Description and Characterization of the Odontella aurita OAOSH22, a Marine Diatom Rich in Eicosapentaenoic Acid and Fucoxanthin, Isolated from Osan Harbor, Korea
Abstract
:1. Introduction
2. Results and Discussion
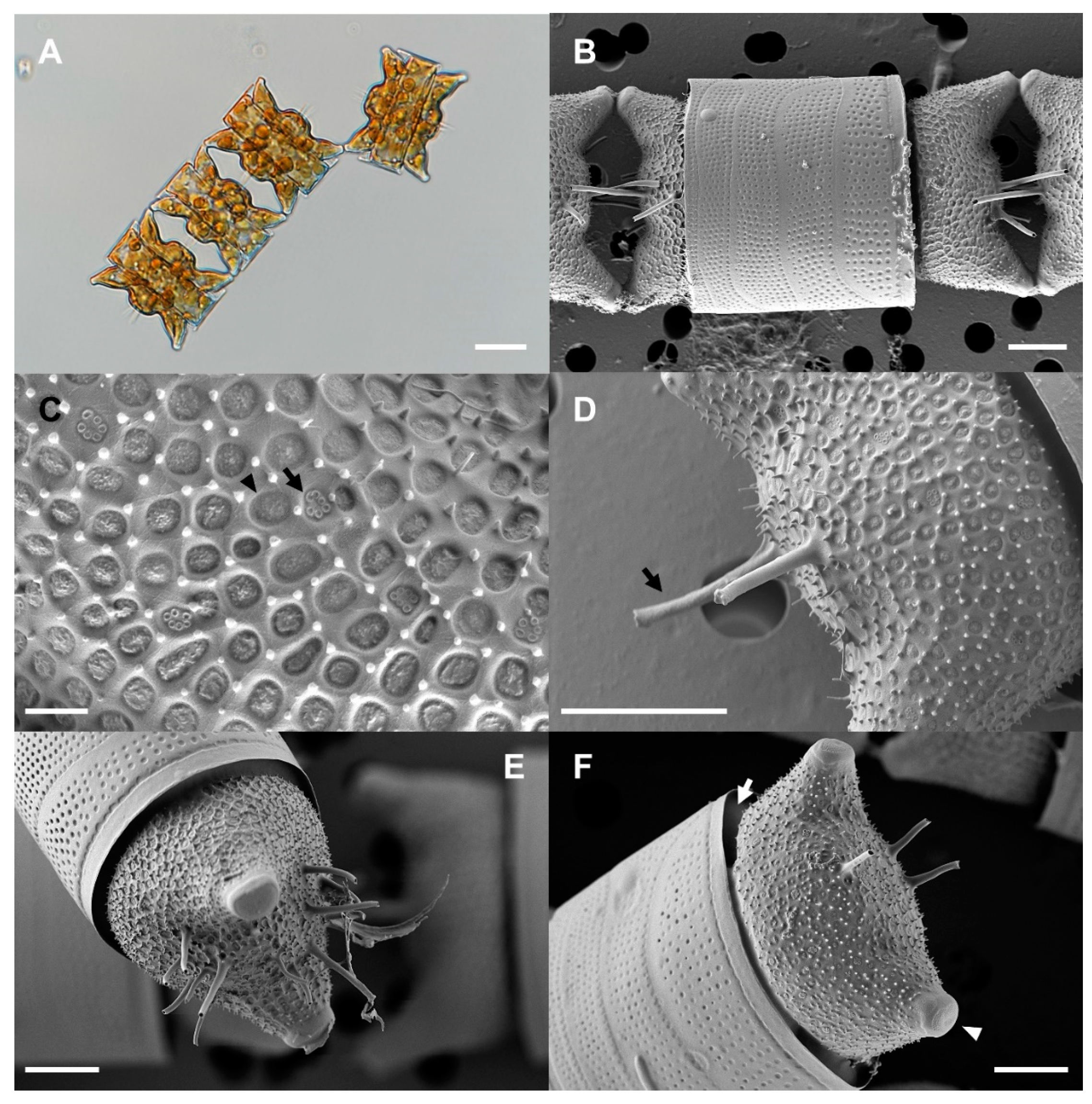
2.1. Morphological Identification of Strain OAOSH22
2.2. Molecular Identification of Strain OAOSH22
2.3. Optimization of Culture Conditions for Strain OAOSH22


2.4. Carotenoid Content of Strain OAOSH22
2.5. Fatty Acids Composition of Strain OAOSH22
3. Materials and Methods
3.1. Sample Collection and Isolation
3.2. Morphological Identification
3.3. Molecular Identification
3.4. Determination of Optimal Culture Conditions
3.5. Determination of Biomass
3.6. Carotenoids Analysis
3.7. Fatty Acid Analysis
3.8. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal pigments: A source of natural food colors. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 81–123. [Google Scholar]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of microalgae for biomaterial production and environmental applications at biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Chandra, R.; Iqbal, H.M.; Vishal, G.; Lee, H.S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Boyd, P.W.; Strzepek, R.; Fu, F.; Hutchins, D.A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 2010, 55, 1353–1376. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 133, E1516–E1525. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P.; Minas, H.J. The role of silicate pump in driving new production. Deep Sea Res. Part I 1995, 42, 697–719. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Wang, S.; Verma, S.K.; Hakeem Said, I.; Thomsen, L.; Ullrich, M.S.; Kuhnert, N. Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb. Cell Factories 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; He, Y.; Guo, B.; Sun, H.; Chen, F. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour. Technol. 2019, 294, 122145. [Google Scholar] [CrossRef]
- Sahin, M.S.; Khazi, M.I.; Demirel, Z.; Dalay, M.C. Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal. Agric. Biotechnol. 2019, 17, 390–398. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef] [PubMed]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, P.; Cai, Q.; Zhang, C.; Gao, B. Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor. Bioresour. Technol. 2022, 344, 126260. [Google Scholar] [CrossRef] [PubMed]
- Kraberg, A.; Baumann, M.; Dürselen, C.D. Coastal Phytoplankton: Photo guide for Northern European Seas; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2010; pp. 96–97. [Google Scholar]
- Lavigne, A.S.; Sunesen, I.; Sar, E.A. Morphological, taxonomic and nomenclatural analysis of species of Odontella, Trieres and Zygoceros (Triceratiaceae, Bacillariophyta) from Anegada Bay (Province of Buenos Aires, Argentina). Diatom Res. 2015, 30, 307–331. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosci. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef]
- Pike, I.H.; Jackson, A. Fish oil: Production and use now and in the future. Lipid. Technol. 2010, 22, 59–61. [Google Scholar] [CrossRef]
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; de Carvalho, J.C.M.; Danesi, E.D.G. Microalgae trends toward functional staple food incorporation: Sustainable alternative for human health improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 1–13. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R.; Takahashi, K. Fucoxanthin Production of Microalgae under Different Culture Factors: A Systematic Review. Mar. Drugs 2022, 20, 592. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Gheysen, L.; Kyomugasho, C.; Kermani, Z.J.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Terriente-Palacios, C.; Castellari, M. Levels of taurine, hypotaurine and homotaurine, and amino acids profiles in selected commercial seaweeds, microalgae, and algae-enriched food products. Food Chem. 2022, 368, 130770. [Google Scholar] [CrossRef] [PubMed]
- Avis de l’Agence Française de Sécurité Sanitaire des Aliments Relatif à la Demande D’évaluation de la Démonstration de L’équivalence en Substance D’une Microalgue Odontella aurita avec des Algues Autorisées (AFSSA Saisine no. 2001-SA-0082). Available online: https://www.anses.fr/fr/system/files/AAAT2001sa0082.pdf (accessed on 12 September 2023).
- Summary of Notifications Received by the Commission until 31 December 2004 Pursuant to Article 5 of Regulation (EC) No 258/97 of the European Parliament and of the Council (2005/C 208/2). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52005XC0825%2801%29&qid=1674696011584 (accessed on 12 September 2023).
- Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 12 September 2023).
- US Food and Drug Administration. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/cfc/XMLService.cfc?method=downloadxls&set=GRASNotices (accessed on 12 September 2023).
- Moreau, D.; Tomasoni, C.; Jacquot, C.; Kaas, R.; Le Guedes, R.; Cadoret, J.P.; Muller-Feuga, A.; Kontiza, I.; Viagas, C.; Roussis, V.; et al. Cultivated microalgae and carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ. Toxicol. Pharmacol. 2006, 22, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yao, R.; He, X.S.; Liao, Z.H.; Liu, Y.T.; Gao, B.Y.; Zhang, C.W.; Niu, J. Beneficial contribution of the microalga Odontella aurita to the growth, immune response, antioxidant capacity, and hepatic health of juvenile golden pompano (Trachinotus ovatus). Aquaculture 2022, 555, 738206. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 1–18. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Rumin, J.; Gonçalves de Oliveira Junior, R.; Bérard, J.B.; Picot, L. Improving microalgae research and marketing in the European Atlantic area: Analysis of major gaps and barriers limiting sector development. Mar. Drugs 2021, 19, 319. [Google Scholar] [CrossRef]
- Sims, P.A.; Williams, D.M.; Ashworth, M. Examination of type specimens for the genera Odontella and Zygoceros (Bacillariophyceae) with evidence for the new family Odontellaceae and a description of three new genera. Phytotaxa 2018, 382, 1–56. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a novel food. Potential and legal framework. Ernahr. Umsch 2021, 68, 78–85. [Google Scholar]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as food in Europe: An overview of species diversity and their application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Moreno, C.M.; Lin, Y.; Davies, S.; Monbureau, E.; Cassar, N.; Marchetti, A. Examination of gene repertoires and physiological responses to iron and light limitation in Southern Ocean diatoms. Polar Biol. 2018, 41, 679–696. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Bashir, F.; Rehman, A.U.; Szabó, M.; Vass, I. Singlet oxygen damages the function of Photosystem II in isolated thylakoids and in the green alga Chlorella sorokiniana. Photosynth. Res. 2021, 149, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of unicellular algae to irradiance. an analysis of strategies. N. Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994; p. 509. [Google Scholar]
- Masojídek, J.; Koblížek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 20–39. [Google Scholar]
- Roleda, M.Y.; Slocombe, S.P.; Leakey, R.J.; Day, J.G.; Bell, E.M.; Stanley, M.S. Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour. Technol. 2013, 129, 439–449. [Google Scholar] [CrossRef]
- Platt, T.G.C.L.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Elbrächter, M.; Drebes, G. Marine Phytoplankton Selected Microphytoplankton Species from the North Sea around Helgoland and Sylt; Schweizerbart Sche Vlgsb.: Stuttgart, Germany, 2009; p. 264. [Google Scholar]
- Baars, J.W.M. Autecological investigations on marine diatoms. 4: Biddulphia aurita (Lyngb.) Brebisson et Godey—A succession of spring diatoms. Hydrobiol. Bull. 1985, 19, 109–116. [Google Scholar] [CrossRef]
- Martens, P. Effects of the severe winter 1995/96 on the biological oceanography of the Sylt-Rømø tidal basin. Helgol. Mar. Res. 2001, 55, 166–169. [Google Scholar] [CrossRef]
- Pasquet, V.; Ulmann, L.; Mimouni, V.; Guihéneuf, F.; Jacquette, B.; Morant-Manceau, A.; Tremblin, G. Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J. Appl. Phycol. 2014, 26, 2265–2271. [Google Scholar] [CrossRef]
- Peng, X.; Meng, F.; Wang, Y.; Yi, X.; Cui, H. Effect of pH, temperature, and CO2 concentration on growth and lipid accumulation of Nannochloropsis sp. MASCC 11. J. Ocean Univ. China 2020, 19, 1183–1192. [Google Scholar] [CrossRef]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. Effect of light intensity, pH, and temperature on triacylglycerol (TAG) accumulation induced by nitrogen starvation in Scenedesmus obliquus. Bioresour. Technol. 2013, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McQuoid, M.R. Influence of salinity on seasonal germination of resting stages and composition of microplankton on the Swedish west coast. Mar. Ecol. Prog. Ser. 2005, 289, 151–163. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H.; Karuna, M.S.L. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 13437–13451. [Google Scholar] [CrossRef]
- Haris, N.; Manan, H.; Jusoh, M.; Khatoon, H.; Katayama, T.; Kasan, N.A. Effect of different salinity on the growth performance and proximate composition of isolated indigenous microalgae species. Aquac. Rep. 2022, 22, 100925. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Takahashi, S.; Miyairi, S.; Suzuki, I.; Murata, N. Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol. 2005, 137, 263–273. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined Production of Fucoxanthin and EPA from Two Diatom Strains Phaeodactylum tricornutum and Cylindrotheca fusiformis Cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2019, 46, 153–158. [Google Scholar] [CrossRef]
- Xia, S.; Wan, L.; Li, A.; Sang, M.; Zhang, C. Effects of nutrients and light intensity on the growth and biochemical composition of a marine microalga Odontella aurita. Chin. J. Oceanol. Limnol. 2013, 31, 1163–1173. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Huysman, M.J.; Vyverman, W.; De Veylder, L. Molecular regulation of the diatom cell cycle. J. Exp. Bot. 2014, 65, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of nutraceutical fatty acids by the oleaginous marine microalgae Phaeodactylum tricornutum utilizing hydrolysates from organosolv-pretreated birch and spruce biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Traller, J.C.; Abbriano, R. The place of diatoms in the biofuels industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, W.; Xie, X. Effects of nitrogen, phosphorus, iron and silicon on growth of five species of marine benthic diatoms. Acta Ecol. Sin. 2014, 34, 311–319. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A Novel Strategy for Isolation and Purification of Fucoxanthinol and Fucoxanthin from the Diatom Nitzschia laevis. Food Chem. 2019, 277, 566–572. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.W.; Chen, F.; Liu, B. A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Kosakowska, A.; Lewandowska, J.; Stoń, J.; Burkiewicz, K. Qualitative and quantitative composition of pigments in Phaeodactylum tricornutum (Bacillariophyceae) stressed by iron. BioMetals 2004, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wilken, S.; Hoffmann, B.; Hersch, N.; Kirchgessner, N.; Dieluweit, S.; Rubner, W.; Hoffmann, L.J.; Merkel, R.; Peeken, I. Diatom frustules show increased mechanical strength and altered valve morphology under iron limitation. Limnol. Oceanogr. 2011, 56, 1399–1410. [Google Scholar] [CrossRef]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.Y.; Varjani, S.; Chang, J.S. Producing fucoxanthin from algae–Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2022, 344, 126170. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]
- Maeda, H.; Fukuda, S.; Izumi, H.; Saga, N. Anti-oxidant and fucoxanthin contents of brown alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Mar. Drugs 2018, 16, 255. [Google Scholar] [CrossRef]
- Mal, N.; Srivastava, K.; Sharma, Y.; Singh, M.; Rao, K.M.; Enamala, M.K.; Chandrasekhar, K.; Chavali, M. Facets of diatom biology and their potential applications. Biomass Convers. Biorefin. 2022, 12, 1959–1975. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What Do We Know about Antimicrobial Activity of Astaxanthin and Fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Marella, T.K.; López-Pacheco, I.Y.; Parra-Saldívar, R.; Dixit, S.; Tiwari, A. Wealth from waste: Diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Sci. Total Environ. 2020, 724, 137960. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar]
- Gao, F.; Teles, I.; Wijffels, R.H.; Barbosa, M.J. Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour. Technol. 2020, 315, 123894. [Google Scholar] [CrossRef] [PubMed]
- Tokushima, H.; Inoue-Kashino, N.; Nakazato, Y.; Masuda, A.; Ifuku, K.; Kashino, Y. Advantageous characteristics of the diatom Chaetoceros gracilis as a sustainable biofuel producer. Biotechnol. Biofuels 2016, 9, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X. Storage Carbon Metabolism of Isochrysis zhangjiangensis under Different Light Intensities and Its Application for Co-Production of Fucoxanthin and Stearidonic Acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Lavaud, J.; Rousseau, B.; van Gorkom, H.J.; Etienne, A.L. Influence of the Diadinoxanthin Pool Size on Photoprotection in the Marine Planktonic Diatom Phaeodactylum tricornutum. Plant Physiol. 2002, 129, 1398–1406. [Google Scholar] [CrossRef]
- Artamonova, E.Y.; Svenning, J.B.; Vasskog, T.; Hansen, E.; Eilertsen, H.C. Analysis of phospholipids and neutral lipids in three common northern cold water diatoms: Coscinodiscus concinnus, Porosira glacialis, and Chaetoceros socialis by ultra-high performance liquid chromatography-mass spectrometry. J. Appl. Phycol. 2017, 29, 1241e1249. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: The disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638. [Google Scholar] [CrossRef]
- Roessler, P.G. Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J. Phycol. 1988, 24, 394–400. [Google Scholar] [CrossRef]
- Barone, G.D.; Cernava, T.; Ullmann, J.; Liu, J.; Lio, E.; Germann, A.T.; Nakielski, A.; Russo, D.A.; Chavkin, T.; Knufmann, K.; et al. Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 2023, 9, e14708. [Google Scholar] [CrossRef] [PubMed]
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal. Agric. Biotechnol. 2018, 16, 320–325. [Google Scholar] [CrossRef]
- Pushpabharathi, N.; Jayalakshmi, M.; Amudha, P.; Vanitha, V. Identification of bioactive compounds in Cymodocea serrulata-a seagrass by gas chromatography–mass spectroscopy. Asian J. Pharm. Clin. Res. 2018, 11, 317–320. [Google Scholar]
- Tovar, R.; Gavito, A.L.; Vargas, A.; Soverchia, L.; Hernandez-Folgado, L.; Jagerovic, N.; Baixeras, E.; Ciccocioppo, R.; de Fonseca, F.R.; Decara, J. Palmitoleoylethanolamide is an efficient anti-obesity endogenous compound: Comparison with oleylethanolamide in diet-induced obesity. Nutrients 2021, 13, 2589. [Google Scholar] [CrossRef]
- Lynch, E.D.; Lee, M.K.; Morrow, J.E.; Welcsh, P.L.; LeoÂn, P.E.; King, M.C. Nonsyndromic Deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science 1997, 278, 1315–1318. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Raja, R.; Iswarya, S.H.; Balasubramanyam, D.; Rengasamy, R. PCR-identification of Dunaliella salina (Volvocales, Chlorophyta) and its growth characteristics. Microbiol. Res. 2007, 162, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Noh, J.H.; Kim, J.H.; Kang, N.S. Ultrastructural and Molecular Characterization of Surirella atomus Hustedt 1955 (Bacillariophyta, Surirellalceae), A Newly Recorded Species in Korea. Ocean Polar Res. 2021, 43, 245–253. [Google Scholar]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Bhola, V.; Desikan, R.; Santosh, S.K.; Subburamu, K.; Sanniyasi, E.; Bux, F. Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J. Biosci. Bioeng. 2011, 111, 377–382. [Google Scholar] [CrossRef]
- Barceló Villalobos, M. Optimización de la Producción de Microalgas en Reactores Abiertos de Escala Industrial. Ph.D. Thesis, University of Almería, Almería, Spain, 2020. [Google Scholar]
- Consalvey, M.; Perkins, R.G.; Paterson, D.M.; Underwood, G.J. PAM fluorescence: A beginners guide for benthic diatomists. Diatom Res. 2005, 20, 1–22. [Google Scholar] [CrossRef]
- Malapascua, J.R.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef]
- Kang, N.S.; Cho, K.; An, S.M.; Kim, E.S.; Ki, H.; Lee, C.H.; Choi, G.; Hong, J.W. Taxonomic and Biochemical Characterization of Microalga Graesiella emersonii GEGS21 for Its Potential to Become Feedstock for Biofuels and Bioproducts. Energies 2022, 15, 8725. [Google Scholar] [CrossRef]
- Garces, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | Amount (mg g−1 DCW) | Composition (%) | ||
|---|---|---|---|---|
| SFA | Myristic acid | C14:0 | 9.65 ± 0.23 | 15.61 ± 1.20 |
| Palmitic acid | C16:0 | 15.96 ± 0.27 | 25.76 ± 0.95 | |
| Stearic acid | C18:0 | 0.68 ± 0.02 | 1.10 ± 0.09 | |
| MUFA | Palmitoleic acid | C16:1n7 | 22.52 ± 0.36 | 36.34 ± 1.36 |
| Oleic acid | C18:1n9 | 0.93 ± 0.02 | 1.50 ± 0.05 | |
| PUFA | Linoleic acid | C18:2n6 cis | 0.75 ± 0.03 | 1.21 ± 0.01 |
| Gamma-linolenic acid (GLA) | C18:3n6 | 0.30 ± 0.03 | 0.48 ± 0.03 | |
| Arachidonic acid (AA) | C20:4n6 | 0.22 ± 0.06 | 0.33 ± 0.09 | |
| Eicosapentaenoic acid (EPA) | C20:5n3 | 11.07 ± 2.63 | 17.66 ± 3.28 | |
| Experimental Conditions | |
|---|---|
| Temperature (°C) | 5, 10, 15, 20, 25 |
| Salinity (psu) | 24, 27, 30, 33, 36 |
| Nutrients (mg L−1) | |
| Nitrate (NaNO3) | 75, 150, 300, 600 |
| Silicate (Na2SiO3•9H2O) | 15, 30, 60, 120 |
| Phosphate (NaH2PO4•H2O) | 5, 10, 20, 40 |
| Iron (FeCl3•6H2O) | 3.15, 6.3, 12.6, 25.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.M.; Cho, K.; Kim, E.S.; Ki, H.; Choi, G.; Kang, N.S. Description and Characterization of the Odontella aurita OAOSH22, a Marine Diatom Rich in Eicosapentaenoic Acid and Fucoxanthin, Isolated from Osan Harbor, Korea. Mar. Drugs 2023, 21, 563. https://doi.org/10.3390/md21110563
An SM, Cho K, Kim ES, Ki H, Choi G, Kang NS. Description and Characterization of the Odontella aurita OAOSH22, a Marine Diatom Rich in Eicosapentaenoic Acid and Fucoxanthin, Isolated from Osan Harbor, Korea. Marine Drugs. 2023; 21(11):563. https://doi.org/10.3390/md21110563
Chicago/Turabian StyleAn, Sung Min, Kichul Cho, Eun Song Kim, Hyunji Ki, Grace Choi, and Nam Seon Kang. 2023. "Description and Characterization of the Odontella aurita OAOSH22, a Marine Diatom Rich in Eicosapentaenoic Acid and Fucoxanthin, Isolated from Osan Harbor, Korea" Marine Drugs 21, no. 11: 563. https://doi.org/10.3390/md21110563







