Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Protein Hydrolysate of Antarctic Krill
2.1.1. Screening of Protease Species
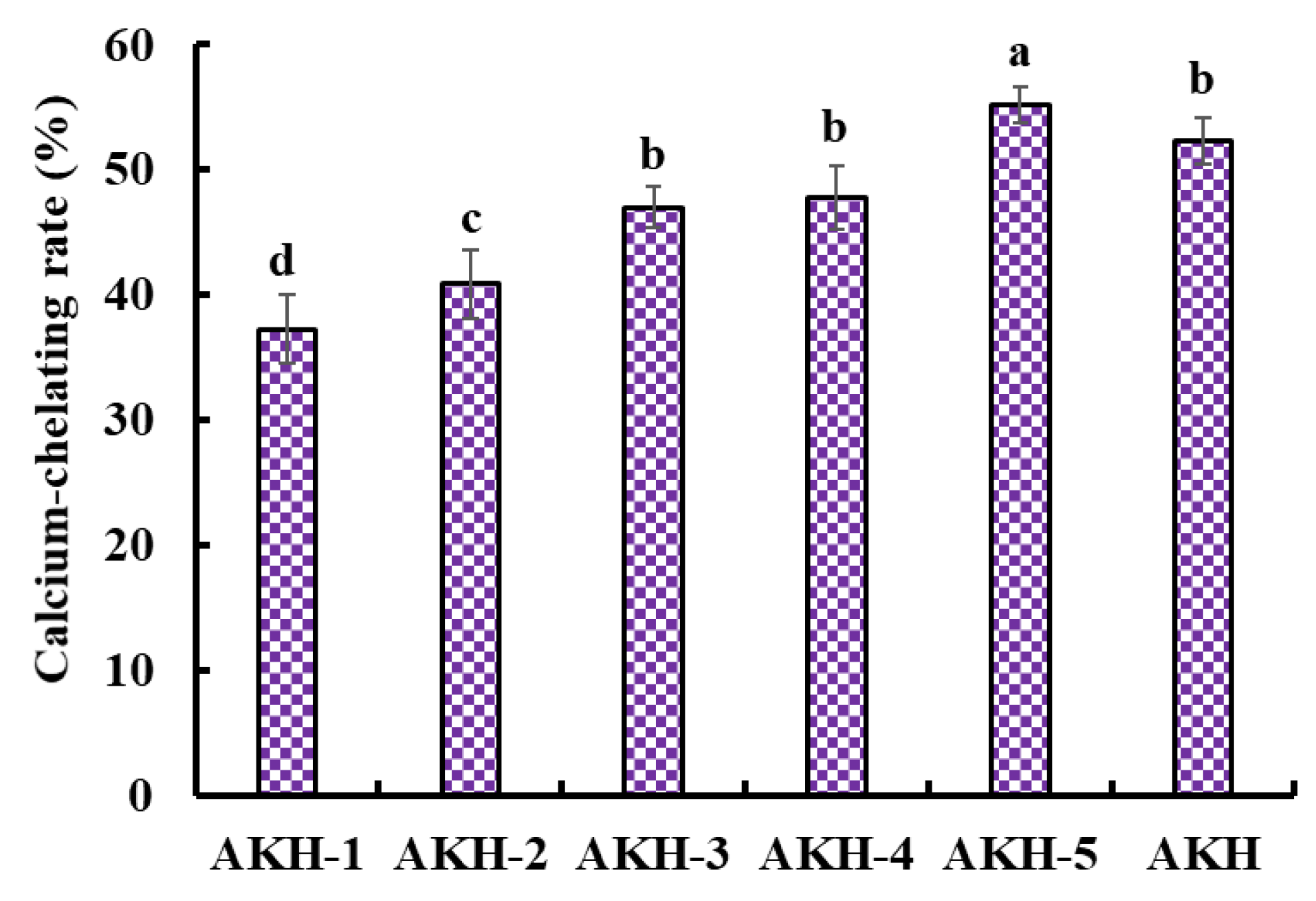
2.1.2. Optimized the Chelating Conditions of Ca with AKH
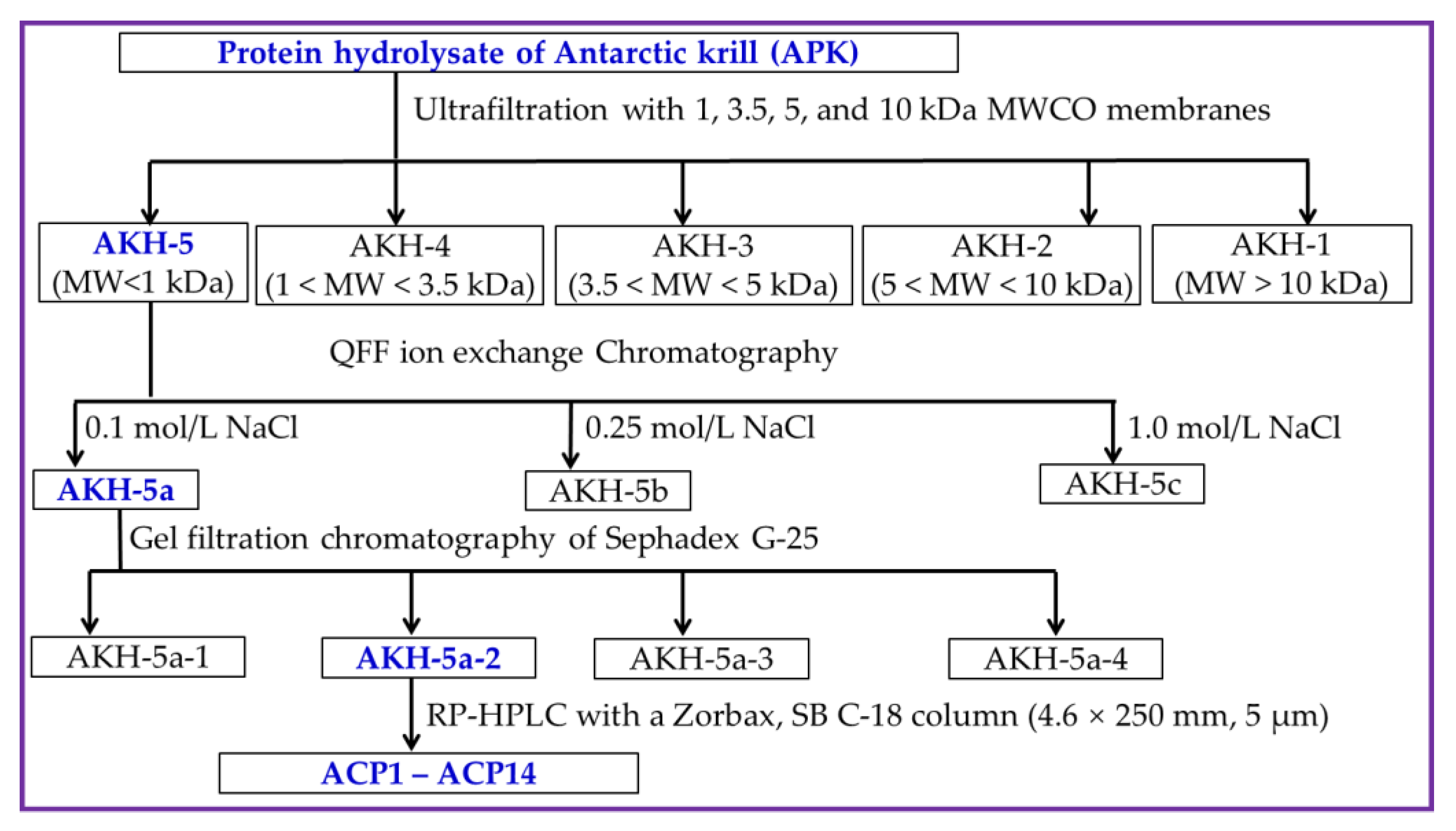
2.2. Preparation of Ca-Chelating Peptides from AKH
2.2.1. Ultrafiltration
2.2.2. Anion-Exchange Chromatography of AKH-5
2.2.3. Gel Permeation Chromatography (GPC) of AKH-5a
2.2.4. RP-HPLC Purification of AKH-5a-2
2.3. Determination of Sequences and MWs of Ca-Chelating Peptides (ACP1–ACP14)
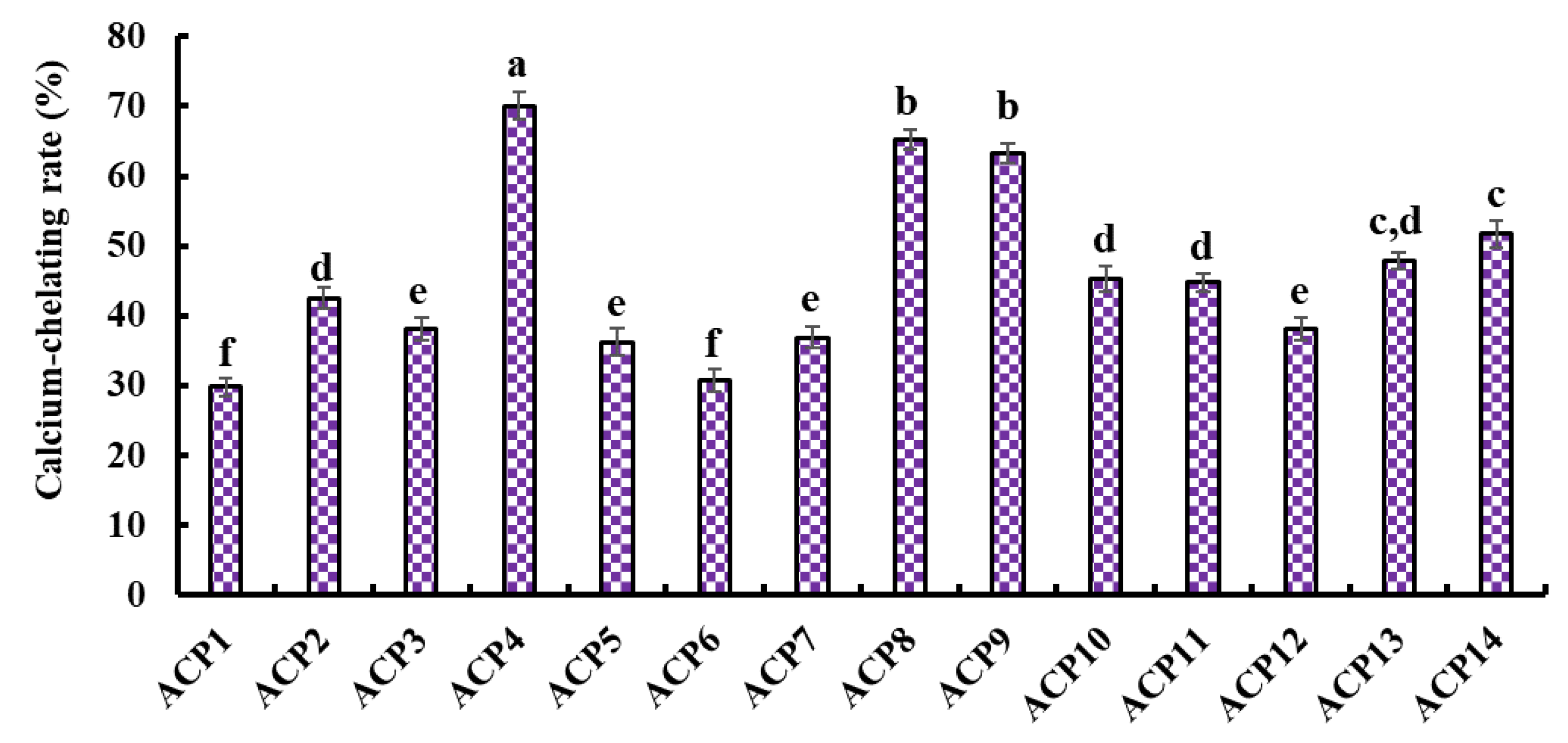
2.4. Ca-Chelating Ability of Fourteen Isolated Peptides (ACP1–ACP14)
2.5. Characterization of VERG-Ca Chelate
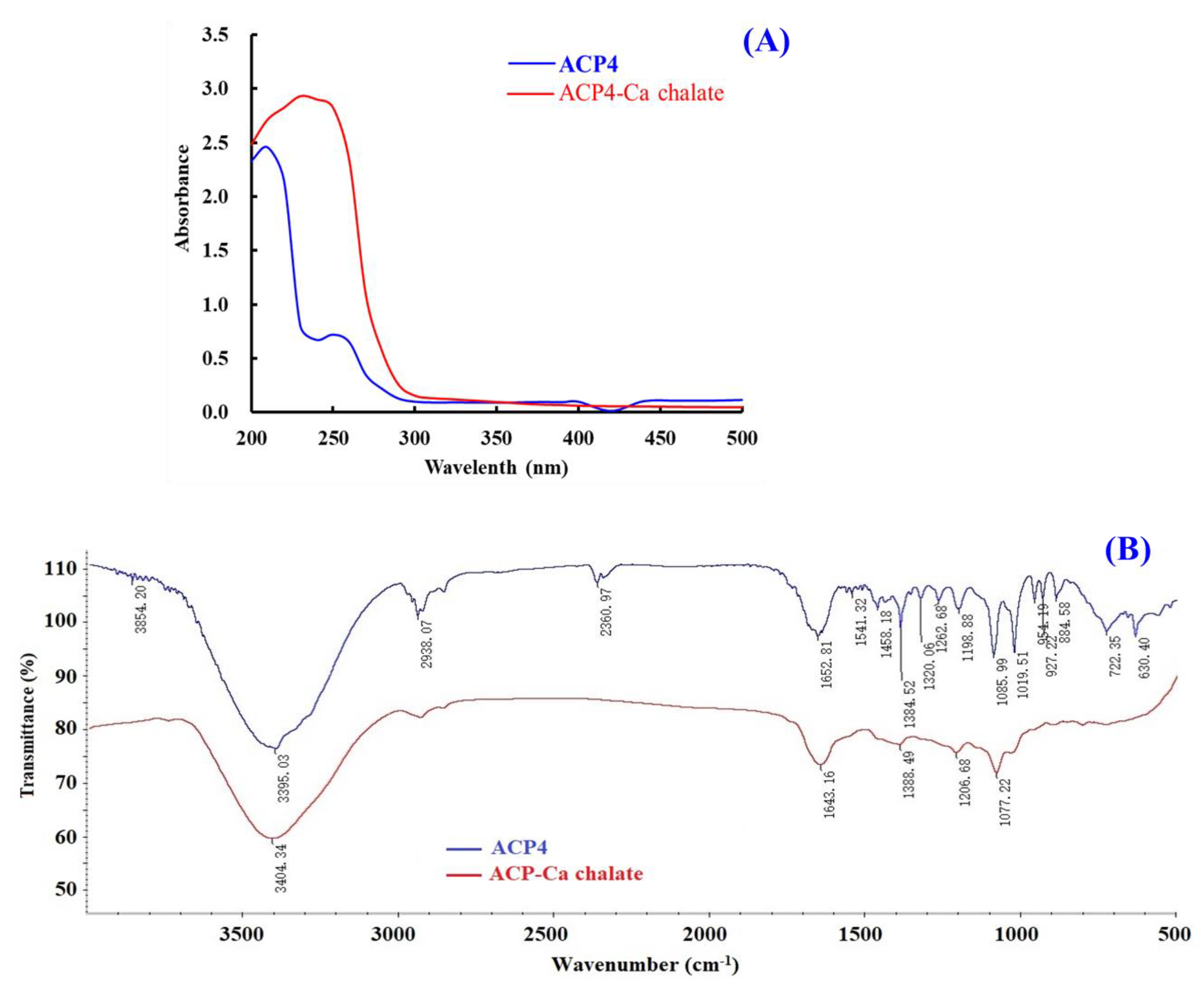
2.5.1. UV Absorption Spectroscopy
2.5.2. FTIR Spectroscopy
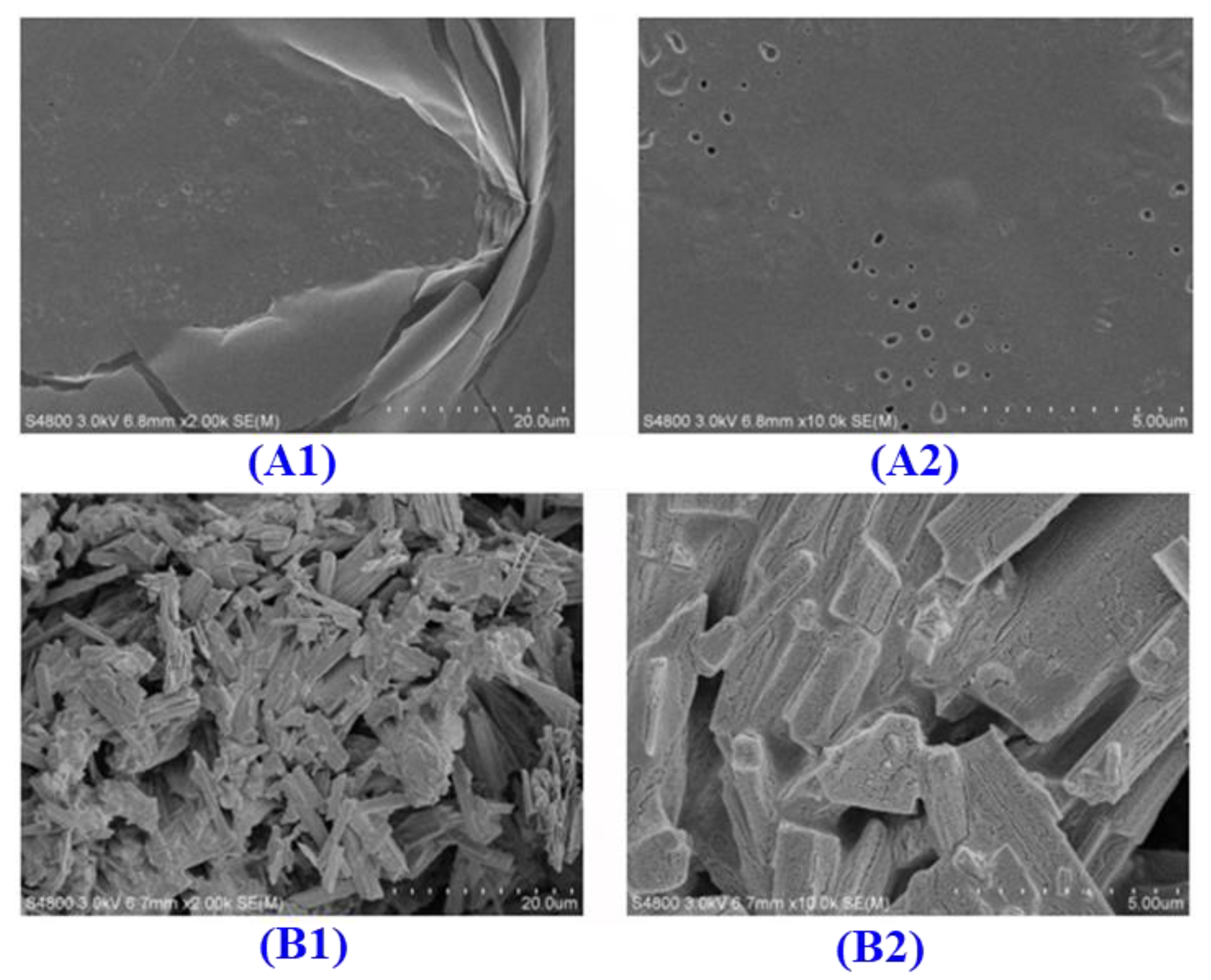
2.5.3. Scanning Electron Microscope (SEM)
2.6. Stability Analysis of VERG-Ca Chelate
2.7. Transport of VERG-Ca Chelate across the Caco-2 Cell Monolayer
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Protein Hydrolysate of Antarctic Krill
3.2.1. Screening of Protease Species
3.2.2. Optimization of the Chelating Conditions of Ca with AKH
3.2.3. Preparation of Peptide-Ca Chelates and Determination of Ca-Chelating Rate
3.2.4. Separation Process of Ca-Chelating Peptides from AKH
3.3. Identification of Peptides (ACP1to ACP14) from AKH
3.4. Characterization of VERG-Ca Chelate
3.4.1. UV Absorption Spectroscopy Analysis
3.4.2. FTIR Spectroscopy Analysis
3.4.3. Scanning Electron Microscopy (SEM) Analysis
3.5. Stability Analysis of VERG-Ca Chelate against Simulated Gastrointestinal Digestion
3.6. Ca Transport Effect of VERG-Ca Chelate in Caco-2 Cell Monolayers
3.6.1. Culture of Caco-2 Cells and Establishment of Caco-2 Cell Monolayer Model
3.6.2. Cytotoxicity Assay
3.6.3. Ca Transport Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Q.J.; Fan, Y.; Hao, L.; Wang, J.; Xia, C.S.; Hou, H. A comprehensive review of calcium and ferrous ions chelating peptides: Preparation, structure and transport pathways. Crit. Rev. Food Sci. Nutr. 2023, 63, 4418–4430. [Google Scholar] [CrossRef]
- Luo, J.Q.; Zhou, Z.S.; Yao, X.T.; Fu, Y. Mineral-chelating peptides derived from fish collagen: Preparation, bioactivity and bioavailability. LWT 2020, 134, 110209. [Google Scholar] [CrossRef]
- Qu, W.J.; Li, Y.H.; Xiong, T.; Feng, Y.H.; Ma, H.L.; Akpabli-Tsigbe, N.D.K. Calcium-chelating improved zein peptide stability, cellular uptake, and bioactivity by influencing the structural characterization. Food Res. Int. 2022, 162, 112033. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, A.; Chen, Y.; Zhang, X.Y.; Li, S.H.; Chen, Y. Preparation of cucumber seed peptide-calcium chelate by liquid state fermentation and its characterization. Food Chem. 2017, 229, 487–494. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhao, L.Y.; Shen, Q.S.; Qi, L.W.; Jiang, S.; Richel, A. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability. LWT 2021, 144, 111264. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.J.; Wang, Z.P.; Pei, X.; Tao, W.J.; Ao, T.Y. Comparison of inorganic and organically bound trace minerals on tissue mineral deposition and fecal excretion in broiler breeders. Biol. Trace Elem. Res. 2019, 189, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegria, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2020, 61, 1470–1489. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhao, W.; Chen, X.; Li, J.; Zhang, L.; Jin, H. Ameliorative Effects of Peptide Phe-Leu-Ala-Pro on Acute Liver and Kidney Injury Caused by CCl4 via Attenuation of Oxidative Stress and Inflammation. ACS Omega 2022, 7, 44796–44803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Zhao, Y.Q.; Wang, Y.M.; Yang, X.R.; Chi, C.F.; Wang, B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022, 50, 102138. [Google Scholar] [CrossRef]
- López-Medina, J.A.; López-Rodriguez, C.; Estornell-Gualde, M.A.; Rey-Fernández, L.; Gómez-Senent, S.; Ballesteros-Pomar, M.D. Relationship between nutritional treatment compliance and nutritional status improvements in patients with gastrointestinal impairment taking an oral peptide-based supplement. Nutrition 2022, 102, 111734. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F. Bioactive food-derived peptides for functional nutrition: Effect of fortification, processing and storage on peptide stability and bioactivity within food matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Lin, S.; Zhao, W.; Chen, Y.; Jin, H. Collagen peptides from Acaudina molpadioides prevent CCl4 -induced liver injury via Keap1/Nrf2-ARE, PI3K/AKT, and MAPKs pathways. J. Food Sci. 2022, 87, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Wang, Y.Z.; Zhao, Y.Q.; Chi, C.F.; Zhu, W.Y.; Wang, B. High Fischer ratio oligopeptides from hard-shelled mussel: Preparation and hepatoprotective effect against acetaminophen-induced liver injury in mice. Food Biosci. 2023, 53, 102638. [Google Scholar] [CrossRef]
- Wu, M.-F.; Xi, Q.-H.; Sheng, Y.; Wang, Y.-M.; Wang, W.-Y.; Chi, C.-F.; Wang, B. Antioxidant peptides from monkfish swim bladders: Ameliorating NAFLD in vitro by suppressing lipid accumulation and oxidative stress via regulating AMPK/Nrf2 pathway. Mar. Drugs 2023, 21, 360. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, F.; Yao, S.; Bi, L.; Jiang, G.; Huang, J.; Tang, Y. Effects of low molecular weight peptides from monkfish (Lophius litulon) roe on immune response in immunosuppressed mice. Front. Nutr. 2022, 9, 929105. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Hu, X.-M.; Cai, W.-W.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef]
- Wu, X.P.; Wang, F.F.; Cai, X.X.; Wang, S.Y. Characteristics and osteogenic mechanism of glycosylated peptides-calcium chelate. Curr. Res. Food Sci. 2022, 5, 1965–1975. [Google Scholar] [CrossRef]
- Miao, J.Y.; Liao, W.W.; Pan, Z.Y.; Wang, Q.; Duan, S.; Cao, Y. Isolation and identification of iron-chelating peptides from casein hydrolysates. Food Funct. 2019, 10, 2372–2381. [Google Scholar] [CrossRef]
- Budseekoad, S.; Yupanqui, C.T.; Sirinupong, N.; Alashi, A.M.; Aluko, R.E.; Youravong, W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods 2018, 49, 333–341. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.W.; Hou, H.; Zhang, H.W.; Li, B.F. Purification and characterization of a novel calcium-biding decapeptide from Pacific cod (Gadus Macrocephalus) bone: Molecular properties and calcium chelating modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Ke, H.L.; Ma, R.J.; Liu, X.U.; Xie, Y.P.; Chen, J.F. Highly effective peptide-calcium chelate prepared from aquatic products processing wastes: Stickwater and oyster shells. LWT 2022, 168, 113947. [Google Scholar] [CrossRef]
- El Hajj, S.; Irankunda, R.; Camaño Echavarría, J.A.; Arnoux, P.; Paris, C.; Canabady-Rochelle, L. Metal-chelating activity of soy and pea protein hydrolysates obtained after different enzymatic treatments from protein isolates. Food Chem. 2023, 405 Pt A, 134788. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, Y.Q.; Li, X.R.; Wang, B. High Fischer ratio oligopeptides determination from Antartic krill: Preparation, peptides profiles, and in vitro antioxidant activity. J. Food Biochem. 2019, 43, e12827. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, Y.Q.; Cai, W.Z.; Bai, X.L.; Dong, P.; Wang, J.F. Antarctic krill peptide alleviates liver fibrosis via downregulating the secondary bile acid mediated NLRP3 signaling pathway. Food Funct. 2022, 13, 7740–7749. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, C.Y.; Jiang, P.F.; Qi, L.B.; Sun, N.K.; Lin, S.Y. Antarctic krill antioxidant peptides show inferior IgE-binding ability and RBL-2H3 cell degranulation. Food Sci. Hum. Well. 2023, 12, 1772–1778. [Google Scholar] [CrossRef]
- Yao, M.K.; Gai, X.L.; Zhang, M.S.; Liu, X.; Cui, T.T.; Jia, A.R. Two proteins prepared from defatted Antarctic krill (Euphausia superba) powder: Composition, structure and functional properties. Food Hydrocoll. 2023, 145, 109009. [Google Scholar] [CrossRef]
- Han, L.H.; Mao, X.Z.; Wang, K.; Li, Y.Y.; Zhao, M.H.; Xue, C.H. Phosphorylated peptides from Antarctic krill (Euphausia superba) ameliorated osteoporosis by activation of osteogenesis-related MAPKs and PI3K/AKT/GSK-3β pathways in dexamethasone-treated mice. J. Funct. Foods 2018, 47, 447–456. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Park, S.Y.; Han, E.J.; Kim, H.S.; Kang, D.S.; Ahn, G. Isolation of an antioxidant peptide from krill protein hydrolysates as a novel agent with potential hepatoprotective effects. J. Funct. Foods 2020, 67, 103889. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhao, Y.Q.; Wang, Y.M.; Zhao, W.H.; Wang, P.; Wang, B. Antioxidant peptides from Antarctic krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Yue, H.; Cai, W.; Yin, H.; Tian, Y.; Dong, P.; Wang, J. Peptides from Antarctic krill (Euphausia superba) ameliorate acute liver injury in mice induced by carbon tetrachloride via activating the Nrf2/HO-1 pathway. Food Funct. 2023, 14, 3526–3537. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.K.; Zhu, X.; Li, Q.K.; Fan, Y.; Cheng, D.; Li, B.F. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Hu, S.; Lin, S.; Liu, Y.; He, X.; Zhang, S.; Sun, N. Exploration of iron-binding mode, digestion Kinetics, and iron absorption behavior of Antarctic Krill-derived heptapeptide-iron complex. Food Res. Int. 2022, 154, 110996. [Google Scholar] [CrossRef]
- Zheng, J.R.; Gao, Y.H.; Ding, J.; Sun, N.; Lin, S.Y. Antarctic krill peptides improve scopolamine-induced memory impairment in mice. Food Biosci. 2022, 49, 101987. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.H.; Ji, H.W. Two novel bioactive peptides from Antarctic krill with dual angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Luo, Q.B.; Suo, S.K.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Preparation, characterization, and cytoprotective effects on HUVECs of fourteen novel angiotensin-I-converting enzyme inhibitory peptides from protein hydrolysate of tuna processing by-products. Front. Nutr. 2022, 9, 868681. [Google Scholar] [CrossRef]
- Beaubier, S.; Durand, E.; Lenclume, C.; Fine, F.; Aymes, A.; Framboisier, X.; Kapel, R.; Villeneuve, P. Chelating peptides from rapeseed meal protein hydrolysates: Identification and evaluation of their capacity to inhibit lipid oxidation. Food Chem. 2023, 422, 136187. [Google Scholar] [CrossRef]
- Hou, T.; Liu, Y.S.; Guo, D.J.; Li, B.; He, H. Collagen peptides from crucian skin improve calcium bioavailability and structural characterization by HPLC-ESI-MS/MS. J. Agric. Food Chem. 2017, 65, 8847–8854. [Google Scholar] [CrossRef]
- Fan, C.Z.; Ge, X.F.; Hao, J.Y.; Wu, T.; Liu, R.; Zhang, M. Identification of high iron-chelating peptides with unusual antioxidant effect from sea cucumbers and the possible binding mode. Food Chem. 2023, 399, 133912. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel antioxidant collagen peptides of Siberian sturgeon (Acipenserbaerii) cartilages: The preparation, characterization, and cytoprotection of H2O2-damaged human umbilical vein endothelial cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-D.; Xi, Q.-H.; Kong, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from the collagens of monkfish (Lophius litulon) swim bladders: Isolation, characterization, molecular docking analysis and activity evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef]
- Huang, G.R.; Ren, L.; Jiang, J.X. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur. Food Res. Technol. 2011, 232, 281–287. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Reddy, I.M.; Mahoney, A.W. Solution visible difference spectral properties of Fe3+-L-amino acid complexes at pH 6.60. J. Agric. Food Chem. 1995, 43, 1436–1443. [Google Scholar] [CrossRef]
- Zhao, L.N.; Cai, X.X.; Huang, S.L.; Wang, S.Y.; Huang, Y.F.; Rao, P.F. Isolation and identification of a whey protein-sourced calcium-binding tripeptide Tyr-Asp-Thr. Int. Dairy J. 2015, 40, 16–23. [Google Scholar] [CrossRef]
- Jin, H.X.; Xu, H.P.; Li, Y.; Zhang, Q.W.; Xie, H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber Acaudina molpadioides obtained from Zhejiang Province in China. Mar. Drugs. 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive peptides from Skipjack tuna cardiac arterial bulbs: Preparation, identification, antioxidant activity, and stability against thermal, pH, and simulated gastrointestinal digestion treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.; FitzGerald, R.J. Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef]
- Liu, B.T.; Zhuang, Y.L.; Sun, L.P. Identification and characterization of the peptides with calcium-binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. J. Food. Sci. 2020, 85, 114–122. [Google Scholar]
- Suo, S.K.; Zhao, Y.Q.; Wang, Y.M.; Pan, X.Y.; Chi, C.F.; Wang, B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from the protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.X.; Huai, H.P.; Hou, W.Y.; Qi, Y.; Min, W.H. Purification, identification, chelation mechanism, and calcium absorption activity of a novel calcium-binding peptide from peanut (Arachis hypogaea) protein hydrolysate. J. Agric. Food Chem. 2023, 71, 11970–11981. [Google Scholar] [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and characterization of a novel pentadecapeptide from protein hydrolysates of Cyclina sinensis and its immunomodulatory effects on RAW264.7 cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and molecular docking study of a novel angiotensin-I converting enzyme inhibitory peptide derived from enzymatic hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef]
- Liao, W.W.; Liu, S.J.; Liu, X.R.; Duan, S.; Xiao, S.Y.; Miao, J.Y. The purification, identification and bioactivity study of a novel calcium-binding peptide from casein hydrolysate. Food Funct. 2019, 10, 7724–7732. [Google Scholar] [CrossRef]
- Zhang, X.W.; Jia, Q.; Li, M.Y.; Liu, H.P.; Wang, Q.; Liu, Z.T. Isolation of a novel calcium-binding peptide from phosvitin hydrolysates and the study of its calcium chelation mechanism. Food Res. Int. 2021, 141, 110169. [Google Scholar] [CrossRef]
- He, S.; Xu, Z.; Li, J.; Guo, Y.; Lin, Q.; Jin, H. Peptides from Harpadon nehereus protect against hyperglycemia-induced HepG2 via oxidative stress and glycolipid metabolism regulation. J. Funct. Foods 2023, 108, 105723. [Google Scholar] [CrossRef]
- Xu, B.; Ye, L.; Tang, Y.; Zheng, J.; Tian, X.; Yang, Y.; Yang, Z. Preparation and purification of an immunoregulatory peptide from Stolephorus chinensis of the East Sea of China. Process Biochem. 2020, 98, 151–159. [Google Scholar] [CrossRef]
- Lee, S.; Song, K. Isolation of a calcium-binding peptide from enzymatic hydrolysates of porcine blood plasma protein. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 290–294. [Google Scholar] [CrossRef]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide-calcium complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef]
- Huang, W.; Lan, Y.; Liao, W.; Lin, L.; Liu, G.; Miao, J. Preparation, characterization and biological activities of egg white peptides-calcium chelate. LWT 2021, 149, 112035. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, Z.; Xu, H.Y.; Li, X.Y.; Hao, X.D. Preparation of sheep bone collagen peptide-calcium chelate using enzymolysis-fermentation methodology and its structural characterization and stability analysis. RSC Adv. 2020, 10, 11624–11633. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Lin, S.; Han, W.; Jiang, P.; Zhu, B.; Sun, N. Calcium delivery system assembled by a nanostructured peptide derived from the sea cucumber ovum. J. Agric. Food Chem. 2019, 67, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A. Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India—An in vitro study. Food Chem. 2015, 170, 430–436. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Deng, Y.Y.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Chen, H.; Jin, W.; Yang, Z.; Cao, Y.; Miao, J. Three newly isolated calcium-chelating peptides from tilapia bone collagen hydrolysate enhance calcium absorption activity in intestinal caco-2 cells. J. Agric. Food Chem. 2020, 68, 2091–2098. [Google Scholar] [CrossRef] [PubMed]




| Protease | Enzymolysis Condition | Ca-Chelating Rate (%) | |||
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Enzyme Dose (%) | pH | ||
| FlavourzymeAPePepsin | 50 | 4 | 2.0 | 7.0 | 30.93 ± 1.37 b |
| 37.5 | 4 | 2.0 | 2.0 | 24.11 ± 2.16 c | |
| Trypsin | 37.5 | 4 | 2.0 | 7.8 | 37.91 ± 2.96 a |
| Papain | 55 | 4 | 2.0 | 7.0 | 23.69 ± 1.98 c |
| Alcalase | 55 | 4 | 2.0 | 9.5 | 32.24 ± 2.31 b |
| No. | pH | Time (min) | Temperature (°C) | Peptide/Ca Ratio | Ca-Chelating Rate (%) |
|---|---|---|---|---|---|
| 1 | 7 | 40 | 40 | 1:5 | 42.38 |
| 2 | 7 | 50 | 50 | 1:10 | 47.11 |
| 3 | 7 | 60 | 60 | 1:15 | 46.29 |
| 4 | 8 | 40 | 50 | 1:15 | 51.95 |
| 5 | 8 | 50 | 60 | 1:5 | 53.71 |
| 6 | 8 | 60 | 40 | 1:10 | 49.17 |
| 7 | 9 | 40 | 60 | 1:10 | 47.35 |
| 8 | 9 | 50 | 40 | 1:15 | 49.15 |
| 9 | 9 | 60 | 50 | 1:5 | 47.86 |
| K1 | 135.78 | 141.68 | 140.7 | 143.95 | |
| K2 | 154.83 | 149.97 | 146.92 | 143.63 | |
| K3 | 144.36 | 143.32 | 147.35 | 147.39 | |
| Best level | A2 | B2 | C3 | D3 | |
| R | 19.05 | 8.29 | 6.65 | 3.72 | |
| R order | A > B > C > D | ||||
| No. | Retention Time (min) | Amino Acid Sequence | Observed/Theoretical MW (Da) |
|---|---|---|---|
| ACP1 | 5.29 | Ala-Lys (AK) | 217.27/217.27 |
| ACP2 | 6.18 | Glu-Ala-Arg (EAR) | 374.40/374.39 |
| ACP3 | 7.02 | Ala-Glu-Ala (AEA) | 289.29/289.29 |
| ACP4 | 7.95 | Val-Glu-Arg-Gly (VERG) | 459.50/459.50 |
| ACP5 | 8.76 | Val-Ala-Ser (VAS) | 275.30/275.30 |
| ACP6 | 10.01 | Gly-Pro-Lys (GPK) | 300.36/300.35 |
| ACP7 | 10.58 | Ser-Pro (SP) | 202.21/202.21 |
| ACP8 | 11.91 | Gly-Pro-Lys-Gly (GPKG) | 357.41/357.41 |
| ACP9 | 13.60 | Ala-Pro-Arg-Gly-His (APRGH) | 536.59/536.58 |
| ACP10 | 14.11 | Gly-Val-Pro-Gly (GVPG) | 328.37/328.36 |
| ACP11 | 14.85 | Leu-Glu-Pro-Gly-Pro (LEPGP) | 511.58/511.57 |
| ACP12 | 15.22 | Leu-Glu-Lys-Gly-Ala (LEKGA) | 516.60/516.59 |
| ACP13 | 16.98 | Phe-Pro-Pro-Gly-Arg (FPPGR) | 572.66/572.66 |
| ACP14 | 22.46 | Gly-Glu-Pro-Gly (GEPG) | 358.35/358.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.-X.; Chen, R.-P.; Zhang, L.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model. Mar. Drugs 2023, 21, 579. https://doi.org/10.3390/md21110579
Ge M-X, Chen R-P, Zhang L, Wang Y-M, Chi C-F, Wang B. Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model. Marine Drugs. 2023; 21(11):579. https://doi.org/10.3390/md21110579
Chicago/Turabian StyleGe, Ming-Xue, Ru-Ping Chen, Lun Zhang, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2023. "Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model" Marine Drugs 21, no. 11: 579. https://doi.org/10.3390/md21110579
APA StyleGe, M.-X., Chen, R.-P., Zhang, L., Wang, Y.-M., Chi, C.-F., & Wang, B. (2023). Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model. Marine Drugs, 21(11), 579. https://doi.org/10.3390/md21110579







