Transcriptomic Profiling of Tetrodotoxin-Induced Neurotoxicity in Human Cerebral Organoids
Abstract
:1. Introduction
2. Results
2.1. Cerebral Organoid Formation and Cultivation
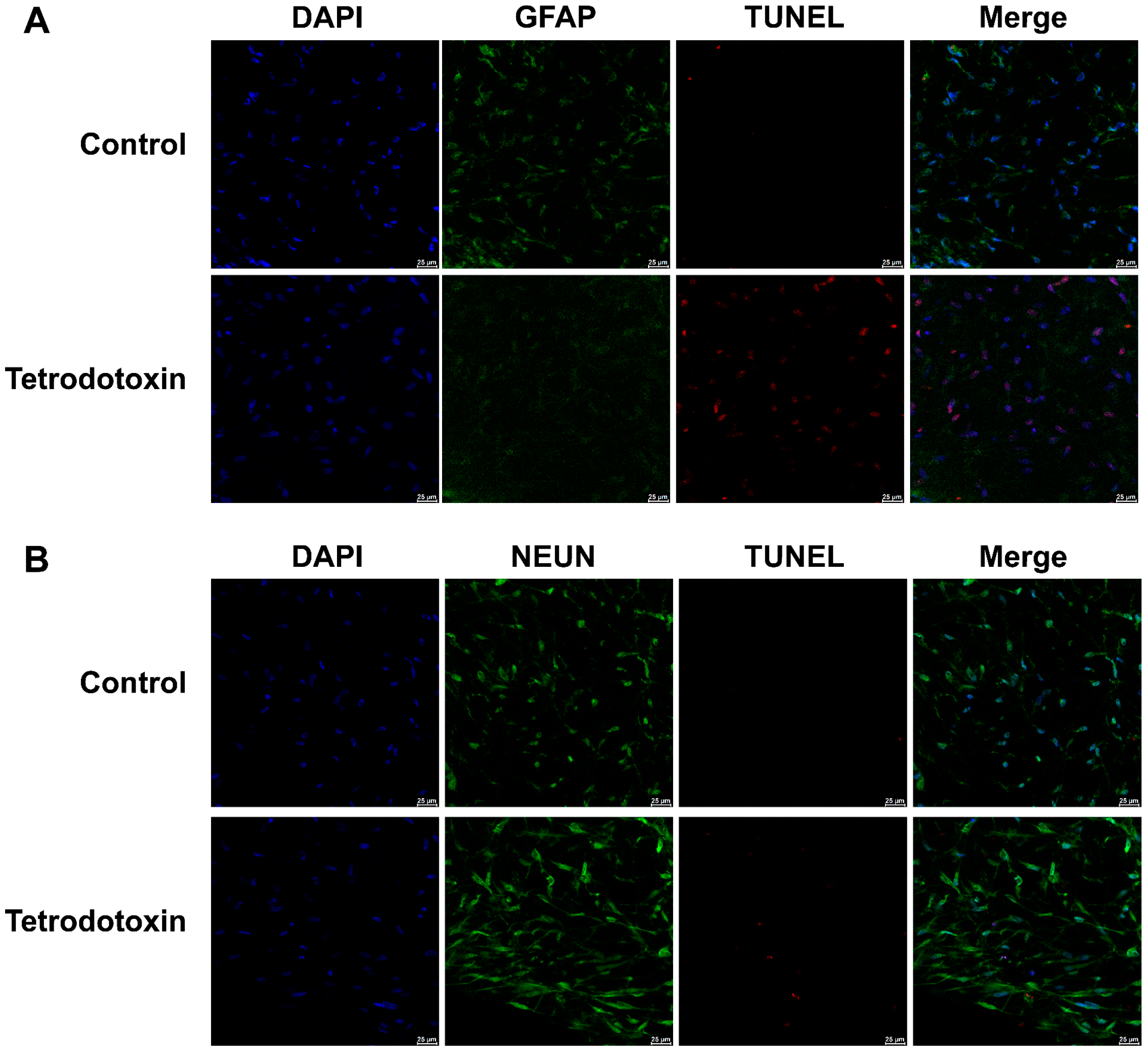
2.2. Tetrodotoxin Exposure Causes Changes in Cerebral Organoid Cell Viability
2.3. Tetrodotoxin Exposure Induces Disruption in RNA Expression within Cerebral Organoids
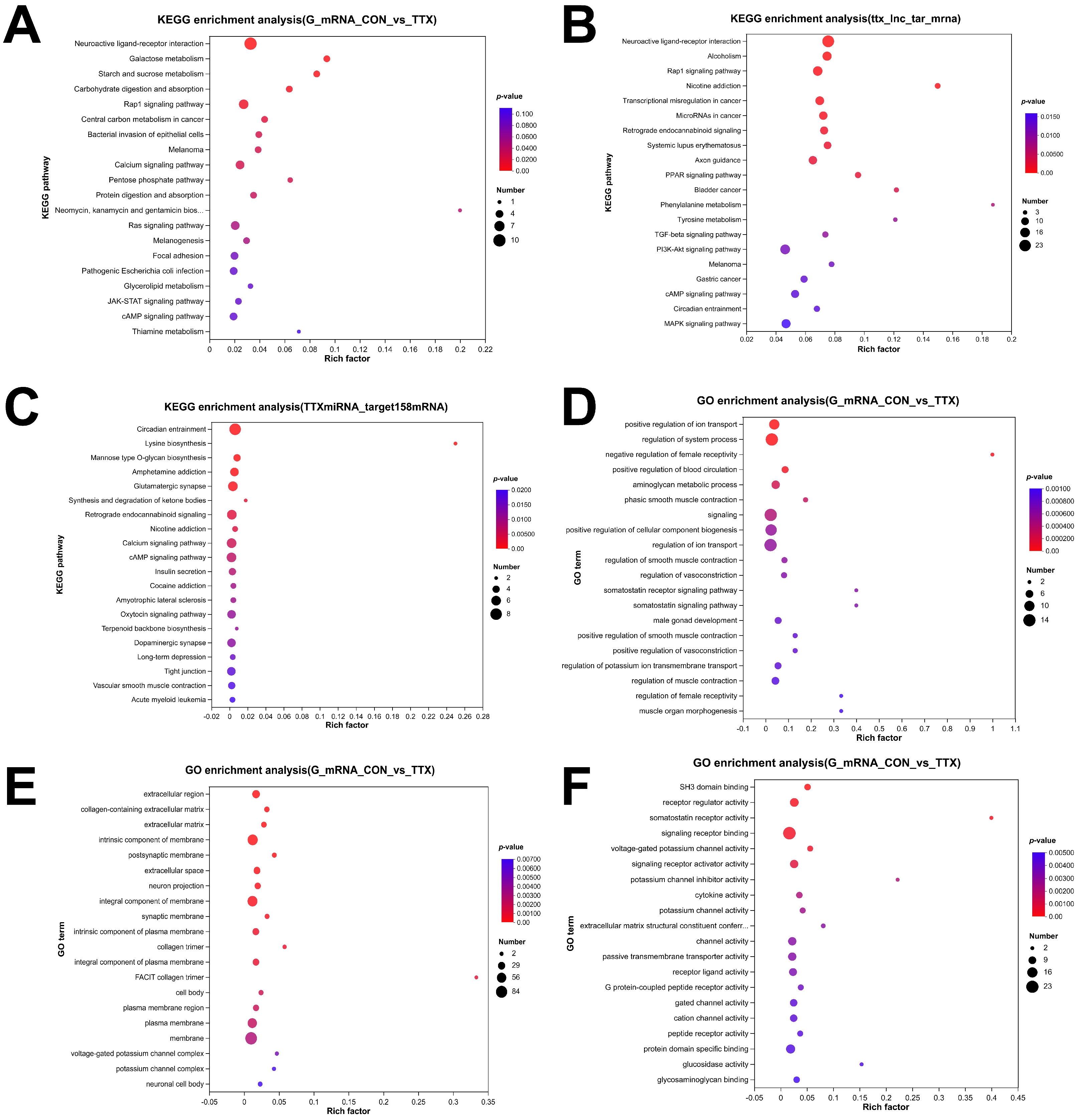
2.4. Tetrodotoxin Exposure Results in Dysregulated RNA Enrichment in GO and KEGG Pathways
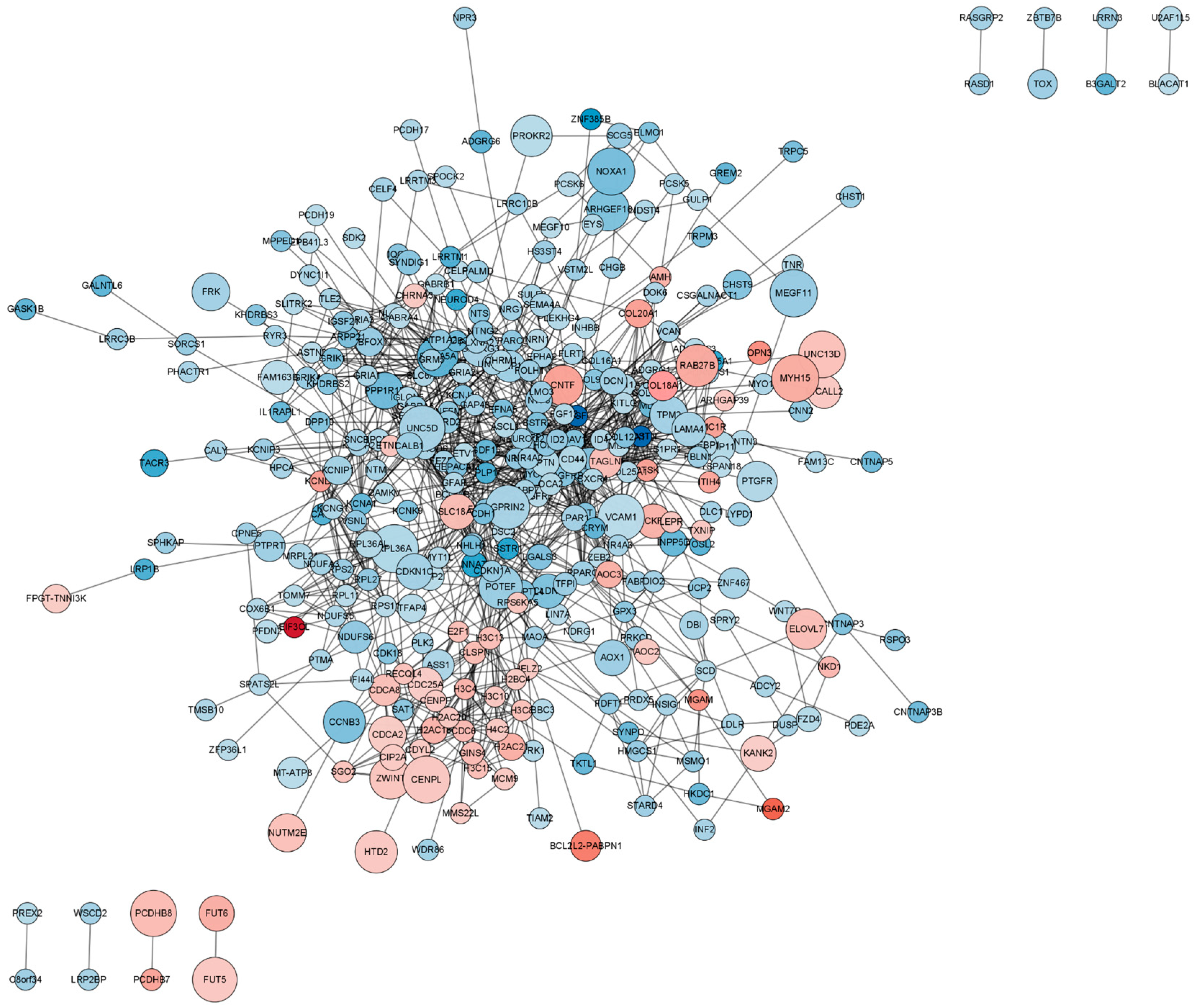
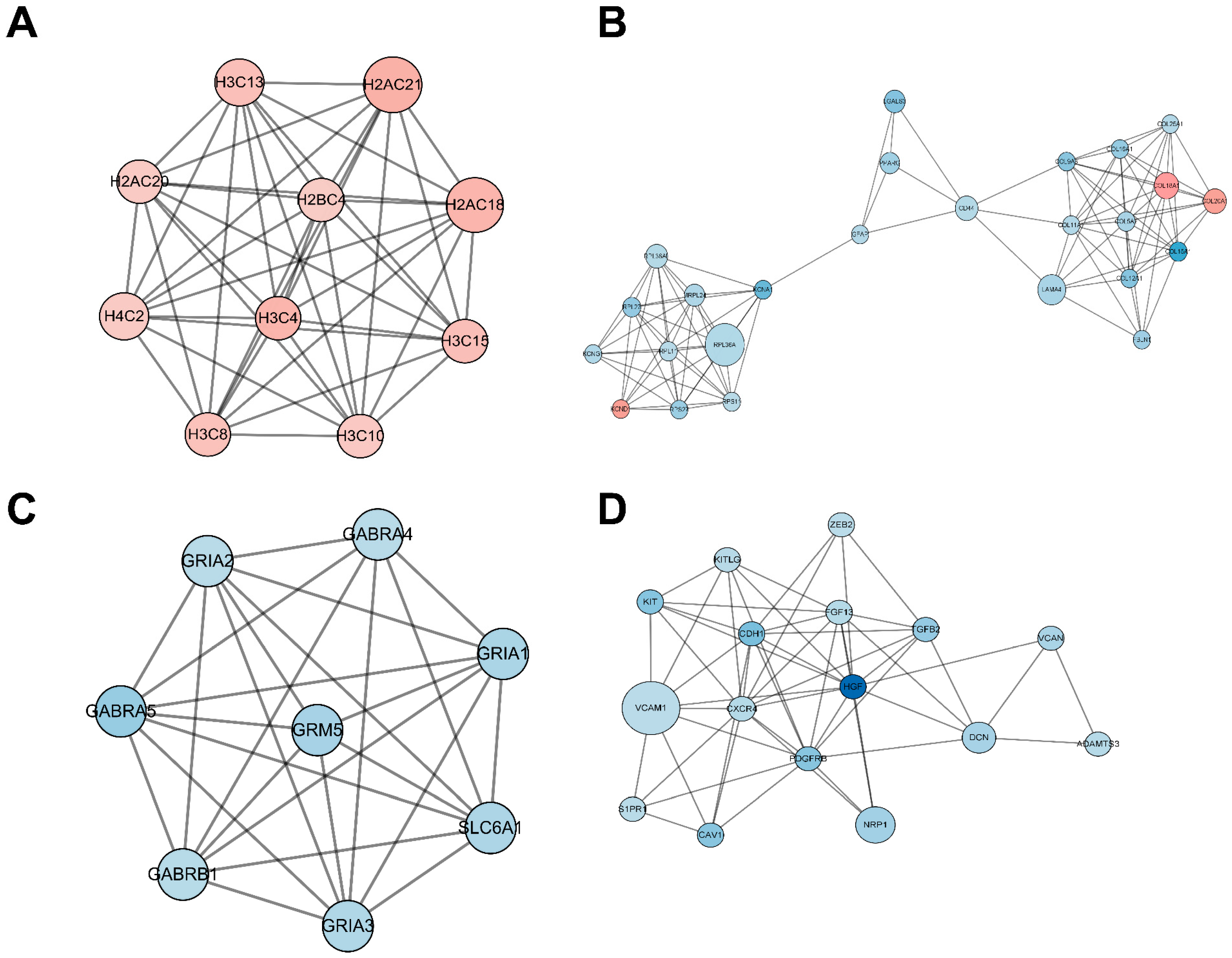
2.5. Functional Analysis of the Protein–Protein Interaction (PPI) Network
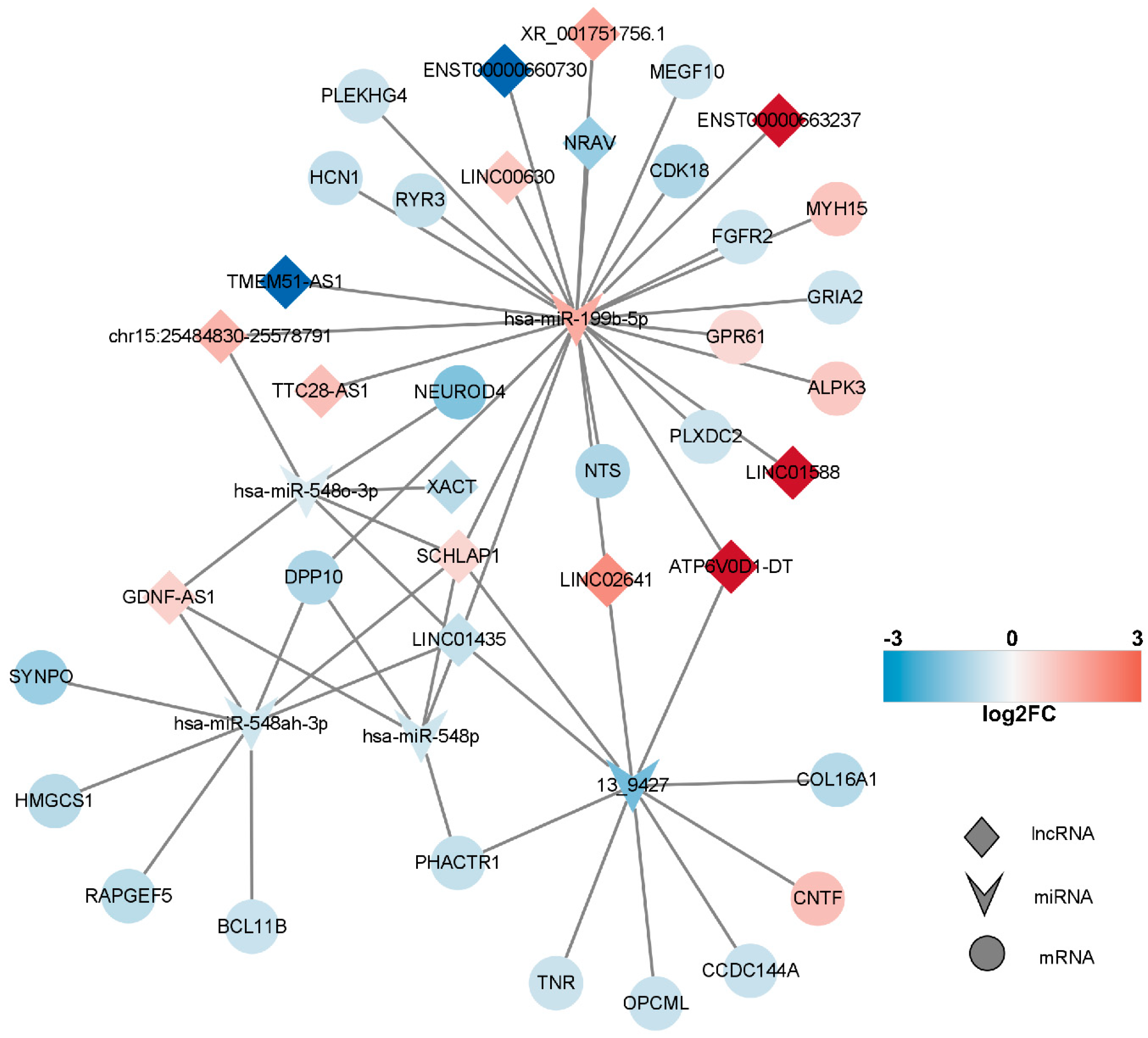
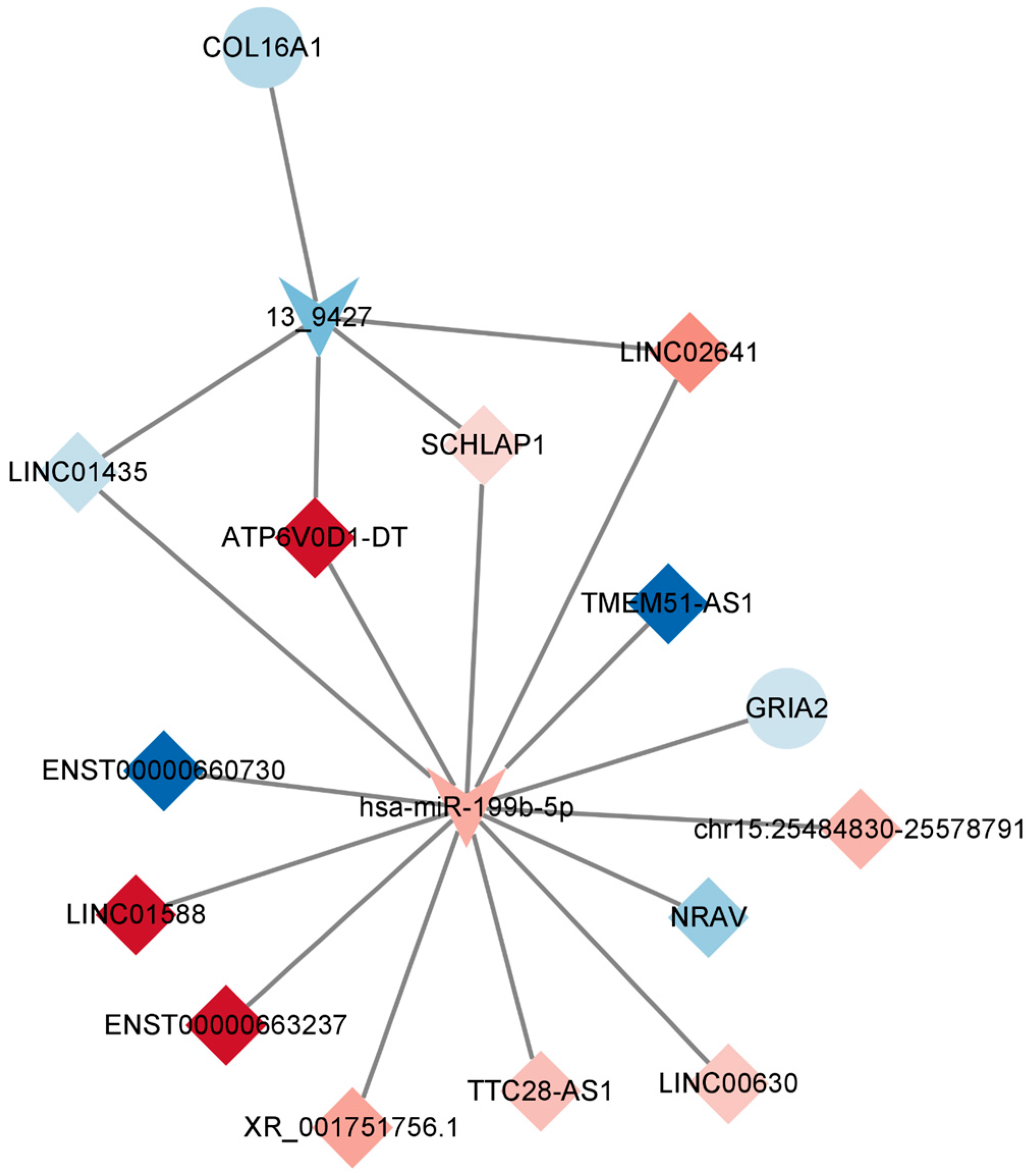
2.6. Competitive Endogenous RNA (ceRNA) Network of DEmRNAs, DElncRNAs, and DEmiRNAs
2.7. Construction of the Key lncRNA–miRNA–mRNA Subnetwork
2.8. TTX-Induced Alterations in Postsynaptic Membrane Homeostasis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cultivation of 3D Cerebral Organoids Derived from iPSCs
4.3. Immunofluorescence Characterization of Cerebral Organoids
4.4. Treatment of Cerebral Organoids with Tetrodotoxin
4.4.1. Cell Viability Assessment
4.4.2. Cytotoxicity Assessment
4.4.3. TUNEL Assay
4.5. Whole-Transcriptome Sequencing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campas, M.; Ozogul, F. An Updated Review of Tetrodotoxin and Its Peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef]
- Liu, S.H.; Tseng, C.Y.; Lin, C.C. Is neostigmine effective in severe pufferfish-associated tetrodotoxin poisoning? Clin. Toxicol. 2015, 53, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, A.; Santamaria, C.M.; Shomorony, A.; Ji, T.; Wei, T.; Gordon, A.; Elofsson, H.; Mehta, M.; Yang, R.; et al. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat. Commun. 2019, 10, 2566. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; He, J.; Le, Q.; Bai, K.; Chen, Y.; Huang, W. Combination Formulation of Tetrodotoxin and Lidocaine as a Potential Therapy for Severe Arrhythmias. Mar. Drugs 2019, 17, 685. [Google Scholar] [CrossRef]
- Shi, J.; Liu, T.T.; Wang, X.; Epstein, D.H.; Zhao, L.Y.; Zhang, X.L.; Lu, L. Tetrodotoxin reduces cue-induced drug craving and anxiety in abstinent heroin addicts. Pharmacol. Biochem. Behav. 2009, 92, 603–607. [Google Scholar] [CrossRef]
- Hong, B.; He, J.; Sun, J.; Le, Q.; Bai, K.; Mou, Y.; Zhang, Y.; Chen, W.; Huang, W. Analgesia Effect of Enteric Sustained-Release Tetrodotoxin Pellets in the Rat. Pharmaceutics 2020, 12, 32. [Google Scholar] [CrossRef]
- Liang, S.; Yin, N.; Faiola, F. Human Pluripotent Stem Cells as Tools for Predicting Developmental Neural Toxicity of Chemicals: Strategies, Applications, and Challenges. Stem Cells Dev. 2019, 28, 755–768. [Google Scholar] [CrossRef]
- Koo, B.; Choi, B.; Park, H.; Yoon, K.J. Past, Present, and Future of Brain Organoid Technology. Mol. Cells 2019, 42, 617–627. [Google Scholar] [CrossRef]
- Li, M.; Gong, J.; Gao, L.; Zou, T.; Kang, J.; Xu, H. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022, 235, 113429. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wang, Y.; Xu, M.; Han, X.; Liu, Y. The Application of Brain Organoids in Assessing Neural Toxicity. Front. Mol. Neurosci. 2022, 15, 799397. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021, 8, e2101462. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Depla, J.A.; Mulder, L.A.; de Sa, R.V.; Wartel, M.; Sridhar, A.; Evers, M.M.; Wolthers, K.C.; Pajkrt, D. Human Brain Organoids as Models for Central Nervous System Viral Infection. Viruses 2022, 14, 634. [Google Scholar] [CrossRef]
- Zhang, P.; Lisman, J.E. Activity-dependent regulation of synaptic strength by PSD-95 in CA1 neurons. J. Neurophysiol. 2012, 107, 1058–1066. [Google Scholar] [CrossRef]
- Ehrlich, I.; Klein, M.; Rumpel, S.; Malinow, R. PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. USA 2007, 104, 4176–4181. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, K.; Oh, J.Y.; Lee, H.; Park, H.; Park, Y.S.; Kim, H.K. Postsynaptic density protein 95 (PSD-95) is transported by KIF5 to dendritic regions. Mol. Brain 2019, 12, 97. [Google Scholar] [CrossRef]
- Kao, C.; Levinson, S. Tetrodotoxin, Saxitoxin, and the Molecular Biology of the Sodium Channel; New York Academy of Sciences: New York, NY, USA, 1986; Volume 479. [Google Scholar]
- Catterall, W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992, 72, S15–S48. [Google Scholar] [CrossRef]
- Hong, H.; Zhao, K.; Huang, S.; Huang, S.; Yao, A.; Jiang, Y.; Sigrist, S.; Zhao, L.; Zhang, Y.Q. Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis. J. Neurosci. 2020, 40, 2817–2827. [Google Scholar] [CrossRef]
- Jin, Y.H.; Andresen, M.C. GABA(B) restrains release from singly-evoked GABA terminals. Neuroscience 2011, 193, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Nabekura, J.; Koizumi, S. Astrocyte-mediated synapse remodeling in the pathological brain. Glia 2017, 65, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Fellin, T.; Zhu, Y.; Lee, S.Y.; Auberson, Y.P.; Meaney, D.F.; Coulter, D.A.; Carmignoto, G.; Haydon, P.G. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci. 2007, 27, 10674–10684. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Shigetomi, E.; Parajuli, B.; Nagatomo, H.; Shinozaki, Y.; Hirayama, Y.; Saito, K.; Kubota, Y.; Danjo, Y.; Lee, J.H.; et al. Adenosine A(2B) receptor down-regulates metabotropic glutamate receptor 5 in astrocytes during postnatal development. Glia 2021, 69, 2546–2558. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Min, C.K.; Yeom, D.R.; Lee, K.E.; Kwon, H.K.; Kang, M.; Kim, Y.S.; Park, Z.Y.; Jeon, H.; Kim, D.H. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012, 447, 371–379. [Google Scholar] [CrossRef]
- Ruiz, A.; Matute, C.; Alberdi, E. Endoplasmic reticulum Ca2+ release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium 2009, 46, 273–281. [Google Scholar] [CrossRef]
- Fudalej, E.; Justyniarska, M.; Kasarello, K.; Dziedziak, J.; Szaflik, J.P.; Cudnoch-Jedrzejewska, A. Neuroprotective Factors of the Retina and Their Role in Promoting Survival of Retinal Ganglion Cells: A Review. Ophthalmic Res. 2021, 64, 345–355. [Google Scholar] [CrossRef]
- Li, K.; Chen, C.; Gao, R.; Yu, X.; Huang, Y.; Chen, Z.; Liu, Z.; Chen, S.; Luo, G.; Huang, X.; et al. Inhibition of BCL11B induces downregulation of PTK7 and results in growth retardation and apoptosis in T-cell acute lymphoblastic leukemia. Biomark. Res. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, P.; Przybylski, G.K.; Depke, M.; Volker, U.; Bahr, J.; Assmus, K.; Broker, B.M.; Walther, R.; Schmidt, C.A. Inhibition of BCL11B expression leads to apoptosis of malignant but not normal mature T cells. Oncogene 2007, 26, 3797–3810. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Shen, Q.; Chen, S.; Yang, L.; Grabarczyk, P.; Przybylski, G.K.; Schmidt, C.A.; Li, Y. Down regulation of BCL11B expression inhibits proliferation and induces apoptosis in malignant T cells by BCL11B-935-siRNA. Hematology 2011, 16, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, L.; Xu, Z.; Chen, H.; Ju, S.; Ding, J.; Guo, Y.; Tian, H. Phosphatase Actin Regulator-1 (PHACTR-1) Knockdown Suppresses Cell Proliferation and Migration and Promotes Cell Apoptosis in the bEnd.3 Mouse Brain Capillary Endothelial Cell Line. Med. Sci. Monit. 2019, 25, 1291–1300. [Google Scholar] [CrossRef]
- Xing, X.; Cai, W.; Ma, S.; Wang, Y.; Shi, H.; Li, M.; Jiao, J.; Yang, Y.; Liu, L.; Zhang, X.; et al. Down-regulated expression of OPCML predicts an unfavorable prognosis and promotes disease progression in human gastric cancer. BMC Cancer 2017, 17, 268. [Google Scholar] [CrossRef]
- Sellar, G.C.; Watt, K.P.; Rabiasz, G.J.; Stronach, E.A.; Li, L.; Miller, E.P.; Massie, C.E.; Miller, J.; Contreras-Moreira, B.; Scott, D.; et al. OPCML at 11q25 is epigenetically inactivated and has tumor-suppressor function in epithelial ovarian cancer. Nat. Genet. 2003, 34, 337–343. [Google Scholar] [CrossRef]
- Li, C.; Tang, L.; Zhao, L.; Li, L.; Xiao, Q.; Luo, X.; Peng, W.; Ren, G.; Tao, Q.; Xiang, T. OPCML is frequently methylated in human colorectal cancer and its restored expression reverses EMT via downregulation of smad signaling. Am. J. Cancer Res. 2015, 5, 1635–1648. [Google Scholar]
- Saklani, P.; Khan, H.; Gupta, S.; Kaur, A.; Singh, T.G. Neuropeptides: Potential neuroprotective agents in ischemic injury. Life Sci. 2022, 288, 120186. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, Z.; Sun, D.; Li, Z.; Pu, Y.; Wang, D.; Huang, A.; He, C.; Cao, L. Cyclin-dependent Kinase 18 Promotes Oligodendrocyte Precursor Cell Differentiation through Activating the Extracellular Signal-Regulated Kinase Signaling Pathway. Neurosci. Bull. 2019, 35, 802–814. [Google Scholar] [CrossRef]
- Dhar, S.S.; Liang, H.L.; Wong-Riley, M.T. Nuclear respiratory factor 1 co-regulates AMPA glutamate receptor subunit 2 and cytochrome c oxidase: Tight coupling of glutamatergic transmission and energy metabolism in neurons. J. Neurochem. 2009, 108, 1595–1606. [Google Scholar] [CrossRef]
- Ratkai, A.; Tarnok, K.; Aouad, H.E.; Micska, B.; Schlett, K.; Szucs, A. Homeostatic plasticity and burst activity are mediated by hyperpolarization-activated cation currents and T-type calcium channels in neuronal cultures. Sci. Rep. 2021, 11, 3236. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, S.; Delcourt, N. Marine Neurotoxins’ Effects on Environmental and Human Health: An OMICS Overview. Mar. Drugs 2021, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Huang, H.; Liu, W.; Hu, P.; Huang, X.; Zou, F.; Liu, J. Uncovering the proteome response of murine neuroblastoma cells against low-dose exposure to saxitoxin. Toxicol. Mech. Methods 2018, 28, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, X.; Liu, W.; Li, S.; Zhou, Y.; Yang, X.; Liu, J. Effects of long-term low dose saxitoxin exposure on nerve damage in mice. Aging 2021, 13, 17211–17226. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Boucas, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Z.; Wei, Y.; Shi, J.; Shi, T.; Chen, X.; Li, L. Transcriptomic Profiling of Tetrodotoxin-Induced Neurotoxicity in Human Cerebral Organoids. Mar. Drugs 2023, 21, 588. https://doi.org/10.3390/md21110588
Liu Z, Wang Z, Wei Y, Shi J, Shi T, Chen X, Li L. Transcriptomic Profiling of Tetrodotoxin-Induced Neurotoxicity in Human Cerebral Organoids. Marine Drugs. 2023; 21(11):588. https://doi.org/10.3390/md21110588
Chicago/Turabian StyleLiu, Zhanbiao, Zhe Wang, Yue Wei, Jingjing Shi, Tong Shi, Xuejun Chen, and Liqin Li. 2023. "Transcriptomic Profiling of Tetrodotoxin-Induced Neurotoxicity in Human Cerebral Organoids" Marine Drugs 21, no. 11: 588. https://doi.org/10.3390/md21110588
APA StyleLiu, Z., Wang, Z., Wei, Y., Shi, J., Shi, T., Chen, X., & Li, L. (2023). Transcriptomic Profiling of Tetrodotoxin-Induced Neurotoxicity in Human Cerebral Organoids. Marine Drugs, 21(11), 588. https://doi.org/10.3390/md21110588






