 2019, 87, 291–301. [Google Scholar]
- Geng, Y.F.; Yang, C.; Zhang, Y.; Tao, S.N.; Mei, J.; Zhang, X.C.; Sun, Y.J.; Zhao, B.T. An innovative role for luteolin as a natural quorum sensing inhibitor in Pseudomonas aeruginosa. Life Sci. 2021, 274, 119325–119335. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef]
- Bajire, S.K.; Jain, S.; Johnson, R.P.; Shastry, R.P. 6-Methylcoumarin attenuates quorum sensing and bioflm formation in Pseudomonas aeruginosa PAO1 and its applications on solid surface coatings with polyurethane. Appl. Microbiol. Biotechnol. 2021, 105, 8647–8661. [Google Scholar]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef] [Green Version]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Stévigny, C.; Duez, P.; et al. Pseudomonas aeruginosa biofilm formation and persistence, along with the production of quorum sensing-dependent virulence factors, are disrupted by a triterpenoid coumarate ester isolated from Dalbergia trichocarpa, a tropical legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef]
- Gökalsın, B.; Sesal, N.C. Lichen secondary metabolite evernic acid as potential quorum sensinginhibitor against Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2016, 32, 150–156. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Jia, A.-Q.; Jiang, H.; Li, P.-L.; Chen, H.; Tan, X.-J.; Liu, E.-Q. 1-(4-Amino-2-hydroxyphenyl)ethanone from Phomopsis liquidambari showed quorum sensing inhibitory activity against Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2021, 105, 341–352. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Jedrzejowsk, A.; Kiendrebeogo, M.; Rajaonson, S.; Randriamampionona, D.; Rabemanantsoa, C.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Duez, P.; Jaziri, M.E.; et al. Endemic Malagasy Dalbergia species inhibit quorum sensing in Pseudomonas aeruginosa PAO1. Microbiology 2013, 159, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-G.; Yan, S.-S.; Yu, Y.-M.; Mi, N.; Zhang, L.-X.; Liu, J.; Li, X.-L.; Liu, F.; Xu, J.-F.; Yang, W.-Q.; et al. An aqueous extract of Yunnan Baiyao inhibits the quorum-sensing-related virulence of Pseudomonas aeruginosa. J. Microbiol. 2013, 51, 207–212. [Google Scholar] [CrossRef]
- Peerzada, Z.; Kanhed, A.M.; Desai, K.B. Effects of active compounds from Cassia fistula on quorum sensing mediated virulence and biofilm formation in Pseudomonas aeruginosa. RSC Adv. 2022, 12, 15196–15214. [Google Scholar] [CrossRef]
- Ham, S.-Y.; Kim, H.-S.; Jang, Y.S.; Sun, P.-F.; Park, J.-H.; Lee, J.S.W.; Byun, Y.J.; Park, H.-D. Control of membrane biofouling by 6-gingerol analogs: Quorum sensing inhibition. Fuel 2019, 250, 79–87. [Google Scholar] [CrossRef]
- Shah, M.D.; Kharkar, P.S.; Sahu, N.U.; Peerzada, Z.; Desai, K.B. Potassium 2-methoxy-4-vinylphenolate: A novel hit exhibiting quorum-sensing inhibition in Pseudomonas aeruginosa via LasIR/RhlIR circuitry. RSC Adv. 2019, 9, 40228–40239. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Mishr, A.; Jha, B. Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.X.; Xu, Z.H.; Zhang, Y.Q.; Tian, J.; Weng, L.X.; Wang, L.H. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. J. Microbiol. 2012, 50, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.-S.; Choi, H.-Y.; Yoon, S.-S.; Kim, W.-G. Terrein is an inhibitor of quorum sensing and c-di-GMP in Pseudomonas aeruginosa: A connection between quorum sensing and c-di-GMP. Sci. Rep. 2018, 8, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Borkotoky, S.; Reddy, D.; Mohanty, S.K.; Kumavath, R.; Murali, A.; Suchiang, K.; Busi, S. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: Insights from in vitro, in vivo and in silico studies. Microbiol. Res. 2019, 226, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Ş.; Gökalsın, B.; Şenkardeş, İ.; Doğan, A.; Sesal, N.C. Anti-quorum sensing and anti-biofilm activities of Hypericum perforatum extracts against Pseudomonas aeruginosa. J. Ethnopharmacol. 2019, 235, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Singh, D.; Sharma, D.; Narvi, S.S.; Agarwal, V. Senna alexandriana Mill as a potential inhibitor for quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Phcog. Mag. 2020, 16, 797–802. [Google Scholar]
- Meena, H.; Mishra, R.; Ranganathan, S.; Sarma, V.V.; Ampasala, D.R.; Kalia, V.C.; Lee, J.-K.; Siddhardha, B. Phomopsis tersa as inhibitor of quorum sensing system and biofilm forming ability of Pseudomonas aeruginosa. Indian J. Microbiol. 2020, 60, 70–77. [Google Scholar] [CrossRef]
- Dai, L.; Wu, T.-Q.; Xiong, Y.-S.; Ni, H.-B.; Ding, Y.; Zhang, W.-C.; Chu, S.-P.; Ju, S.-Q.; Yu, J. Ibuprofen-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Life Sci. 2019, 237, 116947–116955. [Google Scholar] [CrossRef]
- El-Mowafy, S.A.; Abd El Galil, K.H.; El-Messery, S.M.; Shaaban, M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 2014, 74, 25–32. [Google Scholar] [CrossRef]
- Saqr, A.A.; Aldawsari, M.F.; Khafagy, E.-S.; Shaldam, M.A.; Hegazy, W.A.H.; Abbas, H.A. A novel use of allopurinol as a quorum-sensing inhibitor in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1385. [Google Scholar] [CrossRef]
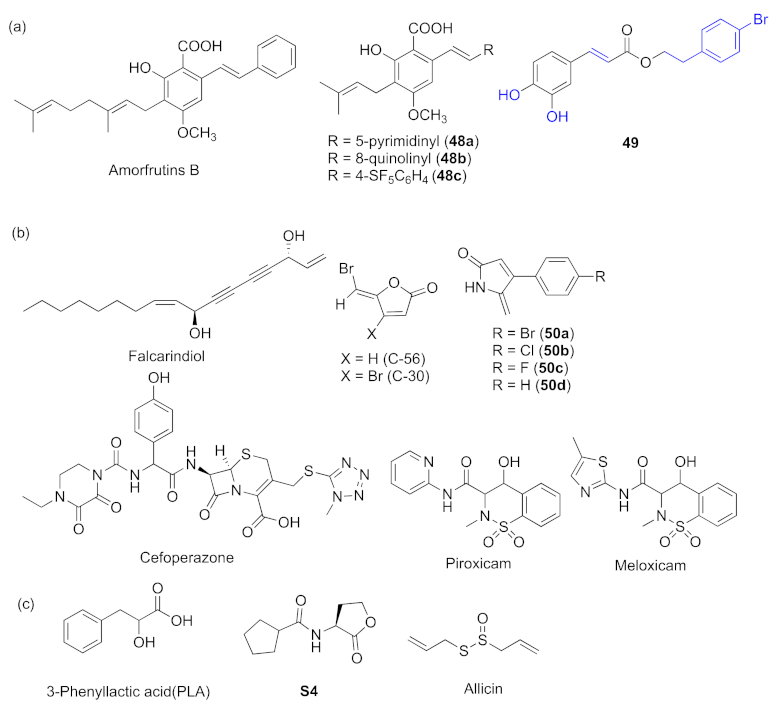
- Xu, X.-J.; Zeng, T.; Huang, Z.-X.; Xu, X.-F.; Lin, J.; Chen, W.-M. Synthesis and biological evaluation of cajaninstilbene acid and amorfrutins A and B as inhibitors of the Pseudomonas aeruginosa quorum sensing system. J. Nat. Prod. 2018, 81, 2621–2629. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Yu, J.-H.; Xu, X.-J.; Xu, X.-F.; Zeng, T.; Lin, J.; Chen, W.-M. Cajaninstilbene acid analogues as novel quorum sensing and biofilm inhibitors of Pseudomonas aeruginosa. Microb. Pathog. 2020, 148, 104414–104422. [Google Scholar] [CrossRef]
- Zeng, Y.-F.; Su, Y.-L.; Liu, W.-L.; Chen, H.-G.; Zeng, S.-G.; Zhou, H.-B.; Chen, W.-M.; Zheng, J.-X.; Sun, P.-H. Design and synthesis of caffeic acid derivatives and evaluation of their inhibitory activity against Pseudomonas aeruginosa. Med. Chem. Res. 2022, 31, 177–194. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zheng, H.D.; Zhou, L.M.; Ji, H.R.; Zhao, L.; Yu, W.G.; Gong, Q.H. Falcarindiol isolated from Notopterygium incisum inhibits the quorum sensing of Pseudomonas aeruginosa. Molecules 2021, 26, 5896–5906. [Google Scholar] [CrossRef]
- Ghosh, R.; Das, A.; Mallik, S. Inhibition of quorum sensing in Pseudomonas aeruginosa: A review. Indian J. Pharm. Sci. 2019, 81, 797–806. [Google Scholar] [CrossRef]
- Das, T.; Sabir, S.; Chen, R.; Farrell, J.; Kriel, F.H.; Whiteley, G.S.; Glasbey, T.O.; Manos, J.; Willcox, M.D.P.; Kumar, N. Halogenated dihydropyrrol-2-one molecules inhibit pyocyanin biosynthesis by blocking the Pseudomonas quinolone signaling system. Molecules 2022, 27, 1169. [Google Scholar] [CrossRef]
- Naga, N.G.; El-Badan, D.E.; Rateb, H.S.; Ghanem, K.M.; Shaaban, M.I. Quorum aensing inhibiting activity of Cefoperazone and its metallic derivatives on Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2021, 11, 716789–716803. [Google Scholar] [CrossRef]
- Soheili, V.; Bazzaz, B.S.F.; Abdollahpour, N.; Hadizadeh, F. Investigation of Pseudomonas aeruginosa quorum-sensing signaling system for identifying multiple inhibitors using molecular docking and structural analysis methodology. Microb. Pathog. 2015, 89, 73–78. [Google Scholar] [CrossRef]
- Chatterjee, M.; D’Morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biswas, R. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef]
- Welsh, M.A.; Eibergen, N.R.; Moore, J.D.; Blackwell, H.E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015, 137, 1510–1519. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.M.; Zhang, H.; Yu, H.; Dai, Q.; Xiong, J.Z.; Sheng, H.L.; Qiu, J.; Jiang, L.; Peng, J.; He, X.M.; et al. Allicin inhibits Pseudomonas aeruginosa virulence by suppressing the rhl and pqs quorum-sensing systems. Can. J. Microbiol. 2019, 65, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Dong, B.Y.; Wang, K.; Cai, S.Q.; Liu, T.J.; Cheng, X.J.; Lei, D.Q.; Chen, Y.L.; Li, Y.N.; Kong, J.L.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.Q.; Yang, D.; Zhao, L.; Shi, F.; Ye, G.; Fu, H.L.; Lin, J.C.; Guo, H.R.; He, R.; Li, J.L.; et al. EGCG-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 4946. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.Q.; Zhang, Y.Z.; Azi, F.; Zhou, J.Z.; Liu, X.L.; Dai, Y.Q.; Wang, Z.; Dong, M.S.; Xia, X.D. Inhibition of biofilm formation and quorum sensing by soy isoflavones in Pseudomonas aeruginosa. Food Control 2022, 133, 108629–108639. [Google Scholar] [CrossRef]
- Hassan, R.; Shaaban, M.I.; Bar, F.M.A.; El-Mahdy, A.M.; Shokralla, S. Quorum sensing inhibiting activity of Streptomyces coelicoflavus isolated from soil. Front. Microbiol. 2016, 7, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Cai, Z.-H.; Lao, Y.-M.; Jin, H.; Ying, K.-Z.; Lin, G.-H.; Zhou, J. Antibiofilm activity substances derived from coral symbiotic bacterial extract inhibit biofouling by the model strain Pseudomonas aeruginosa PAO1. Microb. Biotechnol. 2018, 11, 1090–1105. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Santos, M.M.S.D.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef]
- Li, W.-R.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Sun, T.-L.; Peng, H.; Huang, X.-M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 1, 3222–3232. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gong, Q.H.; Luo, C.Y.; Liang, Y.X.; Kong, X.Y.; Wu, C.L.; Feng, P.X.; Wang, Q.; Zhang, H.; Wireko, M.A. Synthesis and biological evaluation of novel L-homoserine lactone analogs as quorum sensing inhibitors of Pseudomonas aeruginosa. Chem. Pharm. Bull. 2019, 67, 1088–1098. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.; Mortensen, K.T.; Nørskov, A.; Qvortrup, K.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Itaconimides as novel quorum sensing inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2019, 8, 443–453. [Google Scholar]
- El-Mowafy, S.A.; Shaaban, M.I.; Abd El Galil, K.H. Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J. Appl. Microbial. 2014, 117, 1388–1399. [Google Scholar] [CrossRef]
- Fu, R.-Y.; Chu, Y.-W.; Li, J.; Zhao, K.-L.; Lin, J.-F.; Deng, J.-F.; Wang, X.-R. Inhibition activity of d-cycloserine on quorum-sensing system on Pseudomonas aeruginosa. Chin. J. Antibiot. 2021, 46, 545–561. [Google Scholar]
- Das, A.; Das, M.C.; Sandhu, P.; Das, N.; Tribedi, P.; De, U.C.; Akhter, Y.; Bhattacharjee, S. Antibiofilm activity of Parkia javanica against Pseudomonas aeruginosa: A study with fruit extract. RSC Adv. 2017, 7, 5497–5513. [Google Scholar] [CrossRef] [Green Version]
- Noumi, E.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; Martino, L.D.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for evaluating anti-quorum sensing activity of Melaleuca alternifolia essential oil and its main component terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cha, E.; Ham, S.-Y.; Park, J.-H.; Nam, S.J.; Kwon, H.M.; Byun, Y.J.; Park, H.-D. Linoleic acid inhibits Pseudomonas aeruginosa biofilm formation by activating diffusible signal factor-mediated quorum sensing. Biotechnol. Bioeng. 2021, 118, 82–93. [Google Scholar] [CrossRef]
- Amaya, S.; Pereira, J.A.; Borkosky, S.A.; Valdez, J.C.; Bardón, A.; Arena, M.E. Inhibition of quorum sensing in Pseudomonas aeruginosa by sesquiterpene lactones. Phytomedicine 2012, 19, 1173–1177. [Google Scholar] [CrossRef]
- Coelho, P.; Oliveira, J.; Fernandes, I.; Araújo, P.; Pereira, A.R.; Gameiro, P.; Bessa, L.J. Pyranoanthocyanins interfering with the quorum sensing of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 8559. [Google Scholar] [CrossRef]
- Shastry, R.P.; Kanekar, S.; Pandial, A.S.; Rekha, P.D. Isoeugenol suppresses multiple quorum sensing regulated phenotypes and biofilm formation of Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2022, 36, 1663–1667. [Google Scholar] [CrossRef]
- Singh, L.R.; Tripathi, V.C.; Raj, S.; Kumar, A.; Gupta, S.; Horam, S.; Upadhyay, A.; Kushwaha, P.; Arockiaraj, J.; Sashidhara, K.V.; et al. In-house chemical library repurposing: A case example for Pseudomonas aeruginosa antibiofilm activity and quorum sensing inhibition. Drug Dev. Res. 2018, 79, 383–390. [Google Scholar] [CrossRef]
- Jantaruk, P.; Pabuprapap, W.; Nakaew, A.; Kunthalert, D.; Suksamrarn, A. 4-methoxybenzalacetone, the cinnamic acid analog as a potential quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. World J. Microb. Biot. 2021, 37, 153–163. [Google Scholar] [CrossRef]
- Frei, R.; Breitbach, A.S.; Blackwell, H.E. 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew. Chem. Int. Ed. 2012, 51, 5226–5229. [Google Scholar] [CrossRef] [Green Version]
- Önem, E.; Tüzün, B.; Akkoç, S. Anti-quorum sensing activity in Pseudomonas aeruginosa PA01 of benzimidazolium salts: Electronic, spectral and structural investigations as theoretical approach. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Bhadbhade, M.M.; Manefield, M.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of novel acyclic and cyclic glyoxamide based derivatives as bacterial quorum sensing and biofilm inhibitors. Org. Biomol. Chem. 2017, 15, 5743–5755. [Google Scholar] [CrossRef]
- More, P.G.; Karale, N.N.; Lawand, A.S.; Rajmane, S.V.; Pawar, S.V.; Patil, R.H. A 4-(o-methoxyphenyl)-2-aminothiazole: An anti-quorum sensing compound. Med. Chem. Res. 2013, 22, 4183–4191. [Google Scholar] [CrossRef]
- Jiang, K.; Yan, X.L.; Yu, J.H.; Xiao, Z.J.; Wu, H.; Zhao, M.; Yue, Y.D.; Zhou, X.P.; Xiao, J.H.; Lin, F. Design, synthesis, and biological evaluation of 3-amino-2-oxazolidinone derivatives as potent quorum-sensing inhibitors of Pseudomonas aeruginosa PAO1. Eur. J. Med. Chem. 2020, 194, 112252–112264. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, K.L.; Li, H.Y.; Liu, K.H.; Li, J.; Chu, Y.W.; Prithiviraj, B.; Yue, B.; Zhang, X.Y. Antibacterial and anti-virulence efects of furazolidone on Trueperella pyogenes and Pseudomonas aeruginosa. BMC Vet. Res. 2022, 18, 114. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.M.; Jiang, K.; Wang, H.Y.; Lin, F. Inhibitory effects of novel 1,4-disubstituted 1,2,3-triazole compounds on quorum-sensing of P. aeruginosa PAO1. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 373–379. [Google Scholar] [CrossRef]
- Nogaret, P.; Garah, F.E.; Blanc-Potard, A.-B. A novel infection protocol in zebrafish embryo to assess Pseudomonas aeruginosa virulence and validate efficacy of a quorum sensing inhibitor in vivo. Pathogens 2021, 10, 401. [Google Scholar] [CrossRef]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Sabir, S.; Bhadbhade, M.; Black, D.S.; Kumar, N. Synthesis of alkyne-substituted dihydropyrrolones as bacterial quorum-sensing inhibitors of Pseudomonas aeruginosa. Antibiotics 2022, 11, 151. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajamuthu, T.P.; Sarkar, A.; Natrajan, P.; Krishnan, N.; Sakthivelu, M.; Velusamy, P.; Ramasamy, P.; Gopinath, S.C.B. N-acyl-homoserine lactone mediated virulence factor(s) of Pseudomonas aeruginosa inhibited by flavonoids and isoflavonoids. Process Biochem. 2022, 116, 84–93. [Google Scholar] [CrossRef]
- Askoura, M.; Saleh, M.; Abbas, H. An innovative role for tenoxicam as a quorum sensing inhibitor in Pseudomonas aeruginosa. Arch. Microbiol. 2020, 202, 555–565. [Google Scholar] [CrossRef]
- Gi, M.; Jeong, J.H.; Lee, K.; Lee, K.-M.; Toyofuku, M.; Yong, D.E.; Yoon, S.S.; Choi, J.Y. A drug repositioning screening identified pentetic acid as a potential therapeutic agent for suppressing the elastase-mediated virulence of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 7205–7214. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.M.; How, K.Y.; Yin, W.F.; Chan, K.G. The effects of Chinese herbal medicines on the quorum sensing-regulated virulence in Pseudomonas aeruginosa PAO1. Molecules 2018, 23, 972. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Rui, X.; Wang, L.; Guan, Y.; Sun, X.M.; Dong, M.S. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 2014, 42, 125–131. [Google Scholar] [CrossRef]
- Al-Haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Aniti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Rouamba, A.; Compaoré, M.; Sombié, P.A.E.D.; Kiendrebeogo, M. Acacia seyal Del Bark extract reduces quorum sensing-controlled virulence factor production and biofilm formation in Pseudomonas aeruginosa PAO1. Int. J. Curr. Res. Biosci. Plant Biol. 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Dazal, M.V.; Gonzales, E.; Sabino, A.K.; Salazar, R.G.; Samar, B.M.; San Juan, M.Y.; Castillo, A. Controlling biofilm formation by inhibiting the quorum-sensing zctivity of Pseudomonas aeruginosa using the ethanolic extracts of Piper nigrum (Piperaceae) Fruit, Punica granatum (Lythraceae) Pericarp, and Pisum sativum (Fabaceae) Seed. Int. J. Pharm. Sci. Drug Res. 2015, 7, 349–353. [Google Scholar]
- Sepahi, E.; Tarighi, S.; Ahmadi, F.S.; Bagheri, A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol. 2015, 53, 176–180. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit essential oils inhibit quorum sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2019, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- LewisOscar, F.; Nithya, C.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. Microfouling inhibition of human nosocomial pathogen Pseudomonas aeruginosa using marine cyanobacteria. Microb. Pathog. 2018, 14, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.; Rai, V.R. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J. Tradit. Complement. Med. 2018, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, A.D.; Petrović, J.D.; Glamočlija, J.M.; Smiljković, M.S.; Nikolić, M.M.; Stojković, D.S.; Soković, M.D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Inhibition of biofilm and quorum sensing-regulated virulence factors in Pseudomonas aeruginosa by Cuphea carthagenensis (Jacq.) J. F. Macbr. Leaf extract: An in vitro study. J. Ethnopharmacol. 2021, 269, 113699–113711. [Google Scholar] [CrossRef]
- MosaChristas, K.; Kowsalya, E.; Karthick, R.; Jaquline, C.R.I. Antibacterial, antibiofilm and anti-quorum sensing activities of Muntingia calabura L. leaf extract against Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2021, 13595. [Google Scholar] [CrossRef]
- Devi, R.S.; Ganesh, P.S.; Girija, A.S.S.; Priyadharshini, J.V. Attenuation of quorum sensing controlled virulence factors and biofilm formation by edible fruit extract of Coccinia indica against Pseudomonas aeruginosa. JPRI 2021, 33, 756–764. [Google Scholar] [CrossRef]
- Prateeksha; Bajpai, R.; Yusuf, M.A.; Upreti, D.K.; Gupta, V.K.; Singh, B.N. Endolichenic fungus, Aspergillus quandricinctus of Usnea longissima inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa PAO1. Microb. Pathog. 2020, 140, 103841–103933. [Google Scholar] [CrossRef]
- Eris, R.; Ulusoy, S. Rose, clove, chamomile essential oils and pine turpentine inhibit quorum sensing in Chromobacterium violaceum and Pseudomonas aeruginosa. J. Essent. Oil Bear. Plants 2014, 16, 126–135. [Google Scholar] [CrossRef]
- Gilabert, M.; Marcinkevicius, K.; Andujar, S.; Schiavone, M.; Arena, M.E.; Bardón, A. Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine 2015, 22, 77–85. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [Green Version]
- Oluwabusola, E.T.; Katermeran, N.P.; Poh, W.H.; Goh, T.M.B.; Tan, L.T.; Diyaolu, O.; Tabudravu, J.; Ebel, R.; Rice, S.A.; Jaspars, M. Inhibition of the quorum sensing system, elastase production and biofilm formation in Pseudomonas aeruginosa by Psammaplin A and Bisaprasin. Molecules 2022, 27, 1721. [Google Scholar] [CrossRef]
- Jack, A.A.; Khan, S.; Powell, L.C.; Pritcard, M.F.; Beck, K.; Sadh, H.; Sutton, L.; Cavaliere, A.; Florance, H.; Rye, P.D.; et al. Alginate oligosaccharide-induced modification of the lasI-lasR and rhlI-rhlR quorum sensing systems in Pseudomonas aeruginosa. AAC 2018, 62, 2318–2334. [Google Scholar]
- Danaraj, J.; Mariasingarayan, Y.; Ayyappan, S.; Karuppiah, V. Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb. Pathog. 2020, 147, 104392–104401. [Google Scholar]
- Chen, X.C.; Chen, J.W.; Yan, Y.C.; Chen, S.; Xu, X.W.; Zhang, H.W.; Wang, H. Quorum sensing inhibitors from marine bacteria Oceanobacillus sp. XC22919. Nat. Prod. Res. 2019, 33, 1819–1823. [Google Scholar]
- Reina, J.C.; Pérez-Victoria, I.; Martín, J.; Llamas, I. A quorum-sensing inhibitor strain of Vibrio alginolyticus Blocks qs-controlled phenotypes in Chromobacterium violaceum and Pseudomonas aeruginosa. Mar. Drugs 2019, 17, 494. [Google Scholar]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Mao, S.; Wang, H.; Ye, X. The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors. Mar. Drugs 2022, 20, 488. https://doi.org/10.3390/md20080488
Li Q, Mao S, Wang H, Ye X. The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors. Marine Drugs. 2022; 20(8):488. https://doi.org/10.3390/md20080488
Chicago/Turabian StyleLi, Qiaoqiang, Shen Mao, Hong Wang, and Xinyi Ye. 2022. "The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors" Marine Drugs 20, no. 8: 488. https://doi.org/10.3390/md20080488
APA StyleLi, Q., Mao, S., Wang, H., & Ye, X. (2022). The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors. Marine Drugs, 20(8), 488. https://doi.org/10.3390/md20080488